Abstract
Intracerebral hemorrhage (ICH) is one of the most lethal subtypes of stroke, associated with high morbidity and mortality. Prevention of hematoma growth and perihematomal edema expansion are promising therapeutic targets currently under investigation. Despite recent improvements in the management of ICH, the ideal treatments are still to be determined. Early stratification and triage of ICH patients enable the adjustment of the standard of care in keeping with the personalized medicine principles. In recent years, research efforts have been concentrated on the development and validation of blood-based biomarkers. The benefit of looking for blood candidate markers is obvious because of their acceptance in terms of sample collection by the general population compared to any other body fluid. Given their ease of accessibility in clinical practice, blood-based biomarkers have been widely used as potential diagnostic, predictive, and prognostic markers. This review identifies some relevant and potentially promising blood biomarkers for ICH. These blood-based markers are summarized by their roles in clinical practice. Well-designed and large-scale studies are required to validate the use of all these biomarkers in the future.
1. Introduction
Spontaneous intracerebral hemorrhage (ICH) is a common subtype of stroke and accounts for nearly 10% to 30% of all strokes [1]. ICH posed a significant health burden in China in 2019, contributing to 25 million disability-adjusted life years (DALYs). It is associated with an early mortality rate of approximately 30–40% and has shown no reduction in prevalence in recent decades [2]. The higher incidence and mortality of ICH in middle-income and low-income countries may be attributed to limited public awareness regarding preventive measures, as well as challenges in accessing healthcare services [3]. So far, few treatment possibilities have yielded conclusive benefits. However, numerous predictors of poor outcomes after ICH have been suggested, including hematoma volume, hematoma expansion, perihematomal edema (PHE), and the presence and quantity of intraventricular hemorrhage growth [4,5,6,7]. Similarly, researchers continue to search for various blood-based biomarkers to guide management and predict outcomes after acute ICH [8,9,10,11,12]. A growing number of studies have shown that many novel biomarkers, such as C-reactive protein (CRP), S100A12, and interleukin-6, are associated with adverse outcomes in patients with ICH [8,9,10]. Thus, the application of blood-based biomarkers may improve risk stratification and aid clinical decisions in patients with ICH.
Therefore, in this review, we assessed the data available in the literature regarding the diagnostic and prognostic value of circulating blood biomarkers in patients with ICH. For each biomarker, we depicted the detailed characteristics in order to shed light on potential future clinical applications.
2. Diagnostic Blood Biomarkers
Early administration has proven to be effective in the management of acute stroke, including intravenous thrombolysis and mechanical thrombectomy for ischemic stroke, as well as blood pressure reduction therapy for ICH. The efficacy of these treatments is highly dependent on the time from onset to administration. While prehospital treatment shows promise, it necessitates the prior differentiation of stroke subtypes. In addressing this challenge, portable computed tomography has been used; however, achieving the widespread adoption of this costly tool does not seem possible in the near future. Consequently, it is imperative that optimal blood biomarkers demonstrate enough sensitivity and are rapidly available for distinguishing between both stroke subtypes, thereby serving as a valuable diagnostic tool for acute stroke.
2.1. Glial Fibrillary Acidic Protein (GFAP) and Retinol-Binding Protein 4
In a recent study of 272 patients (203 ischemic strokes, 60 ICH, and 9 stroke mimics), it was shown that prehospital plasma GFAP concentrations (pg/mL) could differentiate patients with ischemic stroke from those with ICH. Furthermore, the prehospital GFAP release rate (pg/mL/minute) significantly improved the diagnostic capability of this biomarker. Of note, the median time between the last-seen-well and prehospital sample times was 50 min in this study [13]. Another study demonstrated that GFAP concentrations were associated with both hematoma volume and neurological severity in ICH patients, while no such correlation was observed in ischemic stroke patients [14]. However, in a study of 74 patients, the GFAP levels in patients with a smaller ICH volume often remained in the normal range or were only slightly elevated [15]. These findings suggest that the early release of GFAP may be influenced by the extent of hemorrhagic tissue damage following an ICH event, while it may not be influenced by the extent of tissue ischemia after ischemic stroke. Retinol-binding protein 4 (RBP-4) is a promising biomarker for distinguishing ischemic stroke from ICH. In ischemic stroke patients, RBP4 > 48.75 lg/mL and GFAP < 0.07 ng/mL were found to be independent predictors of stroke subtypes after a multivariate logistic regression analysis [16].
2.2. N-Terminal Pro B-Type Natriuretic Peptide (NT-proBNP)
The stroke-chip study, with a large sample size, investigated the diagnostic accuracy of 21 biomarkers for distinguishing between ischemic and hemorrhagic strokes [17]. NT-proBNP emerged as a remarkable biomarker, with a sensitivity of 44.8% and a specificity of 74.9% in its ability to differentiate between these stroke types. In a study of 189 patients (154 with ischemic stroke and 35 with ICH), it was found that ischemic stroke patients had a higher RBP-4. It was observed that ischemic stroke patients exhibited elevated levels of RBP-4, NT-proBNP, and endostatin, while they had lower levels of GFAP than ICH patients [18].
2.3. S100B
The S100B protein typically reaches its peak concentration on day 2 or 3 after ischemic stroke onset, whereas among ICH patients, it has been shown that there is a rapid increase in plasma S100B levels within a few hours [19]. This distinct temporal pattern allows for an early discrimination between ischemic and hemorrhagic strokes shortly after the onset of symptoms. In a previous study, among the blood samples obtained within 6 h after ictus (n = 337), S100B levels were significantly higher in ICH patients (107.58 vs. 58.70 pg/mL; p < 0.001) compared to those with ischemic stroke. The optimal concentration cut-off point for differentiation was determined to be S100B > 96 pg/mL [20].
3. Etiology Blood Biomarkers
3.1. β-Amyloid-40 and β-Amyloid-42
One study including 29 cerebral amyloid angiopathy (CAA)-associated ICH patients and 21 healthy controls found higher blood β-amyloid-40 and β-amyloid-42 concentrations in probable CAA patients than in control individuals [21]. However, a previous analysis showed contradicting results [22]. These discrepancies may be attributed to differences in the methodology used in the identical detection of antibodies. Together, these findings highlight the potential of body fluid biomarkers as preclinical indicators of CAA, and they suggest that circulating β-amyloid peptides may serve as a valuable marker for assessing microvascular damage.
3.2. Matrix Metalloproteinases (MMPs)
A recent study of plasma matrix MMPs showed that MMP-2 and MMP-9 are highly secreted and expressed in hemorrhagic regions in CAA, no differences were observed in plasma levels between CAA patients and healthy controls [23]. In this study, it was observed that the expression was related to endothelial cells and reactive astrocytes surrounding vessels with CAA, whereas MMP-9 expression was restricted to inflammation cells.
4. Prognostic Blood Biomarkers for Hematoma Expansion
4.1. Serum Calcium Level
Some studies have investigated the effect of admission calcium levels on hematoma growth [24,25,26,27,28]. The definition of hypocalcemia varied across the studies. A retrospective study including 1262 patients reported an association between lower mean calcium levels on admission and hematoma growth in ICH patients [24]. Two other prospective observational cohort studies also demonstrated that lower calcium levels at presentation are associated with hematoma growth in ICH [25,26]. Another study showed an association between ionized calcium < 1.16 mmol/L and hematoma growth [27]. Finally, a large sample size study screening 2103 patients reported that a higher plasma calcium level (calcium > 8.4 mg/dL) on admission was related to a reduced risk of hematoma growth when adjusted for other confounders [28]. Overall, these results suggest a compelling hypothesis that calcium may indeed play a role in the pathophysiology of ICH, with an impaired coagulation cascade serving as a potential biological mechanism.
4.2. Serum Magnesium Level
A retrospective cohort study found an association between lower levels of admission serum magnesium levels and hematoma growth in ICH patients. Lower admission magnesium levels were related to larger baseline hemorrhage sizes, hematoma growth, and 3-month outcomes [29]. Conversely, Goyal et al. concluded that higher serum magnesium levels were independently associated with a lower baseline hematoma size and a lower ICH score. This study found no effect of the baseline plasma magnesium level on hematoma growth [30]. The two studies diverged in their methodologies. The former study included ICH patients with underlying coagulopathy who received hemostatic treatment, which could potentially confound the impact of magnesium on hemostasis. Taken together, magnesium’s potential role in coagulation and platelet function holds promise for ICH treatment.
4.3. Low-Density Lipoprotein Cholesterol (LDL-C)
A previous cohort study suggested that lower LDL-C levels independently predict hematoma growth, early neurological deterioration, and 90-day outcomes after ICH [31]. Recently, several studies have reported that low levels of LDL-C were associated with an increased risk of ICH [32] and hematoma growth [26,32,33,34]. However, the pathophysiological mechanism of LDL-C in relation to ICH remains unclear. Some hypotheses suggest that reduced cholesterol levels may weaken the endothelium, potentially increasing susceptibility to arterial fragility or delayed repair following cerebral microbleeds [31,32,33,34].
4.4. Apolipoprotein E Genotype
In a study including 510 patients with primary ICH, it was found that lobar ICH patients who possessed apolipoprotein ε2 were at an increased risk of hematoma growth in probable or definite CAA-related ICH [35].
5. Blood Biomarkers for Inflammation and Perihematomal Edema
5.1. Neutrophils, Lymphocytes, and Neutrophil–Lymphocyte Ratio (NLR)
Blood-derived inflammatory cells have been strongly recognized as contributors to ICH-mediated secondary brain damage. Accordingly, neuroinflammation plays a critical role in the physiopathological mechanism of perihematomal edema. Neutrophils are the first leukocyte subtype that infiltrates into the hemorrhagic brain and initiates inflammatory reactions [36,37,38,39]. Perihematomal tissue obtained from ICH patients showed that low numbers of neutrophils occurred in less than 8 h after symptom onset [36]. Neutrophil infiltration was observed within 2 days and still manifested at 12 days in a postmortem study of spontaneous ICH patients who survived for 1–12 days after symptom onset [40]. In blood samples from 113 ICH patients, a lower number of neutrophils was associated with a better outcome [41]. Moreover, a large cohort including 1302 patients reported that a higher neutrophil count was associated with a reduced risk of hematoma expansion [11]. A multitude of pathways associated with neutrophils-induced neurotoxicity, including the secretion of cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1beta and interleukin-6, chemokines, free radicals, and other toxic chemicals. These factors ultimately lead to mitochondrial dysfunction, cerebral edema, blood-brain-barrier (BBB) breakdown, and neuronal cell death [38,42,43]. Inflammatory biomarkers were most commonly explored. We found several studies investigating the predictive ability of three blood-borne leukocytes, namely lymphocytes, neutrophils, and monocytes, and the neutrophil–lymphocyte ratio (NLR). NLR was reported as the ratio of absolute neutrophils divided by the absolute lymphocytes and is commonly considered a readily available and inexpensive biomarker to measure systemic inflammation. A few studies have found that increased NLR values are associated with unfavorable outcomes [12,44,45] and perihematomal edema growth [46]. A retrospective study of 177 ICH patients showed that higher neutrophils, lower lymphocytes, and a higher NLR level on admission were independently associated with a poor 3-month outcome [12]. In a study of 213 ICH patients, the authors found a similar association between a higher NLR level and lower lymphocyte counts and unfavorable outcomes at 3 months [45], while one study showed an independent association between early neurological deterioration and an increased risk of in-hospital mortality, but not poor 3-month outcome [44]. This result is consistent with other data: Several research groups did not identify an independent association between a higher white blood cell count, neutrophils, or NLR and a poor outcome [47,48].
5.2. Monocytes
Several clinical studies have tested the association of monocytes with outcomes after ICH [11,47,49,50]. A recent analysis of the Genetic and Environmental Risk Factors for Hemorrhagic Stroke study demonstrated that higher monocyte counts were independently associated with greater odds of 30-day case fatality in ICH patients [49], and a larger cohort from a multi-center study reported similar findings [50]. In another large study, the results showed that a higher peripheral monocyte count was associated with a higher risk of hematoma expansion [11].
5.3. C-Reactive Protein (CRP)
C-reactive protein (CRP) is a classical acute-phase protein and serves as a marker of the inflammatory response featuring a homopentameric structure [51]. The relationship between admission plasma CRP and short-term fatal outcomes was explored in a small sample study, and an increase in the CRP concentration by 1 mg/L led to a 5.2% increase in the odds of first-week mortality [52]. Similarly, a previous study also pointed out that high serum CRP on admission was an independent predictor of unfavorable outcomes [53]. A multi-center study analyzed CRP concentrations and their association with ICH prognosis. Plasma CRP was assessed at admission, 24, 48, and 72 h after the onset of symptoms. Plasma CRP production elevated markedly at 48 to 72 h from admission following ICH and was independently related to poor 30-day functional outcomes. Moreover, the results also concluded that CRP > 10 mg/L independently predicted early hematoma growth and early neurological worsening, both of which were related to increased mortality [8]. Further, another study identified that the addition of CRP concentration to the ICH score significantly improved the accuracy by about 8% [54]. In conclusion, elevated levels of CRP were associated with a higher risk of poor outcomes in ICH patients and were shown to be an independent predictor of mortality [55,56].
5.4. S100A12
S100A12 belongs to the S100 family of calcium-binding proteins, which has newly been identified as a receptor for advanced glycation end products-binding protein. S100A12 triggers inflammatory reactions by promoting cellular and immune signaling pathways [57]. A recent study of 101 patients verified the correlation of serum S100A12 levels with hemorrhagic severity and functional outcomes in patients with acute ICH [9]. Another clinical study showed that serum S100A12 levels were correlated with NIHSS score, ICH volume, and short-term mortality after ICH [58].
5.5. S100B
S100B is a well-established biomarker that is associated with both brain injury and BBB dysfunction. A small case-control study stated that serum S100B had significantly raised after symptom ictus until day 3 in ICH patients compared to controls. This study has shown that there is a correlation between plasma S100B levels and 1-week mortality [59]. Similarly, another study showed that an increased serum level of S100B was closely correlated to the baseline hematoma volume and predicted poor outcome after ICH [19]. In conclusion, elevated S100B levels have been observed following ICH and may potentially contribute to the inflammatory processes associated with ICH in relation to a poorer clinical outcome.
5.6. High Mobility Group Box-1 (HMGB-1)
A single-center study of 65 patients depicted a significant association of HMGB-1 with levels of interleukin-6 and TNF-α and the 10-day National Institutes of Health Stroke Scale score (r = 0.845). This study also concluded that HMGB-1 levels increased dramatically in ICH patients and were associated with disease severity [60]. Serum HMGB1 level was associated with the severity of neurological impairment, indicating that HMGB1 levels could serve as a potential indicator of the extent of brain damage.
5.7. Pentraxin 3 (PTX3)
In a study including 307 ICH patients, it was shown that pentraxin3 (blood samples were drawn within 24 h after ictus) tends to increase after symptom onset. Additionally, high levels of pentraxin3 in ICH patients were correlated with increased mortality during the 5-year follow-up period [61].
5.8. Gelsolin
Plasma gelsolin plays a pivotal role in the removal of actin filaments released from deceased cells into the bloodstream [62]. Furthermore, it has the capacity to bind to a diverse range of proinflammatory and bioactive molecules that act as mediators in neurological development. In a small case-control study group, comparing ICH patients to healthy controls, a decreased concentration of admission serum gelsolin was suggested to be a valuable marker for predicting 6-month poor outcome in ICH patients [62].
5.9. CD163
CD163 expression was recently found to be elevated from 6 to 24 h, peaking at 72 h after ICH ictus in the perihematomal area [63]. Another study concluded that acute serum soluble CD163 levels were associated with hematoma growth, PHE expansion, early or delayed neurological deterioration, and 90-day functional outcomes [64].
5.10. MMP-3/MMP-9
MMPs are produced by activated microglia, infiltrating inflammatory cells following brain injury. This wide distribution implies that MMPs serve various functions in the context of ICH. Clinical studies have shown an independent association between baseline plasma MMP-9 levels and HG [65,66]. Higher elevated admission serum MMP-9 and MMP-3 levels have been found to be associated with PHE volumes, higher NIHSS scores and 90-day poor functional outcomes [67,68,69,70,71].
6. Potential Mechanisms
ICH-induced primary brain injury occurs several minutes after ictus and is due to mechanical disruption from hematoma, resulting in a sharp increase in intracranial pressure, even herniation. Secondary injury following ICH can activate the immune response. The inflammation pathway plays an essential role in exacerbating BBB disruption, mitochondrial dysfunction, neuronal apoptosis, worsened edema, and other pathophysiological consequences [10,42,55,72]. Following the initial brain injury, a cascade of inflammatory changes in the PHE tissue, composed of activated microglia and astrocytes and recruited leucocytes, propagates neuronal apoptosis, and axial degeneration. Crucially, secondary brain damage immediately develops and is mediated by cytotoxicity, excitotoxicity, the oxidative response, and the inflammatory effect of blood lysis [10,73]. Ferrous iron, a major hemoglobin degradation product, is remarkably neurotoxic through generating free radicals [74]. Emerging evidence shows that all these cascades of events result in poor outcomes for ICH. Theoretically, it would seem the most optimal to minimize the cascade of secondary injury and alleviate PHE as early as possible [38]. This concept is supported by animal experiments that have demonstrated that fingolimod might decrease brain edema, atrophy, and cell apoptosis and enhance recovery in ICH rats [75]. A recently published clinical study showed similar results [54,76].
7. Biomarker Panels
To more comprehensively address the complexity of the ICH cascade and enhance the accuracy of biomarkers as diagnostic tools, numerous studies have focused on biomarker panels rather than searching for a single biomarker. Montaner et al. [20] assessed plasma from 1015 patients (915 strokes and 90 mimics) for 10 serum biomarkers using an enzyme-linked immunosorbent assay in an emergency setting. They found that the independent predictors of stroke vs. mimics were caspase-3, D-dimer, soluble receptor for advanced glycation end products (sRAGE), chimerin, secretagogin, and MMP-9. Furthermore, when combining six biomarkers above or below their respective cut-off levels, this model demonstrated a predictive probability of stroke identification exceeding 99%. In a subsequent study conducted by this research team, they found that only S100B and sRAGE could effectively differentiate between acute ischemic stroke and ICH, achieving an area under the receiver operating characteristic curve of 0.76 for blood samples obtained within 3 h after the onset of symptoms [77]. A study published in 2016 with a small sample size found that RBP4 (>61 g/mL) and GFAP (<0.07 ng/mL) distinguished acute ischemic stroke from ICH with a specificity of 100% [16]. However, in a prospective multi-center study, the stroke-chip study, a panel of 21 biomarkers was assessed upon the immediate arrival of patients presenting within 6 h after symptom onset. None of these biomarkers demonstrated the ability to provide an ultra-early accurate differential diagnosis of stroke [17]. In recent decades, biomarkers have exhibited substantial promise for the diagnosis and prognosis of ICH. When integrated with pre-existing clinical and imaging data, they may provide additional information to help early treatment decision-making. From a clinical perspective, it is worth noting that serial biomarker measurements during the acute phases of ICH may offer increased diagnostic and predictive utility. Further validation remains necessary.
8. Surgical Intervention
Hematoma evacuation is widely regarded as a life-saving procedure for supratentorial ICH patients with brain herniation or midline shift [78]. Surgical decompression, whether with or without evacuation, may also potentially reduce mortality in supratentorial ICH patients presenting with moderate hematoma volumes. Surgical intervention after ICH offers the potential to mitigate secondary brain injury associated with the toxicity of hemoglobin degradation products. However, the result from a prospective randomized controlled trial, Surgical Trial in Intracerebral Hemorrhage (STICH I), indicated no significant advantage of surgical procedures compared with conservative treatment. Nevertheless, a subgroup analysis indicated the potential benefits of surgery in patients with lobar hematoma ≤1 cm from the cortical surface [79]. In light of these findings, the STICH II trial was conducted to evaluate the efficacy of early surgical intervention versus medical management in patients with lobar ICH volumes ranging from 10 to 100 mL without intraventricular hemorrhage [80]. There was no statistically significant difference in the rates of favorable outcome. Conventional craniotomy exhibits some drawbacks, including the risk of postoperative rebleeding and additional damage to surrounding brain tissue during surgery, all of which can contribute to increased mortality and morbidity. With the advancement of minimally invasive surgery (MIS), more recent reports have shown greater promise [81,82,83]. A recent meta-analysis of some randomized controlled trials (RCTs) demonstrated that MIS had a positive effect on outcomes compared with medical management and conventional craniotomy for ICH patients [84]. Minimally invasive surgery with thrombolysis in intracerebral hemorrhage evacuation (MISTIE III) was the first randomized controlled trial to investigate the performance of minimally invasive procedures in supratentorial ICH in association with functional outcomes [85]. The study found that patients who had a more significant hematoma reduction (residual hematoma ≤15 mL) had a higher likelihood of a good outcome at 1 year [86]. Furthermore, in the analysis of lobar ICH in MISTIE III and STICH II, end-of-treatment hematoma volume ≤28.8 mL in MISTIE III and ≤30.0 mL in STICH II showed an increased probability of modified Rankin Scale 0 to 3 at 180 days [87]. These findings imply that future advancements in surgical techniques aimed at residual hematoma volumes may yield even more promising results. Moreover, endoscopic evacuation is associated with a lower rate of rebleeding, a shorter hospital stay and the greatest improvement in mortality and functional independence [88,89]. In a retrospective study including 156 patients with spontaneous basal ganglia hemorrhage, it was observed that the mortality of the stereotactic aspiration group was significantly higher than that of the endoscopic aspiration group in the medium (≥40–<80 mL) and large (≥80 mL) hematoma subgroups [88]. Recent breakthroughs in therapeutic devices are significantly advancing the field of ICH treatment. Future studies should aim to develop predictive models or decision-support tools that incorporate both hematoma features and biomarker panels for determining the optimal management strategies for ICH.
9. Future Directions
Specifically, most studies reported on an association between blood biomarkers from single time-point samples after ICH onset and functional outcomes. This may lead to an improved utilization of time-limited treatments. However, as the concentrations of these biomarkers may peak several days after onset, analyzing dynamic changes at a single time point or merely up to 48 or 72 h after ictus may yield limited results. Using a single time point to evaluate a biomarker might not be sufficient to reflect the complex underlying mechanisms. Thus, the post-stroke dynamic changes in serum markers should be sampled at multiple time points. From a clinical perspective, it is crucial to note that sequential measurements of biomarkers are especially informative.
Given the importance of early management [90], considerable emphasis has been delegated to clinicians on making the strategy to expedite the early identification of high-risk patients. The identification of specific biomarkers might provide important information in individualizing the decision to initiate an anti-expansion treatment strategy. Although standardized assessment tools such as the ICH score and 24-point BRAIN score have been validated in clinical practice, these tests remain imperfect [91,92]. The inclusion of blood biomarkers in the prognostic model could be useful for patients with a predicted poor outcome and add key information in the early risk stratification.
10. Conclusions
The blood biomarkers for each category are summarized in Table 1 and Figure 1. Research on the identification of appealing blood markers that are related to ICH prognosis is developing at a rapid pace. Based on the existing studies, the research can be roughly divided into studies on brain edema, such as inflammatory biomarkers, oxidative stress biomarkers, neuron and astrocyte-specific markers, coagulation markers, genetic biomarkers, and metabolic biomarkers. Thus, it is possible that a biomarker or panel of biomarkers will be useful in providing prognostic information and determining individualized treatment decisions in the future. Further investigations with well-designed studies, large sample sizes, and comprehensive data will be needed to validate the findings.

Table 1.
Blood-based Biomarkers in Intracerebral Hemorrhage.
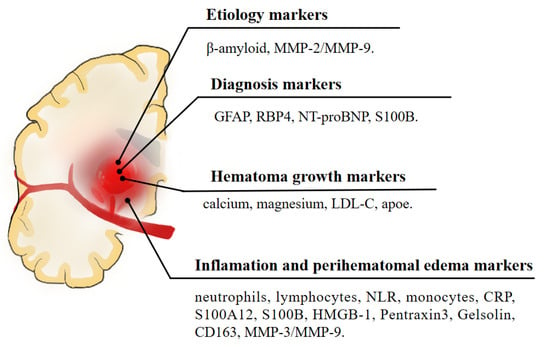
Figure 1.
A schematic illustration of blood biomarkers of intracerebral hemorrhage.
Although research and clinical trials have questioned the availability of targeting inflammatory mediators in the therapy of ICH, the results remain controversial. This reality may be explained by the complex association between cell-mediated immune function and pro- and anti-inflammatory mediators. Thus, a comprehensive understanding of the functional role in the inflammatory reaction in ICH might help to develop and focus new therapeutic targets in the future.
Author Contributions
Q.L. and X.-N.L.: study concept and design. X.-N.L. and Z.-Q.L. drafting of the manuscript. All authors discussed the ideas, interpreted the data, edited the paper and approved the final version. Q.L.: Obtained funding. Q.L. and X.-N.L.: Critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 82071337), the Chongqing Innovation Support Program for Returned Overseas Chinese Scholars (Grant No. cx2020002), Chongqing Science Fund for Distinguished Young Scholars (Grant No. cstc2021jcyj-jqX0029), Research Fund of Anhui Institute of Translational Medicine (Grant No. 2022zhyx-C38), and the First Affiliated Hospital of Chongqing Medical University Ph.D. Innovation Program (Grant No. CYYY-BSYJSCXXM-202314).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Many thanks to Lan Deng for her contributions in discussing the ideas and editing the paper.
Conflicts of Interest
The authors declare no competing interests.
References
- Van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., 3rd; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, H.B.; Chang, Y.; Falcone, G.J.; Cai, X.; Ayres, A.M.; Battey, T.W.; Vashkevich, A.; McNamara, K.A.; Valant, V.; Schwab, K.; et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014, 71, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.N.; Li, Z.Q.; Deng, L.; Yang, W.S.; Li, Y.L.; Huang, Y.J.; Shen, Y.Q.; Xie, X.F.; Li, X.H.; Wang, Z.J.; et al. Early Perihematomal Edema Expansion: Definition, Significance, and Association with Outcomes after Intracerebral Hemorrhage. Oxid. Med. Cell. Longev. 2021, 2021, 6249509. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, R.; Zhao, L.B.; Yang, X.M.; Yang, W.S.; Deng, L.; Lv, X.N.; Wu, G.F.; Tang, Z.P.; Wei, M.; et al. Intraventricular Hemorrhage Growth: Definition, Prevalence and Association with Hematoma Expansion and Prognosis. Neurocrit. Care 2020, 33, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Yogendrakumar, V.; Ramsay, T.; Fergusson, D.A.; Demchuk, A.M.; Aviv, R.I.; Rodriguez-Luna, D.; Molina, C.A.; Silva, Y.; Dzialowski, I.; Kobayashi, A.; et al. PREDICT/Sunnybrook CTA Study Group. Redefining Hematoma Expansion with the Inclusion of Intraventricular Hemorrhage Growth. Stroke 2020, 51, 1120–1127. [Google Scholar] [CrossRef]
- Di Napoli, M.; Godoy, D.A.; Campi, V.; Masotti, L.; Smith, C.J.; Parry Jones, A.R.; Hopkins, S.J.; Slevin, M.; Papa, F.; Mogoanta, L.; et al. C-reactive protein in intracerebral hemorrhage: Time course, tissue localization, and prognosis. Neurology 2012, 79, 690–699. [Google Scholar] [CrossRef]
- Qiu, S.Z.; Zheng, G.R.; Ma, C.Y.; Chen, B.; Huang, J.J.; Huang, G.; Hua, H. High Serum S100A12 Levels Predict Poor Outcome After Acute Primary Intracerebral Hemorrhage. Neuropsychiatr. Dis. Treat. 2021, 17, 3245–3253. [Google Scholar] [CrossRef]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef]
- Morotti, A.; Phuah, C.L.; Anderson, C.D.; Jessel, M.J.; Schwab, K.; Ayres, A.M.; Pezzini, A.; Padovani, A.; Gurol, M.E.; Viswanathan, A.; et al. Leukocyte Count and Intracerebral Hemorrhage Expansion. Stroke 2016, 47, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, S.; Cagnetti, C.; Provinciali, L.; Silvestrini, M. Neutrophil-to-Lymphocyte Ratio Predicts the Outcome of Acute Intracerebral Hemorrhage. Stroke 2016, 47, 1654–1657. [Google Scholar] [CrossRef]
- Mattila, O.S.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Harve-Rytsälä, H.; Pihlasviita, S.; Ritvonen, J.; Sibolt, G.; Nukarinen, T.; Curtze, S.; et al. Ultra-Early Differential Diagnosis of Acute Cerebral Ischemia and Hemorrhagic Stroke by Measuring the Prehospital Release Rate of GFAP. Clin. Chem. 2021, 67, 1361–1372. [Google Scholar] [CrossRef]
- Foerch, C.; Niessner, M.; Back, T.; Bauerle, M.; De Marchis, G.M.; Ferbert, A.; Grehl, H.; Hamann, G.F.; Jacobs, A.; Kastrup, A.; et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin. Chem. 2012, 58, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, M.; Waldschmidt, C.; Kunz, A.; Grittner, U.; Ebinger, M.; Wendt, M.; Winter, B.; Bollweg, K.; Villringer, K.; Fiebach, J.B.; et al. Glial Fibrillary Acidic Protein for Prehospital Diagnosis of Intracerebral Hemorrhage. Cerebrovasc. Dis. 2017, 43, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Llombart, V.; García-Berrocoso, T.; Bustamante, A.; Giralt, D.; Rodriguez-Luna, D.; Muchada, M.; Penalba, A.; Boada, C.; Hernández-Guillamon, M.; Montaner, J. Plasmatic retinol-binding protein 4 and glial fibrillary acidic protein as biomarkers to differentiate ischemic stroke and intracerebral hemorrhage. J. Neurochem. 2016, 136, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, A.; López-Cancio, E.; Pich, S.; Penalba, A.; Giralt, D.; García-Berrocoso, T.; Ferrer-Costa, C.; Gasull, T.; Hernández-Pérez, M.; Millan, M.; et al. Blood Biomarkers for the Early Diagnosis of Stroke: The Stroke-Chip Study. Stroke 2017, 48, 2419–2425. [Google Scholar] [CrossRef]
- Bustamante, A.; Penalba, A.; Orset, C.; Azurmendi, L.; Llombart, V.; Simats, A.; Pecharroman, E.; Ventura, O.; Ribó, M.; Vivien, D.; et al. Blood Biomarkers to Differentiate Ischemic and Hemorrhagic Strokes. Neurology 2021, 96, e1928–e1939. [Google Scholar] [CrossRef]
- Delgado, P.; Alvarez Sabin, J.; Santamarina, E.; Molina, C.A.; Quintana, M.; Rosell, A.; Montaner, J. Plasma S100B level after acute spontaneous intracerebral hemorrhage. Stroke 2006, 37, 2837–2839. [Google Scholar] [CrossRef]
- Montaner, J.; Mendioroz, M.; Delgado, P.; García-Berrocoso, T.; Giralt, D.; Merino, C.; Ribó, M.; Rosell, A.; Penalba, A.; Fernández-Cadenas, I.; et al. Differentiating ischemic from hemorrhagic stroke using plasma biomarkers: The S100B/RAGE pathway. J. Proteom. 2012, 75, 4758–4765. [Google Scholar] [CrossRef]
- Hernandez-Guillamon, M.; Delgado, P.; Penalba, A.; Rodriguez-Luna, D.; Molina, C.A.; Rovira, A.; Alvarez-Sabin, J.; Boada, M.; Montaner, J. Plasma β-amyloid levels in cerebral amyloid angiopathy-associated hemorrhagic stroke. Neurodegener. Dis. 2012, 10, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Cho, H.S.; O’Donnell, H.C.; Rosand, J.; Segal, A.Z.; Younkin, L.H.; Younkin, S.G.; Rebeck, G.W. Plasma beta-amyloid peptide, transforming growth factor-beta 1, and risk for cerebral amyloid angiopathy. Ann. N. Y. Acad. Sci. 2000, 903, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Guillamon, M.; Martinez-Saez, E.; Delgado, P.; Domingues-Montanari, S.; Boada, C.; Penalba, A.; Boada, M.; Pagola, J.; Maisterra, O.; Rodriguez-Luna, D.; et al. MMP-2/MMP-9 plasma level and brain expression in cerebral amyloid angiopathy-associated hemorrhagic stroke. Brain Pathol. 2012, 22, 133–141. [Google Scholar] [CrossRef]
- Tu, L.; Liu, X.; Li, T.; Yang, X.; Ren, Y.; Zhang, Q.; Yao, H.; Qu, X.; Wang, Q.; Tian, T.; et al. Admission Serum Calcium Level as a Prognostic Marker for Intracerebral Hemorrhage. Neurocrit. Care 2019, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Zheng, S.F.; Yao, P.S.; Chen, G.R.; Li, G.H.; Li, S.C.; Zheng, Y.F.; Wang, J.Q.; Kang, D.Z.; Shang-Guan, H.C.; et al. Lower Ionized Calcium Predicts Hematoma Expansion and Poor Outcome in Patients with Hypertensive Intracerebral Hemorrhage. World Neurosurg. 2018, 118, e500–e504. [Google Scholar] [CrossRef] [PubMed]
- Elkhatib, T.H.M.; Shehta, N.; Bessar, A.A. Hematoma Expansion Predictors: Laboratory and Radiological Risk Factors in Patients with Acute Intracerebral Hemorrhage: A Prospective Observational Study. J. Stroke Cerebrovasc. Dis. 2019, 28, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, H.; Wu, T.Y.; Meretoja, A.; Putaala, J.; Tatlisumak, T.; Strbian, D. Effect of baseline hypocalcaemia on volume of intracerebral haemorrhage in patients presenting within 72 hours from symptom onset. J. Neurol. Sci. 2019, 403, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Morotti, A.; Charidimou, A.; Phuah, C.L.; Jessel, M.J.; Schwab, K.; Ayres, A.M.; Romero, J.M.; Viswanathan, A.; Gurol, M.E.; Greenberg, S.M.; et al. Association Between Serum Calcium Level and Extent of Bleeding in Patients With Intracerebral Hemorrhage. JAMA Neurol. 2016, 73, 1285–1290. [Google Scholar] [CrossRef]
- Liotta, E.M.; Prabhakaran, S.; Sangha, R.S.; Bush, R.A.; Long, A.E.; Trevick, S.A.; Potts, M.B.; Jahromi, B.S.; Kim, M.; Manno, E.M.; et al. Magnesium, hemostasis, and outcomes in patients with intracerebral hemorrhage. Neurology 2017, 89, 813–819. [Google Scholar] [CrossRef]
- Goyal, N.; Tsivgoulis, G.; Malhotra, K.; Houck, A.L.; Khorchid, Y.M.; Pandhi, A.; Inoa, V.; Alsherbini, K.; Alexandrov, A.V.; Arthur, A.S.; et al. Serum Magnesium Levels and Outcomes in Patients With Acute Spontaneous Intracerebral Hemorrhage. J. Am. Heart Assoc. 2018, 7, e008698. [Google Scholar] [CrossRef]
- Chang, J.J.; Katsanos, A.H.; Khorchid, Y.; Dillard, K.; Kerro, A.; Burgess, L.G.; Goyal, N.; Alexandrov, A.W.; Alexandrov, A.V.; Tsivgoulis, G. Higher low-density lipoprotein cholesterol levels are associated with decreased mortality in patients with intracerebral hemorrhage. Atherosclerosis 2018, 269, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Luna, D.; Rubiera, M.; Ribo, M.; Coscojuela, P.; Pagola, J.; Piñeiro, S.; Ibarra, B.; Meler, P.; Maisterra, O.; Romero, F.; et al. Serum low-density lipoprotein cholesterol level predicts hematoma growth and clinical outcome after acute intracerebral hemorrhage. Stroke 2011, 42, 2447–2452. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, J.D.; Folsom, A.R.; Longstreth, W.T., Jr.; Shahar, E.; Rosamond, W.D.; Cushman, M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007, 38, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Gu, H.; Yang, K.; Jiang, R.; Li, Z.; Zhao, X.; Wang, Y.; Chinese Stroke Center Alliance Investigators. Lower low-density lipoprotein cholesterol levels are associated with an increased risk of hematoma expansion and ensuing mortality in acute ICH patients. Neurol. Sci. 2022, 43, 3121–3129. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, H.B.; Biffi, A.; Ayres, A.M.; Schwab, K.; Cortellini, L.; Romero, J.M.; Rost, N.S.; Viswanathan, A.; Greenberg, S.M.; Rosand, J.; et al. Apolipoprotein E genotype predicts hematoma expansion in lobar intracerebral hemorrhage. Stroke 2012, 43, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.M.; Clayton, J.A. Early cellular events in the penumbra of human spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc. Dis. 1999, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yong, V.W. Neuroinflammation in intracerebral haemorrhage: Immunotherapies with potential for translation. Lancet Neurol. 2020, 19, 1023–1032. [Google Scholar] [CrossRef]
- Wang, J.; Doré, S. Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2007, 27, 894–908. [Google Scholar] [CrossRef]
- Wang, J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 2010, 92, 463–477. [Google Scholar] [CrossRef]
- Shtaya, A.; Bridges, L.R.; Esiri, M.M.; Lam-Wong, J.; Nicoll, J.A.R.; Boche, D.; Hainsworth, A.H. Rapid neuroinflammatory changes in human acute intracerebral hemorrhage. Ann. Clin. Transl. Neurol. 2019, 6, 1465–1479. [Google Scholar] [CrossRef]
- Kayhanian, S.; Weerasuriya, C.K.; Rai, U.; Young, A.M.H. Prognostic value of peripheral leukocyte counts and plasma glucose in intracerebral haemorrhage. J. Clin. Neurosci. 2017, 41, 50–53. [Google Scholar] [CrossRef]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Czap, A.; Staff, I.; Fortunato, G.; McCullough, L.D. Peripheral leukocyte counts and outcomes after intracerebral hemorrhage. J. Neuroinflamm. 2011, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Giede-Jeppe, A.; Bobinger, T.; Gerner, S.T.; Sembill, J.A.; Sprügel, M.I.; Beuscher, V.D.; Lücking, H.; Hoelter, P.; Kuramatsu, J.B.; Huttner, H.B. Neutrophil-to-Lymphocyte Ratio Is an Independent Predictor for In-Hospital Mortality in Spontaneous Intracerebral Hemorrhage. Cerebrovasc. Dis. 2017, 44, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Z.; Gong, G.; Li, H.; Chen, L.; Song, B.; Liu, X.; Shi, C.; Yang, J.; Yang, T.; et al. Early increased neutrophil-to-lymphocyte ratio is associated with poor 3-month outcomes in spontaneous intracerebral hemorrhage. PLoS ONE 2019, 14, e0211833. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, A.M.; Gialdini, G.; Kone, G.; Baradaran, H.; Merkler, A.E.; Mangat, H.S.; Navi, B.B.; Iadecola, C.; Gupta, A.; Kamel, H.; et al. Neutrophil-Lymphocyte Ratio and Perihematomal Edema Growth in Intracerebral Hemorrhage. Stroke 2017, 48, 2589–2592. [Google Scholar] [CrossRef] [PubMed]
- Mackey, J.; Blatsioris, A.D.; Saha, C.; Moser, E.A.S.; Carter, R.J.L.; Cohen-Gadol, A.A.; Leipzig, T.J.; Williams, L.S. Higher Monocyte Count is Associated with 30-Day Case Fatality in Intracerebral Hemorrhage. Neurocrit. Care 2021, 34, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Arima, H.; Heeley, E.; Delcourt, C.; Krause, M.; Peng, B.; Yang, J.; Wu, G.; Chen, X.; Chalmers, J.; et al. White blood cell count and clinical outcomes after intracerebral hemorrhage: The INTERACT2 trial. J. Neurol. Sci. 2016, 361, 112–116. [Google Scholar] [CrossRef]
- Adeoye, O.; Walsh, K.; Woo, J.G.; Haverbusch, M.; Moomaw, C.J.; Broderick, J.P.; Kissela, B.M.; Kleindorfer, D.; Flaherty, M.L.; Woo, D. Peripheral monocyte count is associated with case fatality after intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2014, 23, e107–e111. [Google Scholar] [CrossRef]
- Walsh, K.B.; Sekar, P.; Langefeld, C.D.; Moomaw, C.J.; Elkind, M.S.; Boehme, A.K.; James, M.L.; Osborne, J.; Sheth, K.N.; Woo, D.; et al. Monocyte Count and 30-Day Case Fatality in Intracerebral Hemorrhage. Stroke 2015, 46, 2302–2304. [Google Scholar] [CrossRef]
- Volanakis, J.E. Human C-reactive protein: Expression, structure, and function. Mol. Immunol. 2001, 38, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, M.L.; Danovska, M.P. Serum C-reactive protein and lipid hydroperoxides in predicting short-term clinical outcome after spontaneous intracerebral hemorrhage. J. Clin. Neurosci. 2011, 18, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Löppönen, P.; Qian, C.; Tetri, S.; Juvela, S.; Huhtakangas, J.; Bode, M.K.; Hillbom, M. Predictive value of C-reactive protein for the outcome after primary intracerebral hemorrhage. J. Neurosurg. 2014, 121, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Godoy, D.A.; Campi, V.; del Valle, M.; Piñero, G.; Mirofsky, M.; Popa-Wagner, A.; Masotti, L.; Papa, F.; Rabinstein, A.A. C-reactive protein level measurement improves mortality prediction when added to the spontaneous intracerebral hemorrhage score. Stroke 2011, 42, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Bader, E.R.; Pana, T.A.; Barlas, R.S.; Metcalf, A.K.; Potter, J.F.; Myint, P.K. Elevated inflammatory biomarkers and poor outcomes in intracerebral hemorrhage. J. Neurol. 2022, 269, 6330–6341. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Parry-Jones, A.R.; Smith, C.J.; Hopkins, S.J.; Slevin, M.; Masotti, L.; Campi, V.; Singh, P.; Papa, F.; Popa-Wagner, A.; et al. C-reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke 2014, 45, 59–65. [Google Scholar] [CrossRef]
- Pietzsch, J.; Hoppmann, S. Human S100A12: A novel key player in inflammation? Amino Acids 2009, 36, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.Q.; He, S.R.; Li, B.B.; Qian, J.; Zheng, X.D. Serum S100A12 and 30-day mortality after acute intracerebral hemorrhage. Clin. Chim. Acta 2018, 477, 1–6. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Dong, X.Q.; Yu, W.H.; Zhang, Z.Y. Change in plasma S100B level after acute spontaneous basal ganglia hemorrhage. Shock 2010, 33, 134–140. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, K.L.; Lin, S.; Zhong, Q.; Lu, F.L.; Liang, H.; Li, J.C.; Wang, J.Z.; Yang, Q.W. Elevation of high-mobility group protein box-1 in serum correlates with severity of acute intracerebral hemorrhage. Mediat. Inflamm. 2010, 2010, 142458. [Google Scholar] [CrossRef]
- Hao, P.H.; Fu, C.; Ma, T.; He, S.M.; Jia, Z.P. Prognostic value of serum pentraxin 3 for intracerebral hemorrhage mortality. J. Integr. Neurosci. 2021, 20, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Q.; Wang, K.; Zhang, H.D.; Li, Y.J. Significant reduction of plasma gelsolin levels in patients with intracerebral hemorrhage. Clin. Chim. Acta 2013, 415, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hu, B.; Shao, S.; Wu, W.; Fan, L.; Bai, G.; Shang, P.; Wang, X. CD163/Hemoglobin Oxygenase-1 Pathway Regulates Inflammation in Hematoma Surrounding Tissues after Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 2015, 24, 2800–2809. [Google Scholar] [CrossRef] [PubMed]
- Roy-O’Reilly, M.; Zhu, L.; Atadja, L.; Torres, G.; Aronowski, J.; McCullough, L.; Edwards, N.J. Soluble CD163 in intracerebral hemorrhage: Biomarker for perihematomal edema. Ann. Clin. Transl. Neurol. 2017, 4, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhuang, X.; Peng, F.; Zheng, W. Relationship of plasma matrix metalloproteinase-9 and hematoma expansion in acute hypertensive cerebral hemorrhage. Int. J. Neurosci. 2016, 126, 213–218. [Google Scholar] [PubMed]
- Silva, Y.; Leira, R.; Tejada, J.; Lainez, J.M.; Castillo, J.; Dávalos, A.; Stroke Project, Cerebrovascular Diseases Group of the Spanish Neurological Society. Molecular signatures of vascular injury are associated with early growth of intracerebral hemorrhage. Stroke 2005, 36, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, M.; Tamborino, C.; De Santis, G.; Garofano, F.; Lupato, A.; Ramponi, V.; Trentini, A.; Casetta, I.; Bellini, T.; Fainardi, E. Timing of serum active MMP-9 and MMP-2 levels in acute and subacute phases after spontaneous intracerebral hemorrhage. Acta Neurochir. Suppl. 2010, 106, 137–140. [Google Scholar]
- Howe, M.D.; Furr, J.W.; Zhu, L.; Edwards, N.J.; McCullough, L.D.; Gonzales, N.R. Sex-specific Association of Matrix Metalloproteinases with Secondary Injury and Outcomes after Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 2019, 28, 1718–1725. [Google Scholar] [CrossRef]
- Alvarez-Sabín, J.; Delgado, P.; Abilleira, S.; Molina, C.A.; Arenillas, J.; Ribó, M.; Santamarina, E.; Quintana, M.; Monasterio, J.; Montaner, J. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: Relationship to clinical and radiological outcome. Stroke 2004, 35, 1316–1322. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.F.; Ma, L.; Worthmann, H.; Wang, Y.L.; Wang, Y.J.; Gao, Y.P.; Raab, P.; Dengler, R.; Weissenborn, K.; et al. Association of molecular markers with perihematomal edema and clinical outcome in intracerebral hemorrhage. Stroke 2013, 44, 658–663. [Google Scholar] [CrossRef]
- Abilleira, S.; Montaner, J.; Molina, C.A.; Monasterio, J.; Castillo, J.; Alvarez-Sabín, J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J. Neurosurg. 2003, 99, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; Lu, H.; Yang, Q.; Wu, H.; Wang, J. Microglial Polarization and Inflammatory Mediators After Intracerebral Hemorrhage. Mol. Neurobiol. 2017, 54, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.; Bagley, J.H.; Di, C.; Friedman, A.H.; Adamson, C. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurg. Focus 2012, 32, E8. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Ossa, N.; Sobrino, T.; Silva, Y.; Blanco, M.; Millán, M.; Gomis, M.; Agulla, J.; Araya, P.; Reverté, S.; Serena, J.; et al. Iron-related brain damage in patients with intracerebral hemorrhage. Stroke 2010, 41, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Barfejani, A.H.; Qin, T.; Dong, Q.; Ayata, C.; Waeber, C. Fingolimod exerts neuroprotective effects in a mouse model of intracerebral hemorrhage. Brain Res. 2014, 1555, 89–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fu, Y.; Hao, J.; Zhang, N.; Ren, L.; Sun, N.; Li, Y.J.; Yan, Y.; Huang, D.; Yu, C.; Shi, F.D. Fingolimod for the treatment of intracerebral hemorrhage: A 2-arm proof-of-concept study. JAMA Neurol. 2014, 71, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.; Mendioroz, M.; Ribó, M.; Delgado, P.; Quintana, M.; Penalba, A.; Chacón, P.; Molina, C.; Fernández-Cadenas, I.; Rosell, A.; et al. A panel of biomarkers including caspase-3 and D-dimer may differentiate acute stroke from stroke-mimicking conditions in the emergency department. J. Intern. Med. 2011, 270, 166–174. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Manoel, A.L. Surgery for spontaneous intracerebral hemorrhage. Crit. Care 2020, 24, 45. [Google Scholar] [CrossRef]
- Mendelow, A.D.; Gregson, B.A.; Fernandes, H.M.; Murray, G.D.; Teasdale, G.M.; Hope, D.T.; Karimi, A.; Shaw, M.D.; Barer, D.H.; STICH Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet 2005, 365, 387–397. [Google Scholar] [CrossRef]
- Mendelow, A.D.; Gregson, B.A.; Rowan, E.N.; Murray, G.D.; Gholkar, A.; Mitchell, P.M.; STICH II Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet 2013, 382, 397–408. [Google Scholar] [CrossRef]
- Cho, D.Y.; Chen, C.C.; Chang, C.S.; Lee, W.Y.; Tso, M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: Comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg. Neurol. 2006, 65, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C.; Cho, D.Y.; Lee, W.Y.; Chen, J.T. Endoscopic evacuation of putaminal hemorrhage: How to improve the efficiency of hematoma evacuation. Surg. Neurol. 2005, 64, 147–153. [Google Scholar] [CrossRef]
- Nagasaka, T.; Tsugeno, M.; Ikeda, H.; Okamoto, T.; Inao, S.; Wakabayashi, T. Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: Preliminary study in comparison with craniotomy. J. Stroke Cerebrovasc. Dis. 2011, 20, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Scaggiante, J.; Zhang, X.; Mocco, J.; Kellner, C.P. Minimally Invasive Surgery for Intracerebral Hemorrhage. Stroke 2018, 49, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Hanley, D.F.; Thompson, R.E.; Rosenblum, M.; Yenokyan, G.; Lane, K.; McBee, N.; Mayo, S.W.; Bistran-Hall, A.J.; Gandhi, D.; Mould, W.A.; et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 2019, 393, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; Tymianski, M. MISTIE III. Stroke 2019, 50, 1634–1635. [Google Scholar] [CrossRef] [PubMed]
- Polster, S.P.; Carrión-Penagos, J.; Lyne, S.B.; Gregson, B.A.; Cao, Y.; Thompson, R.E.; Stadnik, A.; Girard, R.; Money, P.L.; Lane, K.; et al. Intracerebral Hemorrhage Volume Reduction and Timing of Intervention Versus Functional Benefit and Survival in the MISTIE III and STICH Trials. Neurosurgery 2021, 88, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, H.; Tan, Z.; Zhang, X.; Gao, J.; Zhang, L.; Guo, H.; Bai, H.; Cui, W.; Liu, X.; et al. Comparison of endoscopic evacuation, stereotactic aspiration, and craniotomy for treatment of basal ganglia hemorrhage. J. Neurointerv. Surg. 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Li, F.; Zheng, X.; Wang, Q.; Sun, G.; Zhang, J.; Xu, B. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: A comparison with craniotomy. J. Neurosurg. 2018, 128, 553–559. [Google Scholar] [CrossRef]
- Li, Q.; Warren, A.D.; Qureshi, A.I.; Morotti, A.; Falcone, G.J.; Sheth, K.N.; Shoamanesh, A.; Dowlatshahi, D.; Viswanathan, A.; Goldstein, J.N. Ultra-Early Blood Pressure Reduction Attenuates Hematoma Growth and Improves Outcome in Intracerebral Hemorrhage. Ann. Neurol. 2020, 88, 388–395. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Bonovich, D.C.; Besmertis, L.; Manley, G.T.; Johnston, S.C. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef]
- Wang, X.; Arima, H.; Al-Shahi Salman, R.; Woodward, M.; Heeley, E.; Stapf, C.; Lavados, P.M.; Robinson, T.; Huang, Y.; Wang, J.; et al. Clinical prediction algorithm (BRAIN) to determine risk of hematoma growth in acute intracerebral hemorrhage. Stroke 2015, 46, 376–381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).