Revisiting Asthma Obstructive Sleep Apnea Overlap: Current Knowledge and Future Needs
Abstract
:1. Introduction
2. Effects of Asthma on OSA
2.1. How Asthma Affects OSA Phenotype
2.2. Pathological Explanations of How Asthma Affects OSA
2.2.1. Direct Mechanical Effects
2.2.2. Lung Volumes and Airway Resistance
2.2.3. Bronchoconstriction
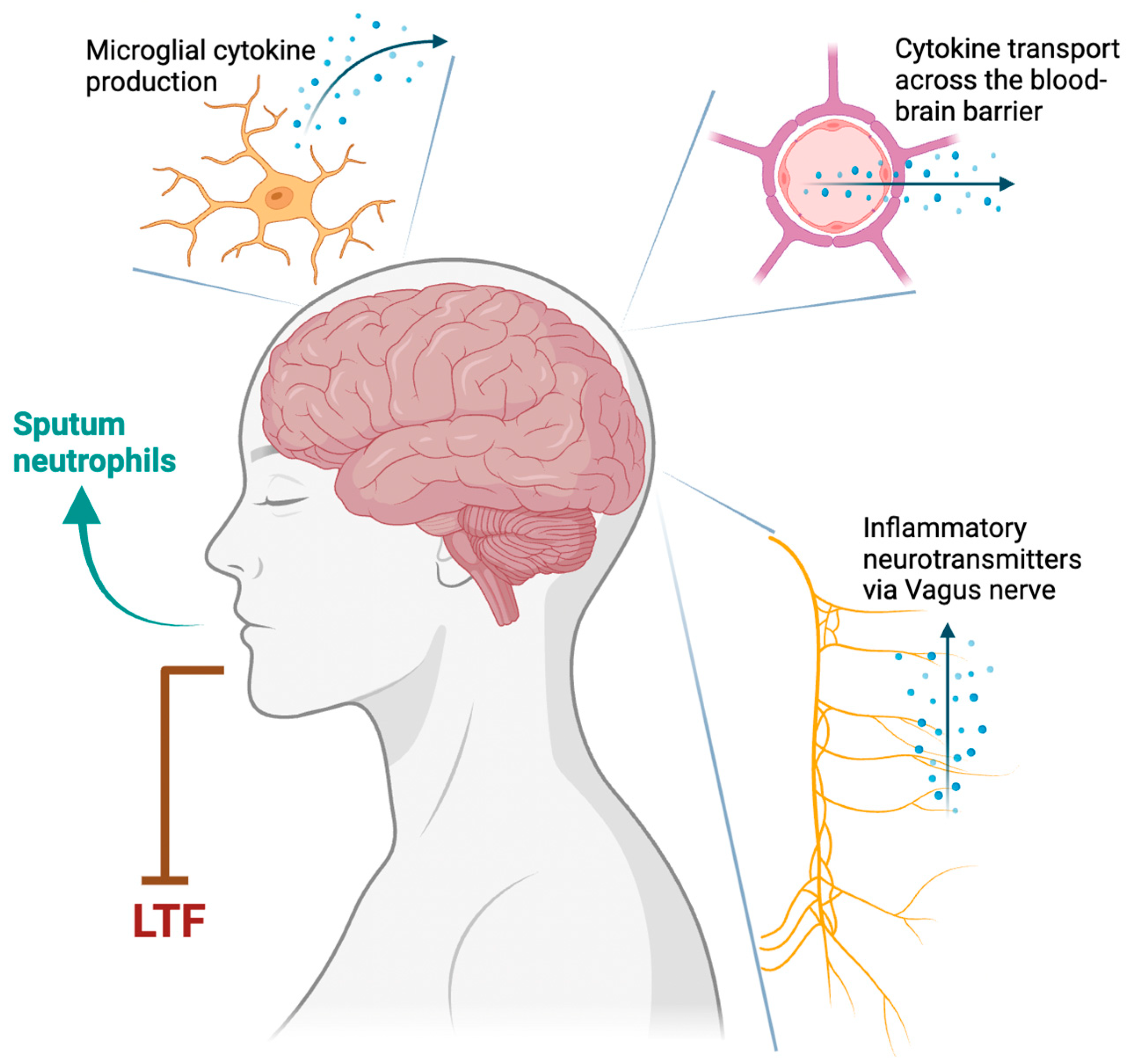
2.2.4. Neuroinflammatory Mechanisms
2.2.5. Type 1 Inflammation
2.2.6. Type 2 Inflammation
2.3. How Asthma Treatments Affect OSA
2.4. Effect of OSA on Asthma
2.5. OSA Affects Asthma Severity and Control
2.6. How OSA Affects Pulmonary Function in Asthmatic Subjects
2.7. Pathological Explanations of How OSA Affects Asthma
2.7.1. Obesity
2.7.2. Hypoxemia
2.7.3. Frequent Arousals and Poor Sleep Quality
2.7.4. Negative Intrathoracic Pressure
2.7.5. Autonomic Nervous System
2.7.6. Airway Inflammation
3. Conclusions
4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Most Recent National Asthma Data|CDC. Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed on 4 September 2023).
- Nurmagambetov, T.; Kuwahara, R.; Garbe, P. The Economic Burden of Asthma in the United States, 2008–2013. Ann. Am. Thorac. Soc. 2018, 15, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Adibi, A.; Safari, A.; FitzGerald, J.M.; Sadatsafavi, M. The Projected Economic and Health Burden of Uncontrolled Asthma in the United States. Am. J. Respir. Crit. Care Med. 2019, 200, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.F. Health Care Savings: The Economic Value of Diagnostic and Therapeutic Care for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2016, 12, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Mukhtar, O.; Kandel, S.; Bhattrai, B.; Dattar, P.; Amgai, B.; Mandal, A.; Alhafidh, O.; Thapa, S.; Khalid, M.; et al. Polysomnographic Variables in Alternate Overlap Syndrome: Data from Sleep Heart Health Study. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, Y.; Chen, R.; Zeng, X.; Zhang, S.; Su, X.; Luo, Y.; Tang, Y.; Li, S.; Zhuang, Z.; et al. The Relationship between Obstructive Sleep Apnea and Asthma Severity and Vice Versa: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2023, 28, 139. [Google Scholar] [CrossRef]
- Knuiman, M.; James, A.; Divitini, M.; Bartholomew, H. Longitudinal Study of Risk Factors for Habitual Snoring in a General Adult Population: The Busselton Health Study. Chest 2006, 130, 1779–1783. [Google Scholar] [CrossRef]
- Janson, C.; De Backer, W.; Gislason, T.; Plaschke, P.; Björnsson, E.; Hetta, J.; Kristbjarnarson, H.; Vermeire, P.; Boman, G. Increased Prevalence of Sleep Disturbances and Daytime Sleepiness in Subjects with Bronchial Asthma: A Population Study of Young Adults in Three European Countries. Eur. Respir. J. 1996, 9, 2132–2138. [Google Scholar] [CrossRef]
- Puthalapattu, S.; Ioachimescu, O.C. Asthma and Obstructive Sleep Apnea: Clinical and Pathogenic Interactions. J. Investig. Med. 2014, 62, 665–675. [Google Scholar] [CrossRef]
- Ragnoli, B.; Pochetti, P.; Raie, A.; Malerba, M. Interrelationship Between Obstructive Sleep Apnea Syndrome and Severe Asthma: From Endo-Phenotype to Clinical Aspects. Front. Med. 2021, 8, 640636. [Google Scholar] [CrossRef]
- Prasad, B.; Nyenhuis, S.M.; Imayama, I.; Siddiqi, A.; Teodorescu, M. Asthma and Obstructive Sleep Apnea Overlap: What Has the Evidence Taught Us? Am. J. Respir. Crit. Care Med. 2020, 201, 1345–1357. [Google Scholar] [CrossRef]
- Kong, D.-L.; Qin, Z.; Shen, H.; Jin, H.-Y.; Wang, W.; Wang, Z.-F. Association of Obstructive Sleep Apnea with Asthma: A Meta-Analysis. Sci. Rep. 2017, 7, 4088. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Pepin, J.-L.; Anttalainen, U.; Schiza, S.E.; Basoglu, O.K.; Pataka, A.; Steiropoulos, P.; Dogas, Z.; Grote, L.; Hedner, J.; et al. Clinical Presentation of Patients with Suspected Obstructive Sleep Apnea and Self-Reported Physician-Diagnosed Asthma in the ESADA Cohort. J. Sleep Res. 2018, 27, e12729. [Google Scholar] [CrossRef] [PubMed]
- Julien, J.Y.; Martin, J.G.; Ernst, P.; Olivenstein, R.; Hamid, Q.; Lemière, C.; Pepe, C.; Naor, N.; Olha, A.; Kimoff, R.J. Prevalence of Obstructive Sleep Apnea-Hypopnea in Severe versus Moderate Asthma. J. Allergy Clin. Immunol. 2009, 124, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Barnet, J.H.; Hagen, E.W.; Palta, M.; Young, T.B.; Peppard, P.E. Association between Asthma and Risk of Developing Obstructive Sleep Apnea. JAMA 2015, 313, 156–164. [Google Scholar] [CrossRef]
- Teodorescu, M.; Polomis, D.A.; Teodorescu, M.C.; Gangnon, R.E.; Peterson, A.G.; Consens, F.B.; Chervin, R.D.; Jarjour, N.N. Association of Obstructive Sleep Apnea Risk or Diagnosis with Daytime Asthma in Adults. J. Asthma 2012, 49, 620–628. [Google Scholar] [CrossRef]
- Bellia, V.; Cuttitta, G.; Insalaco, G.; Visconti, A.; Bonsignore, G. Relationship of Nocturnal Bronchoconstriction to Sleep Stages. Am. Rev. Respir. Dis. 1989, 140, 363–367. [Google Scholar] [CrossRef]
- Ballard, R.D.; Saathoff, M.C.; Patel, D.K.; Kelly, P.L.; Martin, R.J. Effect of Sleep on Nocturnal Bronchoconstriction and Ventilatory Patterns in Asthmatics. J. Appl. Physiol. (1985) 1989, 67, 243–249. [Google Scholar] [CrossRef]
- Antonaglia, C.; Passuti, G.; Giudici, F.; Salton, F.; Ruaro, B.; Radovanovic, D.; Confalonieri, M. Low Arousal Threshold: A Common Pathophysiological Trait in Patients with Obstructive Sleep Apnea Syndrome and Asthma. Sleep Breath. 2023, 27, 933–941. [Google Scholar] [CrossRef]
- Alkhalil, M.; Schulman, E.; Getsy, J. Obstructive Sleep Apnea Syndrome and Asthma: What Are the Links? J. Clin. Sleep Med. 2009, 5, 71–78. [Google Scholar] [CrossRef]
- Thorstensen, W.M.; Sue-Chu, M.; Bugten, V.; Steinsvåg, S.K. Nasal Flow, Volumes, and Minimal Cross Sectional Areas in Asthmatics. Respir. Med. 2013, 107, 1515–1520. [Google Scholar] [CrossRef]
- Owens, R.L.; Macrea, M.M.; Teodorescu, M. The Overlaps of Asthma or COPD with OSA: A Focused Review. Respirology 2017, 22, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Ballard, R.D.; Irvin, C.G.; Martin, R.J.; Pak, J.; Pandey, R.; White, D.P. Influence of Sleep on Lung Volume in Asthmatic Patients and Normal Subjects. J. Appl. Physiol. (1985) 1990, 68, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Tamisier, R.; Pepin, J.L.; Wuyam, B.; Deschaux, C.; Levy, P. Expiratory Changes in Pressure: Flow Ratio during Sleep in Patients with Sleep-Disordered Breathing. Sleep 2004, 27, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Acerbi, I.; Puig, F.; Montserrat, J.M.; Navajas, D.; Farré, R. Upper-Airway Inflammation Triggered by Vibration in a Rat Model of Snoring. Sleep 2007, 30, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Antonaglia, C. Obstructive Sleep Apnea Syndrome (OSAS) and Asthma: A Simple Association: A New Syndrome or a Cluster? J. Biomed. Res. Environ. Sci. 2022, 3, 944–952. [Google Scholar] [CrossRef]
- Shapiro, C.M.; Catterall, J.R.; Montgomery, I.; Raab, G.M.; Douglas, N.J. Do Asthmatics Suffer Bronchoconstriction during Rapid Eye Movement Sleep? Br. Med. J. (Clin. Res. Ed.) 1986, 292, 1161–1164. [Google Scholar] [CrossRef]
- Ravenscroft, K., Jr.; Hartmann, E.L. The Temporal Correlations of Nocturnal Asthmatic Attacks. Psychophysiology 1968, 4, 396–397. [Google Scholar]
- Perez, G.F.; Gutierrez, M.J.; Huseni, S.; Pancham, K.; Rodriguez-Martinez, C.E.; Nino, C.L.; Nino, G. Oximetry Signal Processing Identifies REM Sleep-Related Vulnerability Trait in Asthmatic Children. Sleep Disord. 2013, 2013, 406157. [Google Scholar] [CrossRef]
- Mackay, T.W.; Fitzpatrick, M.F.; Douglas, N.J. Non-Adrenergic, Non-Cholinergic Nervous System and Overnight Airway Calibre in Asthmatic and Normal Subjects. Lancet 1991, 338, 1289–1292. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and Inflammation: Partners in Sickness and in Health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Bjurström, M.F.; Olmstead, R.; Irwin, M.R. Reciprocal Relationship Between Sleep Macrostructure and Evening and Morning Cellular Inflammation in Rheumatoid Arthritis. Psychosom. Med. 2017, 79, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Adrish, M.; Akuthota, P. Approach to Non-Type 2 Asthma. Respir. Med. 2023, 216, 107327. [Google Scholar] [CrossRef] [PubMed]
- Jacono, F.J.; Mayer, C.A.; Hsieh, Y.-H.; Wilson, C.G.; Dick, T.E. Lung and Brainstem Cytokine Levels Are Associated with Breathing Pattern Changes in a Rodent Model of Acute Lung Injury. Respir. Physiol. Neurobiol. 2011, 178, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Ioachimescu, O.C.; Teodorescu, M. Integrating the Overlap of Obstructive Lung Disease and Obstructive Sleep Apnoea: OLDOSA Syndrome. Respirology 2013, 18, 421–431. [Google Scholar] [CrossRef]
- Taillé, C.; Rouvel-Tallec, A.; Stoica, M.; Danel, C.; Dehoux, M.; Marin-Esteban, V.; Pretolani, M.; Aubier, M.; d’Ortho, M.-P. Obstructive Sleep Apnoea Modulates Airway Inflammation and Remodelling in Severe Asthma. PLoS ONE 2016, 11, e0150042. [Google Scholar] [CrossRef]
- Vicente, E.; Marin, J.M.; Carrizo, S.J.; Osuna, C.S.; González, R.; Marin-Oto, M.; Forner, M.; Vicente, P.; Cubero, P.; Gil, A.V.; et al. Upper Airway and Systemic Inflammation in Obstructive Sleep Apnoea. Eur. Respir. J. 2016, 48, 1108–1117. [Google Scholar] [CrossRef]
- Huxtable, A.G.; Vinit, S.; Windelborn, J.A.; Crader, S.M.; Guenther, C.H.; Watters, J.J.; Mitchell, G.S. Systemic Inflammation Impairs Respiratory Chemoreflexes and Plasticity. Respir. Physiol. Neurobiol. 2011, 178, 482–489. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 Inflammation in Asthma—Present in Most, Absent in Many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Murugesan, N.; Saxena, D.; Dileep, A.; Adrish, M.; Hanania, N.A. Update on the Role of FeNO in Asthma Management. Diagnostics 2023, 13, 1428. [Google Scholar] [CrossRef]
- Broytman, O.; Brinkman, J.; Pegelow, D.; Morgan, B.; Teodorescu, M. Ovalbumin—Induced Airway Inflammation Enhances Hypoxic Ventilatory Response in Rats. FASEB J. 2018, 31, 728.9. [Google Scholar] [CrossRef]
- Althoff, M.D.; Jimenez, G.; Peterson, R.; Jin, Y.; Grasemann, H.; Sharma, S.; Federman, A.D.; Wisnivesky, J.P.; Holguin, F. Differences in L-Arginine Metabolism and Asthma Morbidity among Asthma Patients with and without Obstructive Sleep Apnea. Respir. Res. 2022, 23, 230. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.-P.; Aboussouan, L.S.; Minai, O.A.; Paschke, K.; Laskowski, D.; Dweik, R.A. Long-Term Continuous Positive Airway Pressure Therapy Normalizes High Exhaled Nitric Oxide Levels in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2013, 9, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.-C.; Lin, C.-L.; Wei, C.-C.; Chen, C.-H.; Tu, C.-Y.; Hsia, T.-C.; Shih, C.-M.; Hsu, W.-H.; Sung, F.-C.; Kao, C.-H. Risk of Obstructive Sleep Apnea in Adult Patients with Asthma: A Population-Based Cohort Study in Taiwan. PLoS ONE 2015, 10, e0128461. [Google Scholar] [CrossRef]
- Lakser, O.J.; Dowell, M.L.; Hoyte, F.L.; Chen, B.; Lavoie, T.L.; Ferreira, C.; Pinto, L.H.; Dulin, N.O.; Kogut, P.; Churchill, J.; et al. Steroids Augment Relengthening of Contracted Airway Smooth Muscle: Potential Additional Mechanism of Benefit in Asthma. Eur. Respir. J. 2008, 32, 1224–1230. [Google Scholar] [CrossRef]
- Teodorescu, M.; Consens, F.B.; Bria, W.F.; Coffey, M.J.; McMorris, M.S.; Weatherwax, K.J.; Palmisano, J.; Senger, C.M.; Ye, Y.; Kalbfleisch, J.D.; et al. Predictors of Habitual Snoring and Obstructive Sleep Apnea Risk in Patients with Asthma. Chest 2009, 135, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Xie, A.; Sorkness, C.A.; Robbins, J.; Reeder, S.; Gong, Y.; Fedie, J.E.; Sexton, A.; Miller, B.; Huard, T.; et al. Effects of Inhaled Fluticasone on Upper Airway during Sleep and Wakefulness in Asthma: A Pilot Study. J. Clin. Sleep Med. 2014, 10, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2023. Available online: https://ginasthma.org/2023-gina-main-report/ (accessed on 5 October 2023).
- Henao, M.P.; Kraschnewski, J.L.; Bolton, M.D.; Ishmael, F.; Craig, T. Effects of Inhaled Corticosteroids and Particle Size on Risk of Obstructive Sleep Apnea: A Large Retrospective Cohort Study. Int. J. Environ. Res. Public. Health 2020, 17, 7287. [Google Scholar] [CrossRef]
- Yigla, M.; Tov, N.; Solomonov, A.; Rubin, A.-H.E.; Harlev, D. Difficult-to-Control Asthma and Obstructive Sleep Apnea. J. Asthma 2003, 40, 865–871. [Google Scholar] [CrossRef]
- Kiely, J.L.; Nolan, P.; McNicholas, W.T. Intranasal Corticosteroid Therapy for Obstructive Sleep Apnoea in Patients with Co-Existing Rhinitis. Thorax 2004, 59, 50–55. [Google Scholar] [PubMed]
- Santos, C.B.; Hanks, C.; McCann, J.; Lehman, E.B.; Pratt, E.; Craig, T.J. The Role of Montelukast on Perennial Allergic Rhinitis and Associated Sleep Disturbances and Daytime Somnolence. Allergy Asthma Proc. 2008, 29, 140–145. [Google Scholar] [CrossRef]
- Adrish, M.; Hanania, N.A. Choosing and Switching Biological Agents in Severe Asthma. Respirology 2022, 27, 926–928. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, K.-C.; Zuberbier, T. Obstructive Sleep Apnoe Improves on Dupilumab in Patients Treated for Polyposis and Severe Asthma. Eur. Respir. J. 2021, 58, PA752. [Google Scholar] [CrossRef]
- Scioscia, G.; Buonamico, E.; Foschino Barbaro, M.P.; Lacedonia, D.; Sabato, R.; Carpagnano, G.E. Omalizumab as Add-on Therapy in a Patient with Severe Asthma and OSA. Respirol. Case Rep. 2020, 8, e0518. [Google Scholar] [CrossRef] [PubMed]
- Bouloukaki, I.; Fanaridis, M.; Testelmans, D.; Pataka, A.; Schiza, S. Overlaps between Obstructive Sleep Apnoea and Other Respiratory Diseases, Including COPD, Asthma and Interstitial Lung Disease. Breathe 2022, 18, 220073. [Google Scholar] [CrossRef] [PubMed]
- Ioachimescu, O.C.; Janocko, N.J.; Ciavatta, M.-M.; Howard, M.; Warnock, M. V Obstructive Lung Disease and Obstructive Sleep Apnea (OLDOSA) Cohort Study: 10-Year Assessment. J. Clin. Sleep Med. 2020, 16, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Özden Mat, D.; Firat, S.; Aksu, K.; Aksu, F.; Duyar, S.Ş. Obstructive Sleep Apnea Is a Determinant of Asthma Control Independent of Smoking, Reflux, and Rhinitis. Allergy Asthma Proc. 2021, 42, e25–e29. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Polomis, D.A.; Hall, S.V.; Teodorescu, M.C.; Gangnon, R.E.; Peterson, A.G.; Xie, A.; Sorkness, C.A.; Jarjour, N.N. Association of Obstructive Sleep Apnea Risk with Asthma Control in Adults. Chest 2010, 138, 543–550. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Jo, E.-J.; Kang, S.-Y.; Chang, Y.-S.; Yoon, I.-Y.; Cho, S.-H.; Min, K.-U.; Kim, S.-H. Obstructive Sleep Apnea Is Associated with Reduced Quality of Life in Adult Patients with Asthma. Ann. Allergy Asthma Immunol. 2013, 110, 253–257, 257.e1. [Google Scholar] [CrossRef]
- Teodorescu, M.; Polomis, D.A.; Gangnon, R.E.; Fedie, J.E.; Consens, F.B.; Chervin, R.D.; Teodorescu, M.C. Asthma Control and Its Relationship with Obstructive Sleep Apnea (OSA) in Older Adults. Sleep Disord. 2013, 2013, 251567. [Google Scholar] [CrossRef]
- ten Brinke, A.; Sterk, P.J.; Masclee, A.A.M.; Spinhoven, P.; Schmidt, J.T.; Zwinderman, A.H.; Rabe, K.F.; Bel, E.H. Risk Factors of Frequent Exacerbations in Difficult-to-Treat Asthma. Eur. Respir. J. 2005, 26, 812–818. [Google Scholar] [CrossRef]
- Tay, T.R.; Radhakrishna, N.; Hore-Lacy, F.; Smith, C.; Hoy, R.; Dabscheck, E.; Hew, M. Comorbidities in Difficult Asthma Are Independent Risk Factors for Frequent Exacerbations, Poor Control and Diminished Quality of Life. Respirology 2016, 21, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.T.; Stellman, S.D.; Reibman, J.; Farfel, M.R.; Brackbill, R.M.; Friedman, S.M.; Li, J.; Cone, J.E. Factors Associated with Poor Control of 9/11-Related Asthma 10-11 Years after the 2001 World Trade Center Terrorist Attacks. J. Asthma 2015, 52, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, K.; Hu, K.; Yang, J.; Li, Z.; Nie, M.; Dong, Y.; Huang, H.; Chen, J. Impact of Obstructive Sleep Apnea on Severe Asthma Exacerbations. Sleep Med. 2016, 26, 1–5. [Google Scholar] [CrossRef]
- Yii, A.C.A.; Tan, J.H.Y.; Lapperre, T.S.; Chan, A.K.W.; Low, S.Y.; Ong, T.H.; Tan, K.L.; Chotirmall, S.H.; Sterk, P.J.; Koh, M.S. Long-Term Future Risk of Severe Exacerbations: Distinct 5-Year Trajectories of Problematic Asthma. Allergy 2017, 72, 1398–1405. [Google Scholar] [CrossRef]
- Davies, S.E.; Bishopp, A.; Wharton, S.; Turner, A.M.; Mansur, A.H. Does Continuous Positive Airway Pressure (CPAP) Treatment of Obstructive Sleep Apnoea (OSA) Improve Asthma-Related Clinical Outcomes in Patients with Co-Existing Conditions? A Systematic Review. Respir. Med. 2018, 143, 18–30. [Google Scholar] [CrossRef]
- Kauppi, P.; Bachour, P.; Maasilta, P.; Bachour, A. Long-Term CPAP Treatment Improves Asthma Control in Patients with Asthma and Obstructive Sleep Apnoea. Sleep Breath. 2016, 20, 1217–1224. [Google Scholar] [CrossRef]
- Lafond, C.; Sériès, F.; Lemière, C. Impact of CPAP on Asthmatic Patients with Obstructive Sleep Apnoea. Eur. Respir. J. 2007, 29, 307–311. [Google Scholar] [CrossRef]
- Cisneros, C.; Iturricastillo, G.; Martínez-Besteiro, E.; Eiros, J.M.; Marcos, C.; Múgica, V.; Melero, C.; Martínez-Meca, A.; Landete, P.; Zamora, E. Obstructive Sleep Apnea: The Key for a Better Asthma Control? Sleep Med. 2023, 101, 135–137. [Google Scholar] [CrossRef]
- Serrano-Pariente, J.; Plaza, V.; Soriano, J.B.; Mayos, M.; López-Viña, A.; Picado, C.; Vigil, L.; CPASMA Trial Group. Asthma Outcomes Improve with Continuous Positive Airway Pressure for Obstructive Sleep Apnea. Allergy 2017, 72, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.S.; Chan, T.-O.; To, K.-W.; Chan, K.K.P.; Ngai, J.; Yip, W.-H.; Lo, R.L.P.; Ko, F.W.S.; Hui, D.S.C. Continuous Positive Airway Pressure for Obstructive Sleep Apnoea Does Not Improve Asthma Control. Respirology 2018, 23, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Shaarawy, H.; Affara, N. Assessment of the prevalence of obstructive sleep apnea in patients with stable uncontrolled asthma, impact of continuous positive airway pressure treatment. Egypt. J. Chest Dis. Tuberc. 2013, 62, 183–187. [Google Scholar] [CrossRef]
- Shaker, A. Study of obstructive sleep apnea (OSA) in asthmatics. Egypt. J. Chest Dis. Tuberc. 2017, 66, 293–298. [Google Scholar] [CrossRef]
- Zidan, M.; Daabis, R.; Gharraf, H. Overlap of Obstructive Sleep Apnea and Bronchial Asthma: Effect on Asthma Control. Egypt. J. Chest Dis. Tuberc. 2015, 64, 425–430. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Lo, Y.-L.; Lin, S.-M.; Huang, C.-D.; Chung, F.-T.; Lin, H.-C.; Wang, C.-H.; Kuo, H.-P. Obstructive Sleep Apnoea Accelerates FEV1 Decline in Asthmatic Patients. BMC Pulm. Med. 2017, 17, 55. [Google Scholar] [CrossRef]
- Emilsson, Ö.I.; Sundbom, F.; Ljunggren, M.; Benediktsdottir, B.; Garcia-Aymerich, J.; Bui, D.S.; Jarvis, D.; Olin, A.-C.; Franklin, K.A.; Demoly, P.; et al. Association between Lung Function Decline and Obstructive Sleep Apnoea: The ALEC Study. Sleep Breath. 2021, 25, 587–596. [Google Scholar] [CrossRef]
- Devouassoux, G.; Lévy, P.; Rossini, E.; Pin, I.; Fior-Gozlan, M.; Henry, M.; Seigneurin, D.; Pépin, J.-L. Sleep Apnea Is Associated with Bronchial Inflammation and Continuous Positive Airway Pressure-Induced Airway Hyperresponsiveness. J. Allergy Clin. Immunol. 2007, 119, 597–603. [Google Scholar] [CrossRef]
- Lin, H.C.; Wang, C.H.; Yang, C.T.; Huang, T.J.; Yu, C.T.; Shieh, W.B.; Kuo, H.P. Effect of Nasal Continuous Positive Airway Pressure on Methacholine-Induced Bronchoconstriction. Respir. Med. 1995, 89, 121–128. [Google Scholar] [CrossRef]
- Busk, M.; Busk, N.; Puntenney, P.; Hutchins, J.; Yu, Z.; Gunst, S.J.; Tepper, R.S. Use of Continuous Positive Airway Pressure Reduces Airway Reactivity in Adults with Asthma. Eur. Respir. J. 2013, 41, 317–322. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Taheri, S. Excess Weight and Sleep-Disordered Breathing. J. Appl. Physiol. (1985) 2005, 99, 1592–1599. [Google Scholar] [CrossRef]
- Bachour, P.; Bachour, A.; Kauppi, P.; Maasilta, P.; Mäkitie, A.; Palotie, T. Oral appliance in sleep apnea treatment: Respiratory and clinical effects and long-term adherence. Sleep Breath. 2016, 20, 805–812. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal Study of Moderate Weight Change and Sleep-Disordered Breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, Y.; Kong, N.; Zhang, J.; Cheng, G.; Zheng, Y. Weight Gain from Early to Middle Adulthood Increases the Risk of Incident Asthma Later in Life in the United States: A Retrospective Cohort Study. Respir. Res. 2021, 22, 139. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.D.; Ferreira, P.G.; Silva, A.G.; Stelmach, R.; Carvalho-Pinto, R.M.; Fernandes, F.L.A.; Mancini, M.C.; Sato, M.N.; Martins, M.A.; Carvalho, C.R.F. The Role of Exercise in a Weight-Loss Program on Clinical Control in Obese Adults with Asthma. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Pretto, J.J.; Morgan, P.J.; Callister, R.; Wood, L.G. Dietary Restriction and Exercise Improve Airway Inflammation and Clinical Outcomes in Overweight and Obese Asthma: A Randomized Trial. Clin. Exp. Allergy 2013, 43, 36–49. [Google Scholar] [CrossRef] [PubMed]
- van Huisstede, A.; Rudolphus, A.; Castro Cabezas, M.; Biter, L.U.; van de Geijn, G.-J.; Taube, C.; Hiemstra, P.S.; Braunstahl, G.-J. Effect of Bariatric Surgery on Asthma Control, Lung Function and Bronchial and Systemic Inflammation in Morbidly Obese Subjects with Asthma. Thorax 2015, 70, 659–667. [Google Scholar] [CrossRef]
- Okoniewski, W.; Lu, K.D.; Forno, E. Weight Loss for Children and Adults with Obesity and Asthma. A Systematic Review of Randomized Controlled Trials. Ann. Am. Thorac. Soc. 2019, 16, 613–625. [Google Scholar] [CrossRef]
- Araujo, A.M.S.; Duarte, R.L.M.; Gozal, D.; Cardoso, A.P.; Mello, F.C.Q. Predictive Factors for Obstructive Sleep Apnea in Adults with Severe Asthma Receiving Biologics: A Single-Center Cross-Sectional Study. Sleep Breath. 2023, 27, 1091–1098. [Google Scholar] [CrossRef]
- Denjean, A.; Canet, E.; Praud, J.P.; Gaultier, C.; Bureau, M. Hypoxia-Induced Bronchial Responsiveness in Awake Sheep: Role of Carotid Chemoreceptors. Respir. Physiol. 1991, 83, 201–210. [Google Scholar] [CrossRef]
- Teodorescu, M.; Song, R.; Brinkman, J.A.; Sorkness, R.L. Chronic Intermittent Hypoxia Increases Airway Hyperresponsiveness during House Dust Mites Exposures in Rats. Respir. Res. 2023, 24, 189. [Google Scholar] [CrossRef]
- Low, T.; Lin, T.-Y.; Lin, J.-Y.; Lai, C.J. Airway Hyperresponsiveness Induced by Intermittent Hypoxia in Rats. Respir. Physiol. Neurobiol. 2022, 295, 103787. [Google Scholar] [CrossRef]
- Sultonov, D.; Kim, Y.H.; Park, H.; Kim, K.-S. Intermittent Hypoxia on the Attenuation of Induced Nasal Allergy and Allergic Asthma by MAPK Signaling Pathway Downregulation in a Mice Animal Model. Int. J. Mol. Sci. 2022, 23, 9235. [Google Scholar] [CrossRef] [PubMed]
- Sundbom, F.; Janson, C.; Ljunggren, M.; Lindberg, E. Asthma and Asthma-Related Comorbidity: Effects on Nocturnal Oxygen Saturation. J. Clin. Sleep Med. 2022, 18, 2635–2641. [Google Scholar] [CrossRef] [PubMed]
- Sundbom, F.; Janson, C.; Malinovschi, A.; Lindberg, E. Effects of Coexisting Asthma and Obstructive Sleep Apnea on Sleep Architecture, Oxygen Saturation, and Systemic Inflammation in Women. J. Clin. Sleep Med. 2018, 14, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Nishimura, M.; Shinano, H.; Sato, F.; Miyamoto, K.; Kawakami, Y. Effect of Mild Hypoxia on Airway Responsiveness to Methacholine in Subjects with Airway Hyperresponsiveness. Chest 1999, 116, 1653–1658. [Google Scholar] [CrossRef]
- Sarıman, N.; Levent, E.; Cubuk, R.; Yurtlu, S.; Benli Aksungar, F. Bronchial Hyperreactivity and Airway Wall Thickening in Obstructive Sleep Apnea Patients. Sleep Breath. 2011, 15, 341–350. [Google Scholar] [CrossRef]
- Sundbom, F.; Malinovschi, A.; Lindberg, E.; Almqvist, C.; Janson, C. Insomnia Symptoms and Asthma Control-Interrelations and Importance of Comorbidities. Clin. Exp. Allergy 2020, 50, 170–177. [Google Scholar] [CrossRef]
- Al-Harbi, A.; Alanazi, T.; Alghamdi, H.; Alberreet, M.; Alkewaibeen, A.; Alkhalifah, A.; Omair, A.; Khan, M.; Al-Jahdali, H. Prevalence of Insomnia Among Patients with Bronchial Asthma. J. Asthma Allergy 2022, 15, 111–116. [Google Scholar] [CrossRef]
- Luyster, F.S.; Strollo, P.J.; Holguin, F.; Castro, M.; Dunican, E.M.; Fahy, J.; Gaston, B.; Israel, E.; Jarjour, N.N.; Mauger, D.T.; et al. Association Between Insomnia and Asthma Burden in the Severe Asthma Research Program (SARP) III. Chest 2016, 150, 1242–1250. [Google Scholar] [CrossRef]
- Braido, F.; Baiardini, I.; Ferrando, M.; Scichilone, N.; Santus, P.; Petrone, A.; Di Marco, F.; Corsico, A.G.; Zanforlin, A.; Milanese, M.; et al. The Prevalence of Sleep Impairments and Predictors of Sleep Quality among Patients with Asthma. J. Asthma 2021, 58, 481–487. [Google Scholar] [CrossRef]
- Shi, X.; Buysse, D.J.; Ritterband, L.M.; Sereika, S.M.; Strollo, P.J.; Wenzel, S.E.; Luyster, F.S. Solving Insomnia Electronically: Sleep Treatment for Asthma (SIESTA): A Study Protocol for a Randomized Controlled Trial. Contemp. Clin. Trials 2019, 79, 73–79. [Google Scholar] [CrossRef]
- Kasai, T.; Bradley, T.D. Obstructive Sleep Apnea and Heart Failure: Pathophysiologic and Therapeutic Implications. J. Am. Coll. Cardiol. 2011, 57, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kuniyoshi, F.H.S.; Garcia-Touchard, A.; Gami, A.S.; Romero-Corral, A.; van der Walt, C.; Pusalavidyasagar, S.; Kara, T.; Caples, S.M.; Pressman, G.S.; Vasquez, E.C.; et al. Day-Night Variation of Acute Myocardial Infarction in Obstructive Sleep Apnea. J. Am. Coll. Cardiol. 2008, 52, 343–346. [Google Scholar] [CrossRef]
- Desjardin, J.A.; Sutarik, J.M.; Suh, B.Y.; Ballard, R.D. Influence of Sleep on Pulmonary Capillary Volume in Normal and Asthmatic Subjects. Am. J. Respir. Crit. Care Med. 1995, 152, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Leung, R.S.T. Sleep-Disordered Breathing: Autonomic Mechanisms and Arrhythmias. Prog. Cardiovasc. Dis. 2009, 51, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J.A.; Widdicombe, J.G. Reflex Effects of Upper Airway Irritation on Total Lung Resistance and Blood Pressure. J. Appl. Physiol. 1962, 17, 861–865. [Google Scholar] [CrossRef]
- Chrysostomakis, S.I.; Simantirakis, E.N.; Schiza, S.E.; Karalis, I.K.; Klapsinos, N.C.; Siafakas, N.M.; Vardas, P.E. Continuous Positive Airway Pressure Therapy Lowers Vagal Tone in Patients with Obstructive Sleep Apnoea-Hypopnoea Syndrome. Hell. J. Cardiol. 2006, 47, 13–20. [Google Scholar]
- Dissanayake, H.U.; Bin, Y.S.; Ucak, S.; de Chazal, P.; Sutherland, K.; Cistulli, P.A. Association between Autonomic Function and Obstructive Sleep Apnea: A Systematic Review. Sleep Med. Rev. 2021, 57, 101470. [Google Scholar] [CrossRef]
- Dissanayake, H.U.; Bin, Y.S.; Sutherland, K.; Ucak, S.; de Chazal, P.; Cistulli, P.A. The Effect of Obstructive Sleep Apnea Therapy on Cardiovascular Autonomic Function: A Systematic Review and Meta-Analysis. Sleep 2022, 45, zsac210. [Google Scholar] [CrossRef]
- Teodorescu, M.; Broytman, O.; Curran-Everett, D.; Sorkness, R.L.; Crisafi, G.; Bleecker, E.R.; Erzurum, S.; Gaston, B.M.; Wenzel, S.E.; Jarjour, N.N.; et al. Obstructive Sleep Apnea Risk, Asthma Burden, and Lower Airway Inflammation in Adults in the Severe Asthma Research Program (SARP) II. J. Allergy Clin. Immunol. Pract. 2015, 3, 566–575.e1. [Google Scholar] [CrossRef]
- Karamanlı, H.; Özol, D.; Ugur, K.S.; Yıldırım, Z.; Armutçu, F.; Bozkurt, B.; Yigitoglu, R. Influence of CPAP Treatment on Airway and Systemic Inflammation in OSAS Patients. Sleep Breath. 2014, 18, 251–256. [Google Scholar] [CrossRef]
- Aihara, K.; Oga, T.; Chihara, Y.; Harada, Y.; Tanizawa, K.; Handa, T.; Hitomi, T.; Uno, K.; Mishima, M.; Chin, K. Analysis of Systemic and Airway Inflammation in Obstructive Sleep Apnea. Sleep Breath. 2013, 17, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.A.; Schwartzman, I.N.; Mellema, M.S.; Flynt, L.; Imrich, A.; Johnston, R.A. Effect of Leptin on Allergic Airway Responses in Mice. J. Allergy Clin. Immunol. 2005, 115, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.; Winck, J.C.; Guimarães, J.T.; Santos, A.C.; Almeida, J.; Marques, J.A. Autoadjusting-CPAP Effect on Serum Leptin Concentrations in Obstructive Sleep Apnoea Patients. BMC Pulm. Med. 2008, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Imayama, I.; Prasad, B. Role of Leptin in Obstructive Sleep Apnea. Ann. Am. Thorac. Soc. 2017, 14, 1607–1621. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Israel, E.; Reddel, H.K. Severe and Difficult-to-Treat Asthma in Adults. N. Engl. J. Med. 2017, 377, 965–976. [Google Scholar] [CrossRef]
| Proposed Mechanisms | Pathological Pathway |
|---|---|
| Sleep architecture | FRC Long-term facilitation Respiratory phase interdependence |
| Nasal congestion | |
| Type 1 inflammation | Bronchoconstriction Sputum neutrophils |
| Type 2 inflammation | NANC inhibition |
| Treatment Type | Risk of Developing OSA | Mechanisms |
|---|---|---|
| Inhaled corticosteroids (ICS) | Central neck fat distribution, pharyngeal muscle myopathy | |
| ICS extra-fine particles (<2 µm) | Upper airway deposition of particles | |
| Oral corticosteroids | Obesity, central neck fat distribution, pharyngeal muscle myopathy | |
| Intranasal corticosteroids | Airway resistance, airway patency | |
| Antileukotrienes | Airway resistance, airway patency | |
| Biologic therapy | Unclear |
| Author | Year | Sample | Definition of OSA | Findings |
|---|---|---|---|---|
| Cross sectional | ||||
| ten Brinke [62] | 2005 | n = 136 difficult-to-treat asthma | PSG or history of snoring and daytime sleepiness with frequent apnea periods of >10 s | ↑ frequent exacerbation OR 3.4 (1.2–10.4) adjusted for age and asthma duration. ↔ frequent exacerbation after accounting for covariates |
| Teodorescu [59] | 2010 | n = 472 outpatient clinic | Sleep Apnea Scale of the Sleep Disorders Questionnaire (high OSA risk vs. without high risk) | ↑ uncontrolled asthma in high OSA risk. OR 2.87 (95% CI 1.54–5.32) accounting for covariates |
| Kim [60] | 2013 | n = 217 outpatient clinic | Berlin questionnaire (high risk vs. low risk) | ↓ asthma specific quality of life score in high OSA risk (vs. low OSA risk) ↔ asthma control |
| Teodorescu [61] | 2013 | n = 813 outpatient clinic | PSG | ↑ worse asthma severity step (OR 2.91, 95% CI 1.15–7.36) ↑ severe asthma (OR 6.67, 95% CI 1.74–25.56) in older subjects (age 60–75) vs. OR 2.61, 95% CI 1.28–5.33) in younger subjects (age 18–59). |
| Tay [63] | 2016 | n = 90 difficult asthma clinic | Berlin questionnaire (high OSA risk vs. low risk) | ↔ frequent exacerbation, ACT score, and asthma-specific quality of life accounting for increasing BMI and other comorbid conditions |
| Ozden Mat [58] | 2021 | n = 137 outpatient clinic | Berlin questionnaire (high OSA risk vs. low risk) | ↑ 7.9 times increased odds of uncontrolled asthma Odds ratio 7.896, 95% CI 2.902–21.487. |
| Longitudinal | ||||
| Jordan [64] | 2015 | n = 2445 World Trade Center Health Registry, 10–11 years follow up | Physician-diagnosed OSA | ↑ 1.39 and 1.48 times increased risk of poorly controlled asthma and very poorly controlled asthma, respectively, adjusting for covariates |
| Wang [65] | 2016 | n = 146 asthma n = 157 no asthma 1 year follow up | PSG | ↑ AHI increased risk of severe asthma exacerbations (OR 1.322, 95% CI 1.148–1.523) |
| Yii [66] | 2017 | n = 177 Step 4 of GINA treatment ladder 5 years follow up | PSG | ↔ severe asthma exacerbation |
| Author | Year | Sample | Study Design | Findings |
|---|---|---|---|---|
| Lafond [69] | 2007 | n = 20 OSA and asthma | Prospective, 6 weeks after CPAP therapy | CPAP use 6.7 h/d ↔ airway responsiveness, %FEV1, FEV1/FVC ratio ↑ asthma-specific quality of life |
| Teodorescu [16] | 2012 | n = 75 CPAP therapy, OSA and asthma | Cross-sectional | ↓ persistent daytime asthma symptoms ↔ persistent nighttime asthma symptoms |
| Shaarawy [73] | 2013 | n = 15 uncontrolled asthma and OSA | Prospective, 6 weeks after CPAP therapy | ↓ Epworth sleepiness scale ↓ arousal index ↔ % FEV1, %FVC, FEV1/FVC ratio ↔ ACT score |
| Kauppi [68] | 2016 | n = 152 CPAP started after asthma treatment | Cross-sectional, survey questionnaire | CPAP use 6.3 h/d, mean 5.7 years. ↓ self-reported asthma severity and ↑ACT score without significant changes in BMI ↓ daily rescue medication use |
| Serrano-Pariente [71] | 2017 | n = 99 OSA and asthma | Prospective, before and after 6 months of CPAP | ↓ asthma control questionnaire score ↓ % of uncontrolled asthma ↓ % of asthma attacks ↓ GERD symptoms ↓ positive bronchodilation test ↓ FeNO ↑ Asthma control and ↑ quality of life among patients compliant with CPAP (≥4 h/night) vs. noncompliant subjects. |
| Shaker [74] | 2017 | n = 12 OSA and asthma | Prospective, 3 months after CPAP therapy | ↓ daytime and nighttime asthma symptoms ↓ GERD symptoms ↓ difficult to control asthma ↓ Epworth sleepiness scale ↑ %FEV1 ↑ FEV1/FVC ratio ↑ sleep efficiency ↓ total sleep time |
| Ng [72] | 2018 | n = 17 CPAP group n = 20 control group Nocturnal asthma symptoms and OSA | Randomized controlled trial | CPAP use, 5.0 h/d at 1 month and 5.2 h/d at 3 months ↓ Epworth sleepiness score ↑ asthma specific quality of life ↑ vital domain of quality of life ↔ ACT score ↔ asthma exacerbation rate, spirometry, and airway responsiveness |
| Cisneros [70] | 2023 | n = 100 OSA and asthma | Retrospective, before and ≥3 months after CPAP | ↑ clinical asthma control ↑ ACT score |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saxena, D.; Imayama, I.; Adrish, M. Revisiting Asthma Obstructive Sleep Apnea Overlap: Current Knowledge and Future Needs. J. Clin. Med. 2023, 12, 6552. https://doi.org/10.3390/jcm12206552
Saxena D, Imayama I, Adrish M. Revisiting Asthma Obstructive Sleep Apnea Overlap: Current Knowledge and Future Needs. Journal of Clinical Medicine. 2023; 12(20):6552. https://doi.org/10.3390/jcm12206552
Chicago/Turabian StyleSaxena, Damini, Ikuyo Imayama, and Muhammad Adrish. 2023. "Revisiting Asthma Obstructive Sleep Apnea Overlap: Current Knowledge and Future Needs" Journal of Clinical Medicine 12, no. 20: 6552. https://doi.org/10.3390/jcm12206552
APA StyleSaxena, D., Imayama, I., & Adrish, M. (2023). Revisiting Asthma Obstructive Sleep Apnea Overlap: Current Knowledge and Future Needs. Journal of Clinical Medicine, 12(20), 6552. https://doi.org/10.3390/jcm12206552






