FastSkin® Concept: A Novel Treatment for Complex Acute and Chronic Wound Management
Abstract
:1. Introduction
2. Materials and Methods
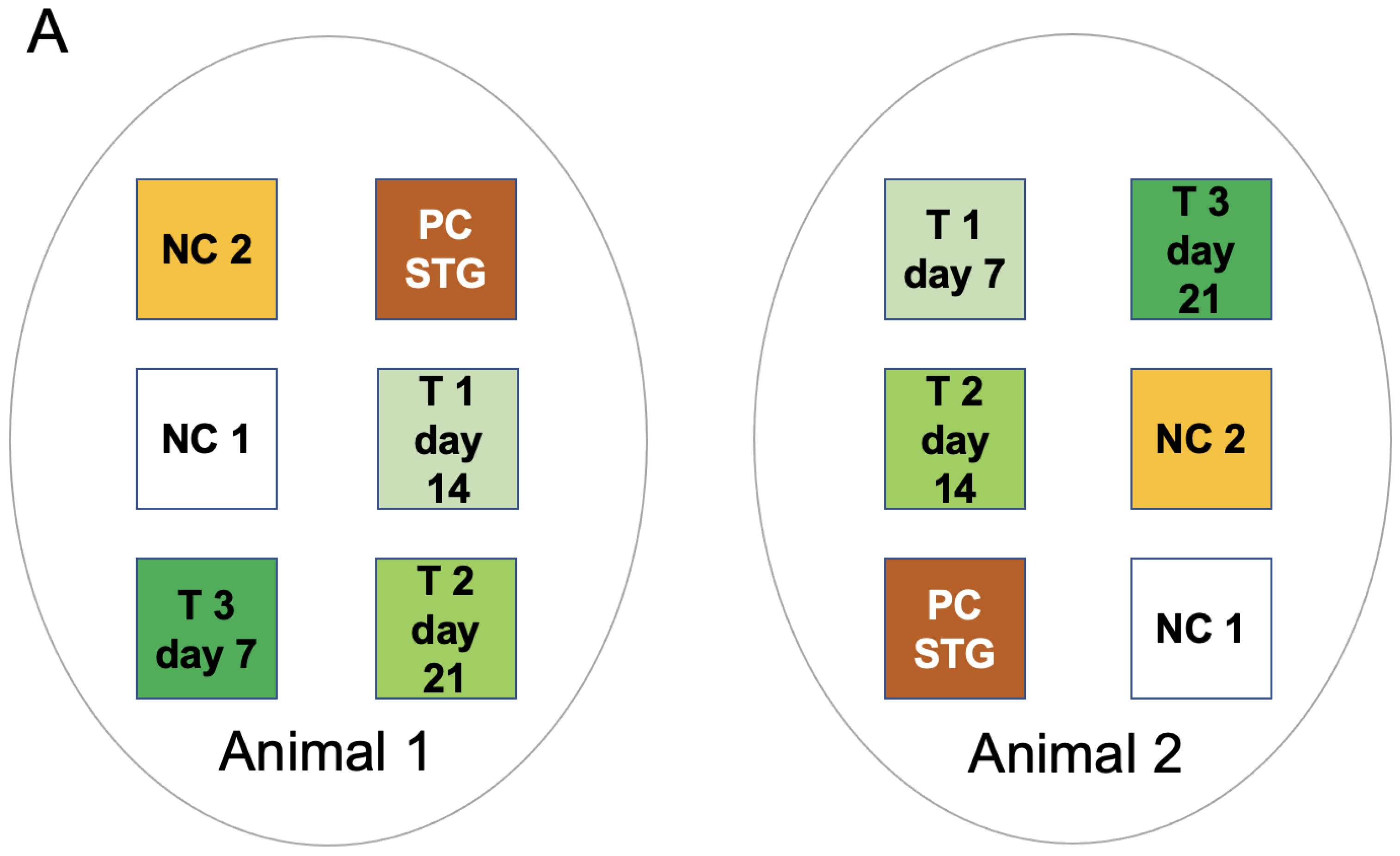
2.1. Study Design
2.2. Animal Care and Surgeries
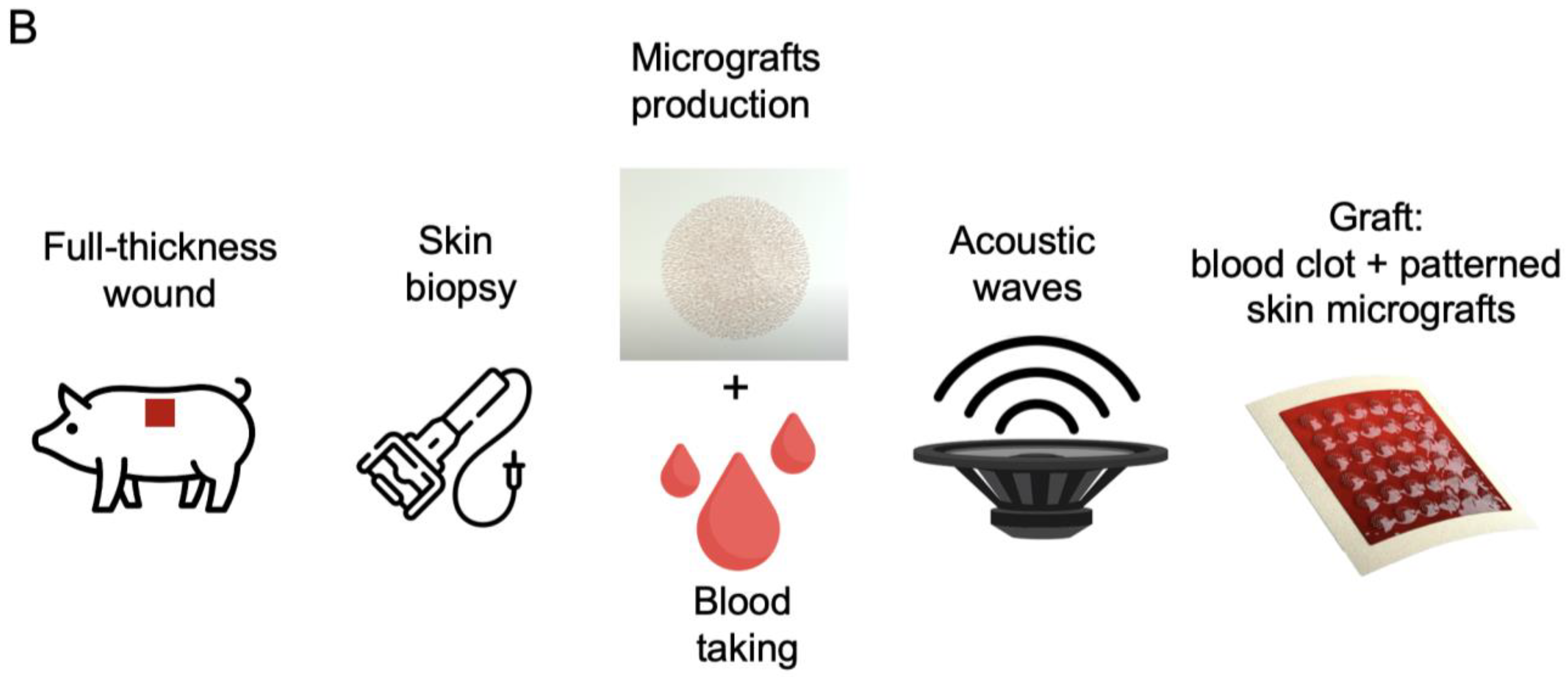
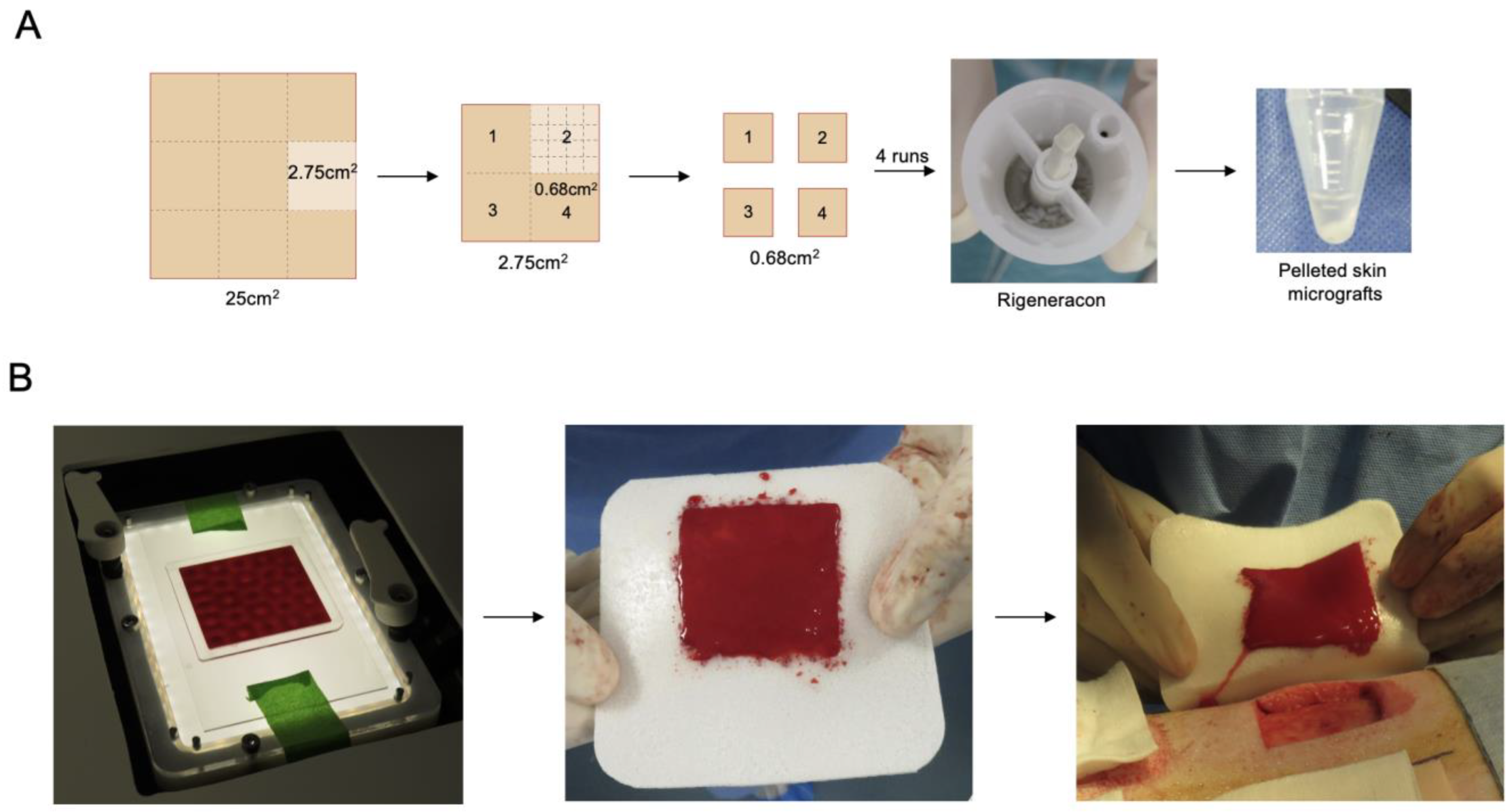
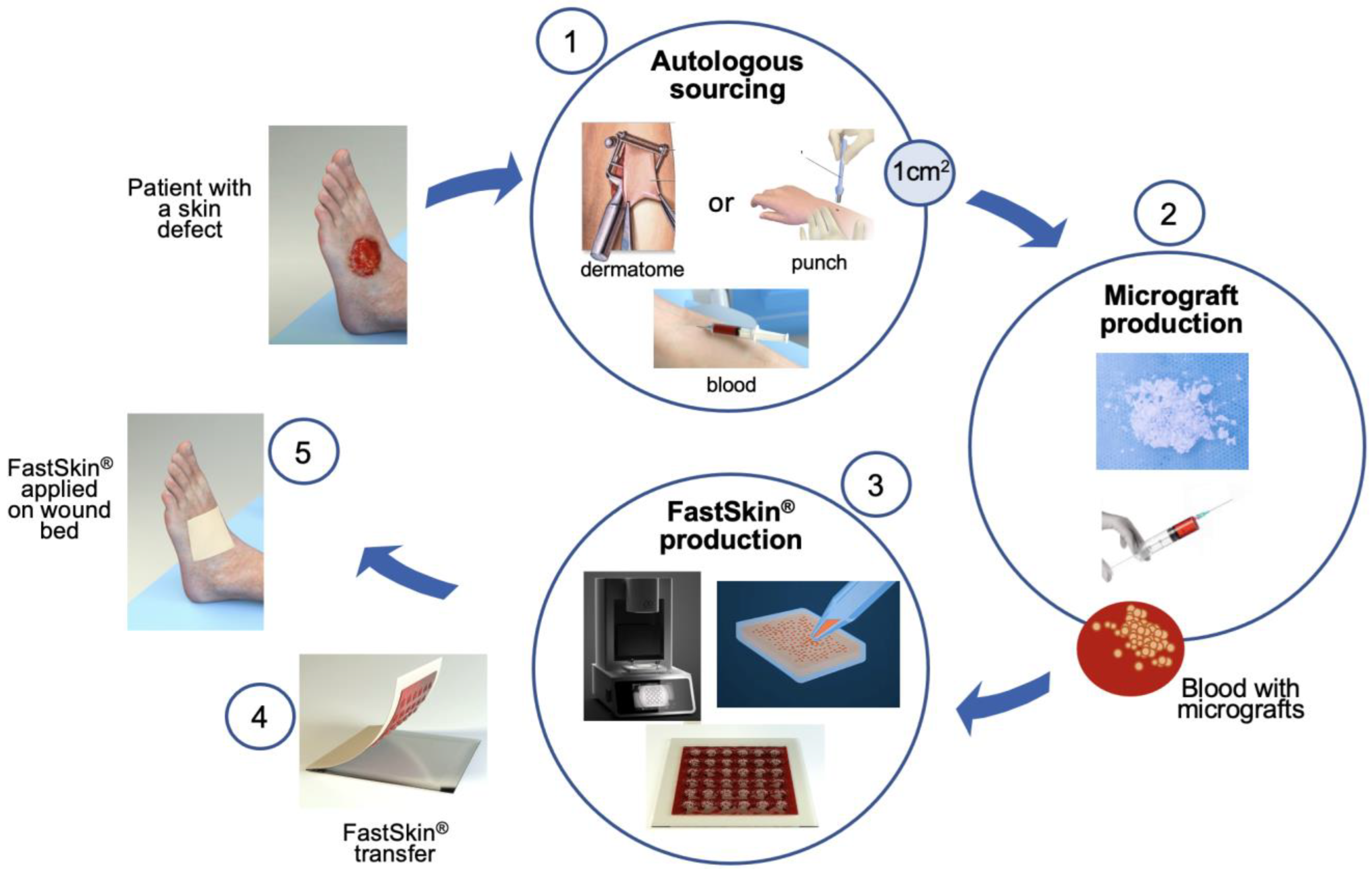
2.3. Preparation of Test and Control Treatments
2.4. Macroscopic Wound Analysis
2.5. Histology
3. Results
3.1. Animal Recovery
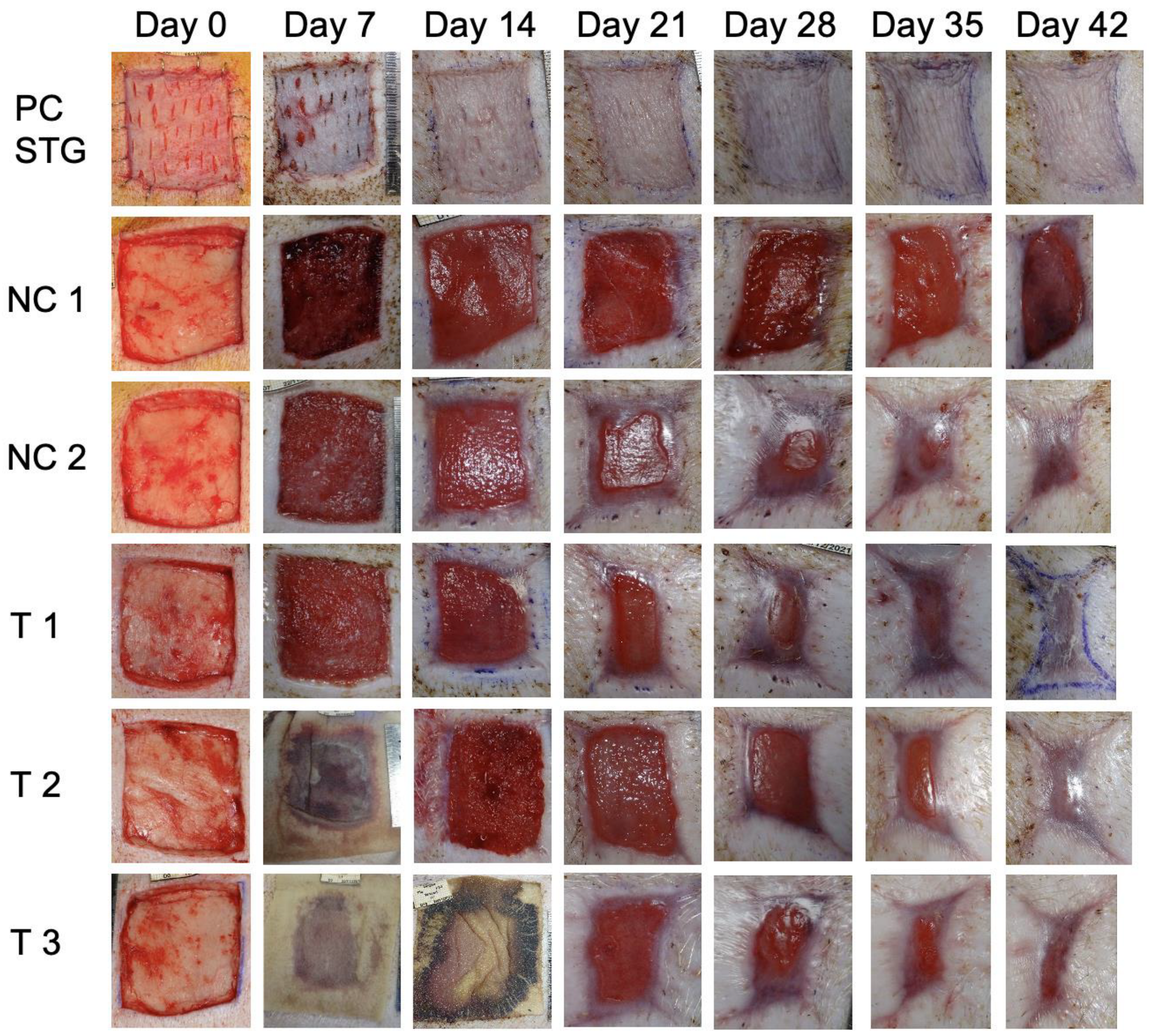
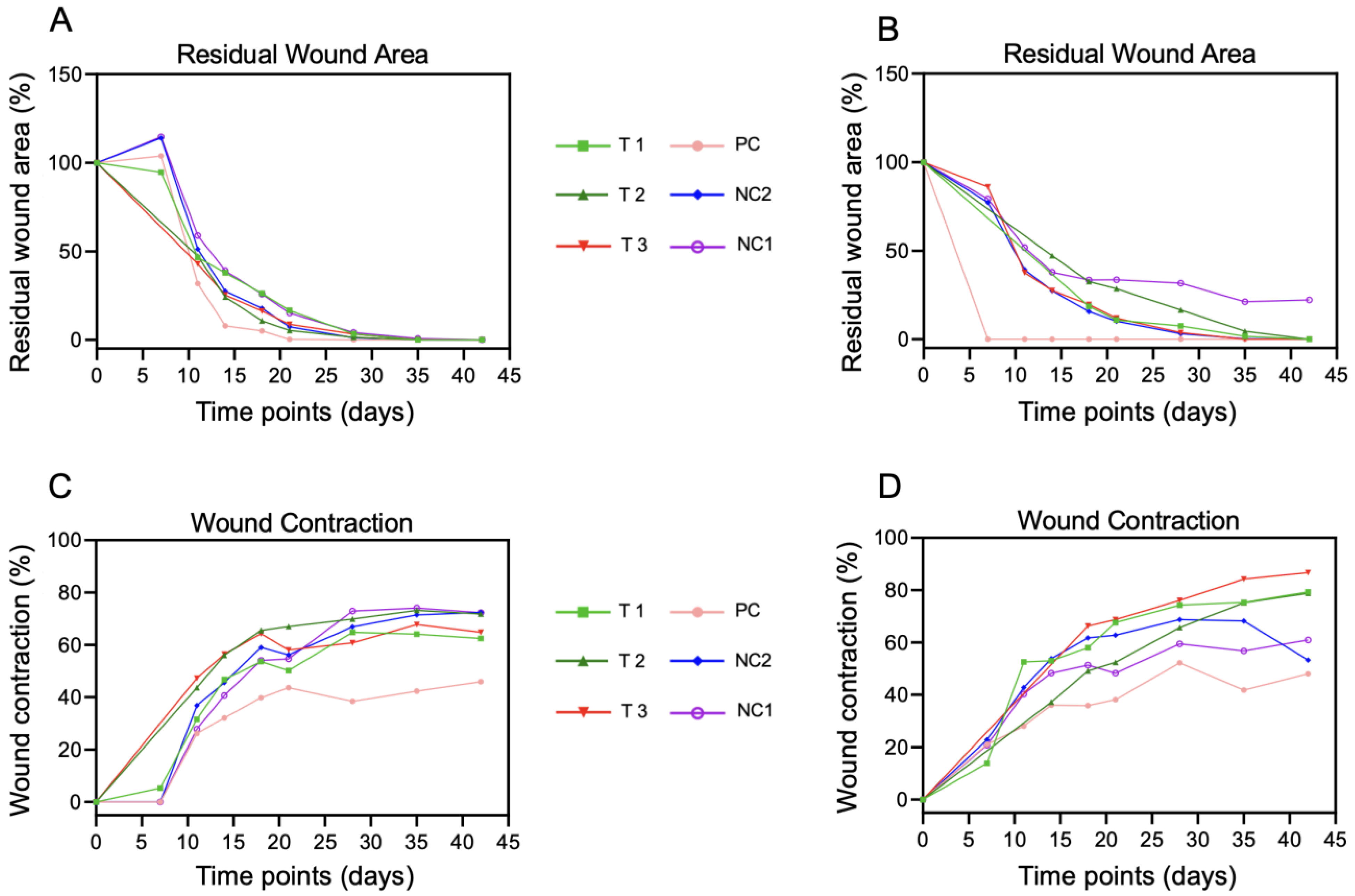
3.2. Wound Healing
3.3. Morphometric Analysis of Wounds over Time
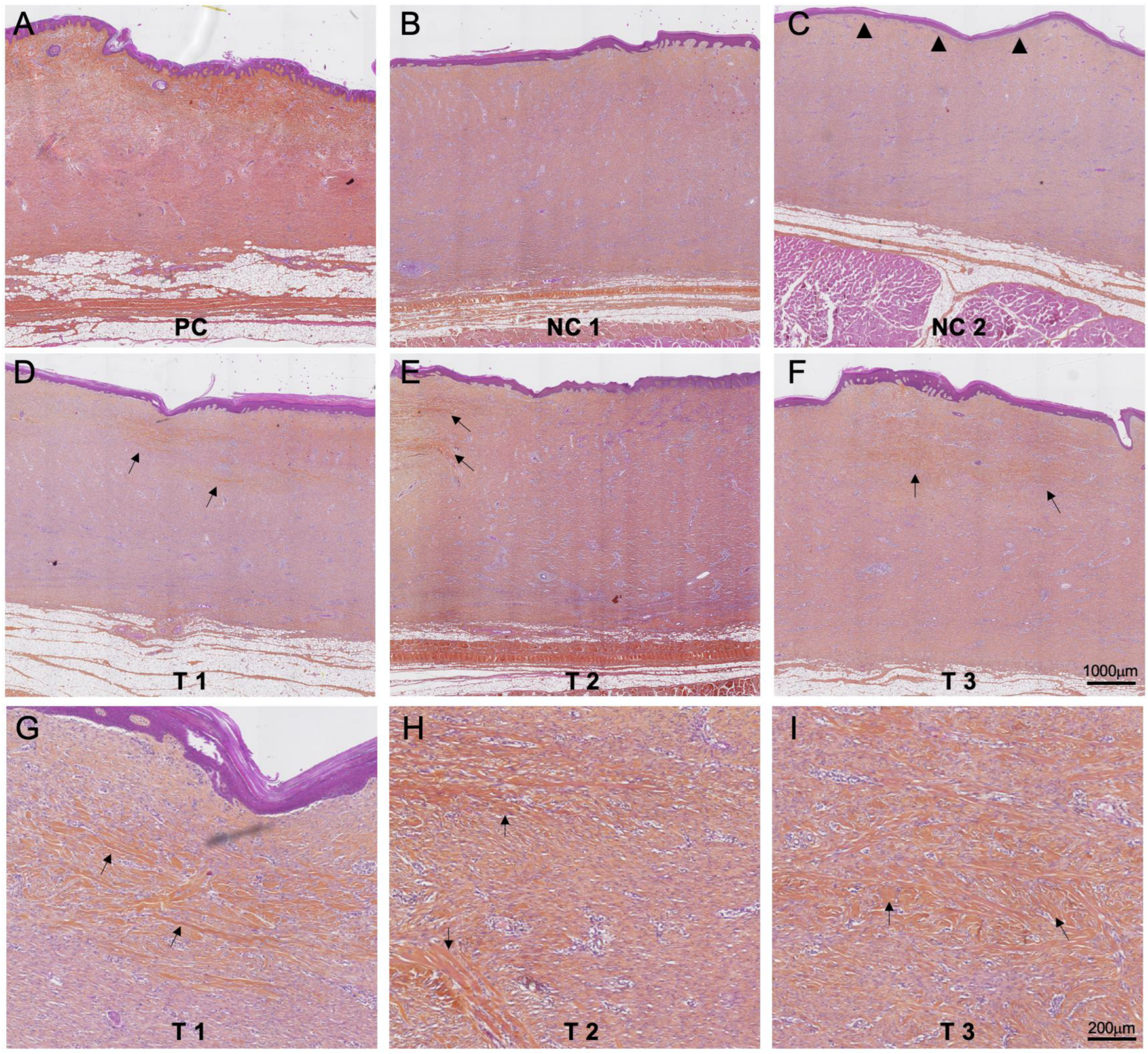
3.4. Histology Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C. Wound healing: Normal and abnormal. In Grabbs & Smith’s Plastic Surgery, 6th ed.; Thorne, C.H., Beasley, R.W., Aston, J.S., Bartlett, S.P., Gurtner, G.C., Spear, S.L., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 15–23. [Google Scholar]
- Singer, A.J. Healing Mechanisms in Cutaneous Wounds: Tipping the Balance. Tissue Eng. Part B Rev. 2022, 28, 1151–1167. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.W.; Mason, S.T.; Schomer, K.; Klein, M.B. Epidemiology and impact of scarring after burn injury: A systematic review of the literature. J. Burn Care Res. 2012, 33, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Agale, S.V. Chronic leg ulcers: Epidemiology, aetiopathogenesis, and management. Ulcers 2013, 2013, 413604. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015, 5, e009283. [Google Scholar] [CrossRef]
- Guest, J.F.; Vowden, K.; Vowden, P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J. Wound Care 2017, 26, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Heyer, K.; Herberger, K.; Protz, K.; Glaeske, G.; Augustin, M. Epidemiology of chronic wounds in Germany: Analysis of statutory health insurance data. Wound Repair Regen. 2016, 24, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M. Cultured epithelial autograft (CEA) in burn treatment: Three decades later. Burns 2007, 33, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Lalley, A.L. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma 2018, 6, 4. [Google Scholar] [CrossRef]
- Lucich, E.A.; Rendon, J.L.; Valerio, I.L. Advances in addressing full-thickness skin defects: A review of dermal and epidermal substitutes. Regen. Med. 2018, 13, 443–456. [Google Scholar] [CrossRef]
- Urciuolo, F.; Casale, C.; Imparato, G.; Netti, P.A. Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. J. Clin. Med. 2019, 8, 2083. [Google Scholar] [CrossRef]
- Hendrickx, B.; Vranckx, J.J.; Luttun, A. Cell-based vascularization strategies for skin tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 13–24. [Google Scholar] [CrossRef]
- Sahota, P.S.; Burn, J.L.; Heaton, M.; Freedlander, E.; Suvarna, S.K.; Brown, N.J.; Mac Neil, S. Development of a reconstructed human skin model for angiogenesis. Wound Repair Regen. 2003, 11, 275–284. [Google Scholar] [CrossRef]
- Baltazar, T.; Merola, J.; Catarino, C.; Xie, C.B.; Kirkiles-Smith, N.C.; Lee, V.; Hotta, S.; Dai, G.; Xu, X.; Ferreira, F.C.; et al. Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells. Tissue Eng. Part A 2020, 26, 227–238. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Miyazaki, H.; Tsunoi, Y.; Akagi, T.; Sato, S.; Akashi, M.; Saitoh, D. A novel strategy to engineer pre-vascularized 3-dimensional skin substitutes to achieve efficient, functional engraftment. Sci. Rep. 2019, 9, 7797. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.J.; Kasper, M.A.; Patel, K.; Carter, M.J.; Kushnir, I.; Kushnir, A.; Serena, T.E. Safety and Efficacy of an Autologous Blood Clot Product in the Management of Texas 1A or 2A Neuropathic Diabetic Foot Ulcers: A Prospective, Multicenter, Open Label Pilot Study. Wounds 2018, 30, 84–89. [Google Scholar] [PubMed]
- Snyder, R.J.; Schultz, G.; Wachuku, C.; Rashid, A.M.; Ead, J.K.K. Proposed Mechanism of Action of Topically Applied Autologous Blood Clot Tissue: A Quintessential Cellular and Tissue Based Therapy. J. Am. Podiatr. Med. Assoc. 2020, 113, 20–140. [Google Scholar] [CrossRef] [PubMed]
- Meek, C.P. Successful microdermagrafting using the Meek-Wall microdermatome. Am. J. Surg. 1958, 96, 557–558. [Google Scholar] [CrossRef]
- Marcarelli, M.; Trovato, L.; Novarese, E.; Riccio, M.; Graziano, A. Rigenera protocol in the treatment of surgical wound dehiscence. Int. Wound J. 2017, 14, 277–281. [Google Scholar] [CrossRef]
- Riccio, M.; Marchesini, A.; Zingaretti, N.; Carella, S.; Senesi, L.; Onesti, M.G.; Parodi, P.C.; Ribuffo, D.; Vaienti, L.; De Francesco, F. A Multicentre Study: The Use of Micrografts in the Reconstruction of Full-Thickness Posttraumatic Skin Defects of the Limbs-A Whole Innovative Concept in Regenerative Surgery. Stem Cells Int. 2019, 2019, 5043518. [Google Scholar] [CrossRef]
- Andreone, A.; den Hollander, D. A Retrospective Study on the Use of Dermis Micrografts in Platelet-Rich Fibrin for the Resurfacing of Massive and Chronic Full-Thickness Burns. Stem Cells Int. 2019, 2019, 8636079. [Google Scholar] [CrossRef]
- Andreone, A.; de Hollander, D. A case report on the effect of micrografting in the healing of chronic and complex burn wounds. Int. J. Burns Trauma 2020, 10, 15–20. [Google Scholar]
- Andreone, A.; Giaquinto-Cillers, M. Reconstruction of scalp defects with exposed bone using dermis template (Integra®) with or without autologous skin micrografts (Rigenera®) and Flowable wound matrix (Integra®). Wounds Int. 2021, 12, 58–65. [Google Scholar]
- Giaccone, M.; Brunetti, M.; Camandona, M.; Trovato, L.; Graziano, A. A New Medical Device, Based on Rigenera Protocol, in the Management of Complex Wounds. J. Stem Cells Res. Rev. Rep. 2013, 1, 3. [Google Scholar]
- Jimi, S.; Takagi, S.; De Francesco, F.; Miyazaki, M.; Saparov, A. Acceleration of Skin Wound-Healing Reactions by Autologous Micrograft Tissue Suspension. Medicina 2020, 56, 321. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.; Farina, E.; Farina, M.A. Micrografting chronic lower extremity ulcers with mechanically disaggregated skin using a micrograft preparation system. J. Wound Care 2018, 27, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Niimi, Y.; Baba, K.; Tsuchida, M.; Takeda, A. A Histological Evaluation of Artificial Dermal Scaffold Used in Micrograft Treatment: A Case Study of Micrograft and NPWT Performed on a Postoperative Ulcer Formation after Tumor Resection. Medicina 2022, 58, 73. [Google Scholar] [CrossRef] [PubMed]
- Trovato, L.; Failla, G.; Serantoni, S.; Palumbo, F.P. Regenerative Surgery in the Management of the Leg Ulcers. J. Cell Sci. Ther. 2016, 7, 1000238. [Google Scholar] [CrossRef]
- Sun, W.; Starly, B.; Daly, A.C.; Burdick, J.A.; Groll, J.; Skeldon, G.; Shu, W.; Sakai, Y.; Shinohara, M.; Nishikawa, M.; et al. The bioprinting roadmap. Biofabrication 2020, 12, 022002. [Google Scholar] [CrossRef]
- Guex, A.G.; Di Marzio, N.; Eglin, D.; Alini, M.; Serra, T. The waves that make the pattern: A review on acoustic manipulation in biomedical research. Mater. Today Bio 2021, 10, 100110. [Google Scholar] [CrossRef]
- Hackl, F.; Bergmann, J.; Granter, S.R.; Koyama, T.; Kiwanuka, E.; Zuhaili, B.; Pomahac, B.; Caterson, E.J.; Junker, J.P.E.; Eriksson, E. Epidermal regeneration by micrograft transplantation with immediate 100-fold expansion. Plast. Reconstr. Surg. 2012, 129, 443e–452e. [Google Scholar] [CrossRef]
- Balli, M.; Vitali, F.; Janiszewski, A.; Caluwe, E.; Cortes-Calabuig, A.; Carpentier, S.; Duelen, R.; Ronzoni, F.; Marcelis, L.; Bosisio, F.M.; et al. Autologous micrograft accelerates endogenous wound healing response through ERK-induced cell migration. Cell Death Differ. 2020, 27, 1520–1538. [Google Scholar] [CrossRef]
- Osborne, S.N.; Schmidt, M.A.; Derrick, K.; Harper, J.R. Epidermal micrografts produced via an automated and minimally invasive tool form at the dermal/epidermal junction and contain proliferative cells that secrete wound healing growth factors. Adv. Skin Wound Care 2015, 28, 397–405. [Google Scholar] [CrossRef]
- Astarita, C.; Arora, C.L.; Trovato, L. Tissue regeneration: An overview from stem cells to micrografts. J. Int. Med. Res. 2020, 48, 300060520914794. [Google Scholar] [CrossRef]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy 2012, 28, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, M.; Heinz, S.M.; Fridman, R.; Hawkins, J.; Wachuku, C.D. Use of autologous whole blood clot in the treatment of complex surgical wounds: A case series. J. Wound Care 2023, 32, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guven, S.; Usta, O.B.; Yarmush, M.L.; Demirci, U. Biotunable acoustic node assembly of organoids. Adv. Healthc. Mater. 2015, 4, 1937–1943. [Google Scholar] [CrossRef]
- Chen, P.; Luo, Z.; Guven, S.; Tasoglu, S.; Ganesan, A.V.; Weng, A.; Demirci, U. Microscale assembly directed by liquid-based template. Adv. Mater. 2014, 26, 5936–5941. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Puetzer, J.L.; Serio, A.; Guex, A.G.; Kapnisi, M.; Breant, A.; Zong, Y.; Assal, V.; Skaalure, S.C.; King, O.; et al. Engineering Anisotropic Muscle Tissue using Acoustic Cell Patterning. Adv. Mater. 2018, 30, e1802649. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, C.; Chen, P.; Guven, S.; Demirtas, T.T.; Nieland, T.J.; Padilla, F.; Demirci, U. A Bio-Acoustic Levitational (BAL) Assembly Method for Engineering of Multilayered, 3D Brain-Like Constructs, Using Human Embryonic Stem Cell Derived Neuro-Progenitors. Adv. Mater. 2016, 28, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Shin, J.; Park, H.J.; Rhyou, C.; Kang, D.; Lee, S.J.; Yoon, Y.S.; Cho, S.W.; Lee, H. High-resolution acoustophoretic 3D cell patterning to construct functional collateral cylindroids for ischemia therapy. Nat. Commun. 2018, 9, 5402. [Google Scholar] [CrossRef]
- Naseer, S.M.; Manbachi, A.; Samandari, M.; Walch, P.; Gao, Y.; Zhang, Y.S.; Davoudi, F.; Wang, W.; Abrinia, K.; Cooper, J.M.; et al. Surface acoustic waves induced micropatterning of cells in gelatin methacryloyl (GelMA) hydrogels. Biofabrication 2017, 9, 015020. [Google Scholar] [CrossRef]
- Figarol, A.; Olive, L.; Joubert, O.; Ferrari, L.; Rihn, B.H.; Sarry, F.; Beyssen, D. Biological Effects and Applications of Bulk and Surface Acoustic Waves on In Vitro Cultured Mammal Cells: New Insights. Biomedicines 2022, 10, 1166. [Google Scholar] [CrossRef]
- Levario-Diaz, V.; Bhaskar, P.; Carmen Galan, M.; Barnes, A.C. Effect of acoustic standing waves on cellular viability and metabolic activity. Sci. Rep. 2020, 10, 8493. [Google Scholar] [CrossRef]
- Petta, D.; Basoli, V.; Pellicciotta, D.; Tognato, R.; Barcik, J.; Arrigoni, C.; Bella, E.D.; Armiento, A.R.; Candrian, C.; Richards, R.G.; et al. Sound-induced morphogenesis of multicellular systems for rapid orchestration of vascular networks. Biofabrication 2020, 13, 015004. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, M. Acoustofluidics 12: Biocompatibility and cell viability in microfluidic acoustic resonators. Lab Chip 2012, 12, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Di Marzio, N.; Ananthanarayanan, P.; Guex, A.G.; Alini, M.; Riganti, C.; Serra, T. Sound-based assembly of a microcapillary network in a saturn-like tumor model for drug testing. Mater. Today Bio 2022, 16, 100357. [Google Scholar] [CrossRef] [PubMed]

| Silicone Removal (Day) | Wound Contraction (% of Initial Wound) D42 | Residual Wound Surface (% of Initial Wound) D28 | Histology Score | |
|---|---|---|---|---|
| PC | 7 | 46 | 0 | 4 |
| PC | 7 | 48 | 0 | 4 |
| NC 1 | 7 | 72 | 4 | 0 |
| NC 1 | 7 | 61 | 32 | 0 * |
| NC 2 | 7 | 72 | 1 | 0 |
| NC 2 | 7 | 53 | 3 | 0 |
| T1 | 7 | 62 | 3 | 1 |
| T1 | 7 | 79 | 4 | 2 |
| T2 | 11 | 72 | 1 | 1 |
| T2 | 14 | 79 | 17 | 1 |
| T3 | 11 | 65 | 3 | 1 |
| T3 | 18 | 87 | 7 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Summa, P.G.; Di Marzio, N.; Jafari, P.; Jaconi, M.E.; Nesic, D. FastSkin® Concept: A Novel Treatment for Complex Acute and Chronic Wound Management. J. Clin. Med. 2023, 12, 6564. https://doi.org/10.3390/jcm12206564
di Summa PG, Di Marzio N, Jafari P, Jaconi ME, Nesic D. FastSkin® Concept: A Novel Treatment for Complex Acute and Chronic Wound Management. Journal of Clinical Medicine. 2023; 12(20):6564. https://doi.org/10.3390/jcm12206564
Chicago/Turabian Styledi Summa, Pietro G., Nicola Di Marzio, Paris Jafari, Marisa E. Jaconi, and Dobrila Nesic. 2023. "FastSkin® Concept: A Novel Treatment for Complex Acute and Chronic Wound Management" Journal of Clinical Medicine 12, no. 20: 6564. https://doi.org/10.3390/jcm12206564
APA Styledi Summa, P. G., Di Marzio, N., Jafari, P., Jaconi, M. E., & Nesic, D. (2023). FastSkin® Concept: A Novel Treatment for Complex Acute and Chronic Wound Management. Journal of Clinical Medicine, 12(20), 6564. https://doi.org/10.3390/jcm12206564








