Effect of Body Mass Index on the Prognostic Value of Atherogenic Index of Plasma in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Measurement
2.3. Follow-Up and Endpoints
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Jia, S.; Liu, Y.; Yuan, J. Evidence in Guidelines for Treatment of coronary artery disease. Adv. Exp. Med. Biol. 2020, 1177, 37–73. [Google Scholar]
- Li, J.; Li, X.; Wang, Q.; Hu, S.; Wang, Y.; Masoudi, F.A.; Spertus, J.A.; Krumholz, H.M.; Jiang, L.; China Peace Collaborative Group. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): A retrospective analysis of hospital data. Lancet 2015, 385, 441–451. [Google Scholar] [CrossRef]
- Tokgözoğlu, L. Dyslipidemia, atherosclerosis, and vulnerable plaques: The effect of atorvastatin on atherosclerosis and plaque structure. Turk. Kardiyol. Dern. Ars. 2009, 37 (Suppl. S2), 11–16. [Google Scholar]
- Guedeney, P.; Claessen, B.E.; Kalkman, D.N.; Aquino, M.; Sorrentino, S.; Giustino, G.; Farhan, S.; Vogel, B.; Sartori, S.; Montalescot, G.; et al. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J. Am. Coll. Cardiol. 2019, 73, 2401–2409. [Google Scholar] [CrossRef]
- Sekimoto, T.; Koba, S.; Mori, H.; Sakai, R.; Arai, T.; Yokota, Y.; Sato, S.; Tanaka, H.; Masaki, R.; Oishi, Y.; et al. Small dense low density lipoprotein cholesterol: A residual risk for rapid progression of nonculprit coronary lesion in patients with acute coronary syndrome. J. Atheroscler. Thromb. 2021, 28, 1161–1174. [Google Scholar] [CrossRef]
- Kokubo, Y.; Watanabe, M.; Higashiyama, A.; Honda-Kohmo, K. Small-dense low-density lipoprotein cholesterol: A subclinical marker for the primary prevention of coronary heart disease. J. Atheroscler. Thromb. 2020, 27, 641–643. [Google Scholar] [CrossRef]
- Kanonidou, C. Small dense low-density lipoprotein: Analytical review. Clin. Chim. Acta 2021, 520, 172–178. [Google Scholar] [CrossRef]
- Dobiasova, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Frohlich, J.; Dobiasova, M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin. Chem. 2003, 49, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Dobiásová, M. AIP--aterogenní index plazmy jako významný prediktor kardiovaskulárního rizika: Od výzkumu do praxe [AIP--atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice]. Vnitr. Lek. 2006, 52, 64–71. [Google Scholar] [PubMed]
- Özcan Abacıoğlu, Ö.; Yıldırım, A.; Koyunsever, N.Y.; Karadeniz, M.; Kılıç, S. Relationship between atherogenic index of plasma and stent thrombosis in patients with acute coronary syndrome. Anatol. J. Cardiol. 2022, 26, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine 2017, 96, E8058. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.S.; Cheah, W.L.; Lwin, S.; Moe Nwe, T.; Win, T.T.; Aung, M. Understanding the Relationship between Atherogenic Index of Plasma and Cardiovascular Disease Risk Factors among Staff of an University in Malaysia. J. Nutr. Metab. 2018, 2018, 7027624. [Google Scholar] [CrossRef]
- Özen, Y.; Bilal Özbay, M.; Yakut, I.; Kanal, Y.; Abdelmottelaeb, W.; Nriagu, B.N.; Salmon, J.T.S.; Quintanilla, B.; Munguía, C.; Reyes Castro, T.; et al. Atherogenic index of plasma and triglyceride-glucose index to predict more advanced coronary artery diseases in patients with the first diagnosis of acute coronary syndrome. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3993–4005. [Google Scholar]
- Sato, F.; Nakamura, Y.; Kayaba, K.; Ishikawa, S. TG/HDL-C ratio as a predictor of stroke in the population with healthy BMI: The Jichi Medical School Cohort Study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1872–1879. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Q.; Wei, Z.; Wei, J.; Cui, M. Atherogenic Index of Plasma and Coronary Artery Disease in the Adult Population: A Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 817441. [Google Scholar] [CrossRef]
- Cai, G.; Liu, W.; Lv, S.; Wang, X.; Guo, Y.; Yan, Z.; Du, Y.; Zhou, Y. Gender-specific associations between atherogenic index of plasma and the presence and severity of acute coronary syndrome in very young adults: A hospital-based observational study. Lipids Health Dis. 2019, 18, 99. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Y.; Cheng, Y.; Shen, H.; Gao, F.; Qi, J.; Yang, L.; Wang, Z.; Shi, D.; Liu, Y.; et al. Prognostic impact of the atherogenic index of plasma in type 2 diabetes mellitus patients with acute coronary syndrome undergoing percutaneous coronary intervention. Lipids Health Dis. 2020, 19, 240. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, C.; Yang, J.; Seery, S.; Qi, Y.; Wang, W.; Zhang, K.; Shao, C.; Tang, Y.D. Atherogenic index of plasma for non-diabetic, coronary artery disease patients after percutaneous coronary intervention: A prospective study of the long-term outcomes in China. Cardiovasc. Diabetol. 2022, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014, 2014, 2354–2394. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 2013, 2013, e362–e425. [Google Scholar]
- Burns, S.F.; Lee, S.J.; Arslanian, S.A. Surrogate lipid markers for small dense low density lipoprotein particles in overweight youth. J. Pediatr. 2012, 161, 991–996. [Google Scholar] [CrossRef]
- Austin, M.A.; King, M.C.; Vranizan, K.M.; Krauss, R.M. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation 1990, 82, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Breslow, J.L.; Hennekens, C.H.; Buring, J.E.; Willett, W.C.; Krauss, R.M. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988, 260, 1917–1921. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef]
- Nishikura, T.; Koba, S.; Yokota, Y.; Hirano, T.; Tsunoda, F.; Shoji, M.; Hamazaki, Y.; Suzuki, H.; Itoh, Y.; Katagiri, T.; et al. Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J. Atheroscler. Thromb. 2014, 21, 755–767. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP); Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Otrante, A.; Bounafaa, A.; Berrougui, H.; Essamadi, A.-K.; Nguyen, M.; Fülöp, T.; Khalil, A. Small Dense LDL Level and LDL/HDL Distribution in Acute Coronary Syndrome Patients. Biomedicines 2023, 11, 1198. [Google Scholar] [CrossRef]
- Li, C.; Ford, E.S.; Meng, Y.X.; Mokdad, A.H.; Reaven, G.M. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc. Diabetol. 2008, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- He, J.; He, S.; Liu, K.; Shi, D.; Chen, X. The TG/HDL-c ratio might be a surrogate for insulin resistance in Chinese non-obese women. Internet J. Endocrinol. 2014, 2014, 105168. [Google Scholar]
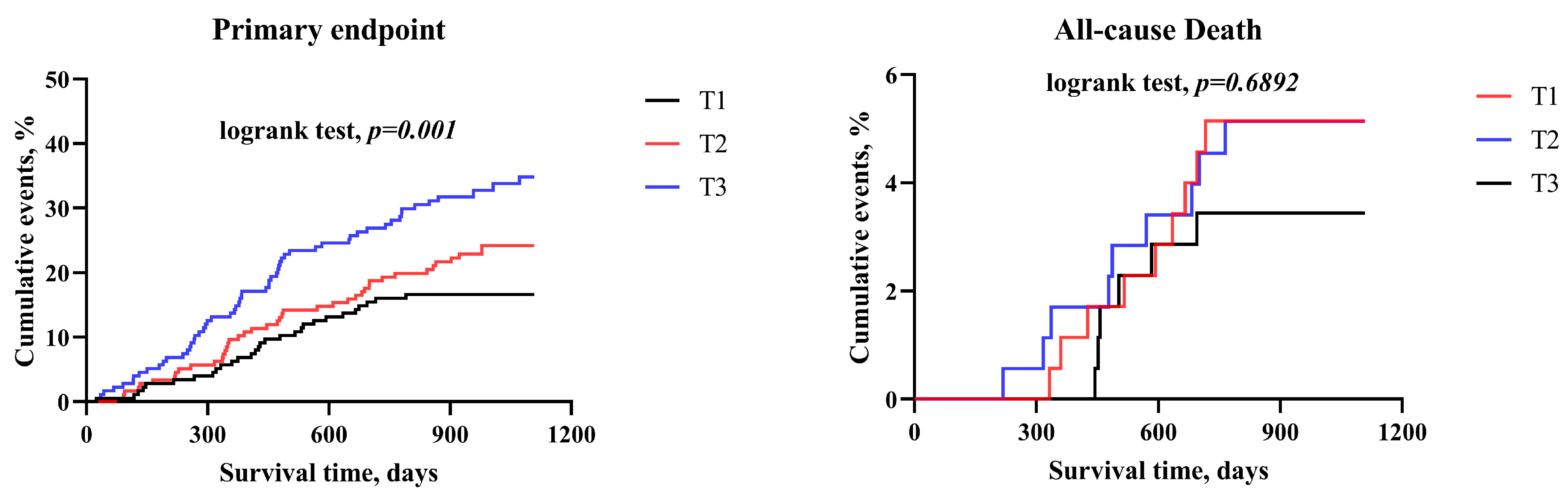
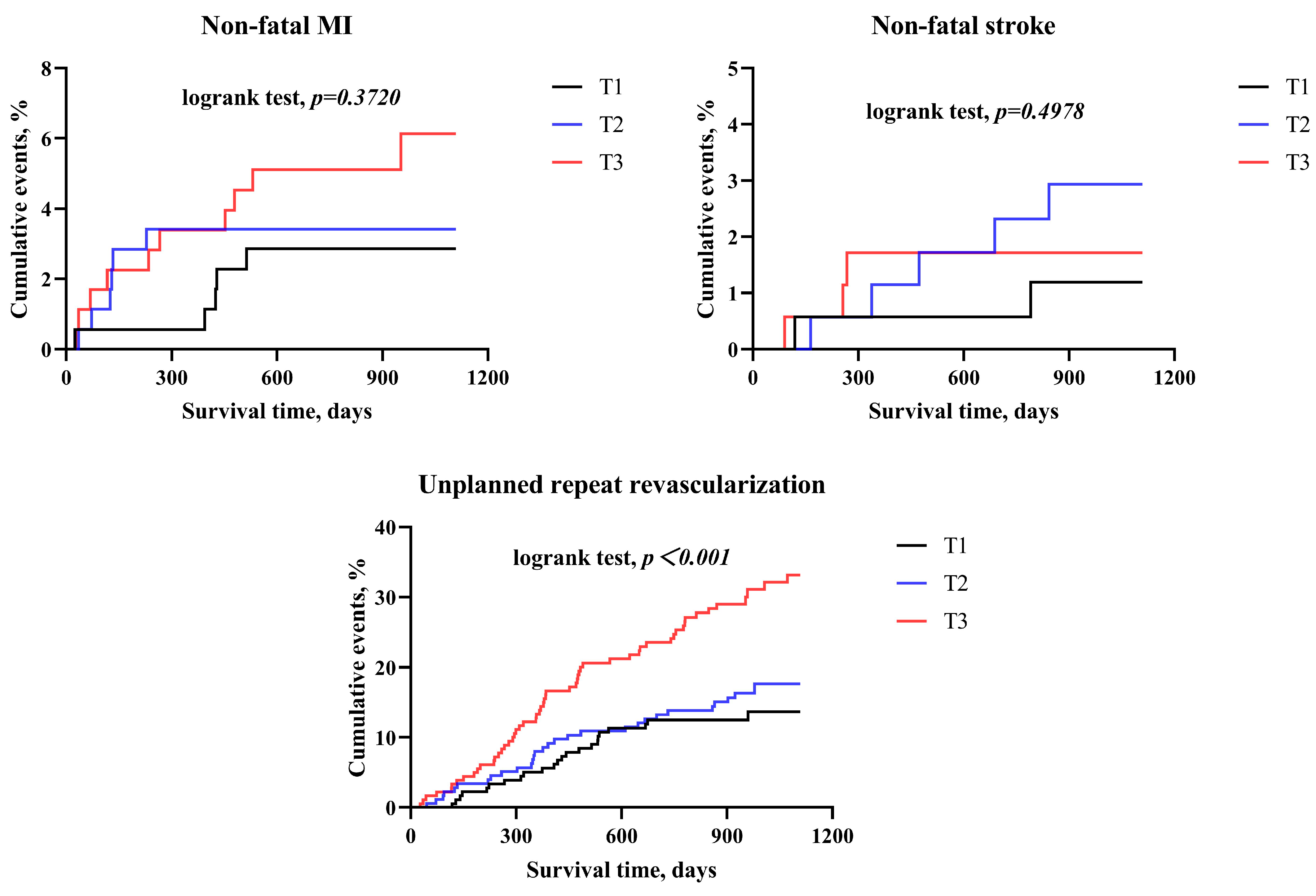
| Variable | BMI < 24 kg/m2 | 24 kg/m2 ≤ BMI < 28 kg/m2 | BMI > 28 kg/m2 | ||||
|---|---|---|---|---|---|---|---|
| Total | T1 | T2 | T3 | p Value | |||
| N = 526 | N = 175 | N = 176 | N = 175 | N = 827 | N = 372 | ||
| Demographics | |||||||
| Age (years) | 62 ± 10 | 63 ± 9 | 61 ± 9 | 60 ± 12 | 0.013 * | 60 ± 10 | 57 ± 11 |
| Male sex, n (%) | 338 (64.3) | 106 (60.6) | 118 (67.0) | 114 (65.1) | 0.429 | 676 (81.7) | 309 (83.1) |
| Risk factors | |||||||
| Current smoking, n (%) | 193 (36.7) | 55 (31.4) | 62 (35.2) | 76 (43.4) | 0.059 | 388 (46.9) | 182 (48.9) |
| Chronically daily drinking, n (%) | 53 (10.1) | 20 (11.4) | 9 (5.1) | 24 (13.7) | 0.021 * | 121 (14.6) | 56 (15.1) |
| Family history of CHD, n (%) | 161 (30.7) | 47 (26.9) | 57 (32.4) | 57 (32.6) | 0.419 | 259 (31.4) | 130 (34.8) |
| Hypertension, n (%) | 302 (57.4) | 104 (59.4) | 101 (57.4) | 97 (55.4) | 0.751 | 528 (63.8) | 269 (72.3) |
| Dyslipidemia, n (%) | 379 (72.1) | 75 (42.9) | 134 (76.1) | 170 (97.1) | <0.001 * | 678 (82.0) | 323 (86.8) |
| Diabetes, n (%) | 219 (41.6) | 57 (32.6) | 89 (50.6) | 73 (41.7) | 0.003 * | 395 (47.8) | 181 (48.7) |
| PAD, n (%) | 60 (11.4) | 15 (8.6) | 29 (16.5) | 16 (9.1) | 0.034 * | 79 (9.6) | 38 (10.2) |
| Cardiac failure, n (%) | 45 (8.6) | 10 (5.7) | 19 (10.8) | 16 (9.1) | 0.222 | 57 (6.9) | 18 (4.8) |
| CKD, n (%) | 57 (10.8) | 20 (11.4) | 15 (8.5) | 22 (12.6) | 0.453 | 34 (4.1) | 9 (2.4) |
| Previous MI, n (%) | 101 (19.2) | 33 (18.9) | 34 (19.3) | 34 (19.4) | 0.990 | 145 (17.5) | 85 (22.8) |
| Past PCI, n (%) | 102 (19.4) | 40 (22.9) | 28 (15.9) | 34 (19.4) | 0.258 | 161 (19.5) | 79 (21.2) |
| LVEF (%) | 65 (60–68) | 65 (61–69) | 64.5 (60–68) | 64 (59–68) | 0.143 | 64 (60–68) | 64 (59–68) |
| Clinical presentation | |||||||
| UA, n (%) | 382 (72.6) | 152 (86.9) | 126 (71.6) | 104 (59.4) | <0.001 * | 622 (75.2) | 277 (74.5) |
| NSTEMI, n (%) | 74 (14.1) | 17 (9.7) | 21 (11.9) | 36 (20.6) | 0.009 * | 95 (11.5) | 50 (13.4) |
| STEMI, n (%) | 70 (13.3) | 6 (3.4) | 29 (16.5) | 35 (20.0) | <0.001 * | 110 (13.3) | 45 (12.1) |
| Laboratory measurements (fasting state) | |||||||
| TC (mmol/L) | 4.15 ± 0.95 | 4.00 ± 0.90 | 4.08 ± 0.92 | 4.36 ± 0.98 | 0.001 * | 4.09 ± 0.99 | 4.27 ± 1.03 |
| TG (mmol/L) | 1.31 (0.91–1.86) | 0.81 ± 0.22 | 1.31 ± 0.29 | 2.42 ± 1.01 | <0.001 * | 1.44 (1.02–2.06) | 1.70 (1.23–2.48) |
| LDL-C (mmol/L) | 2.42 ± 0.79 | 2.23 ± 0.78 | 2.46 ± 0.77 | 2.56 ± 0.79 | <0.001 * | 2.40 ± 0.80 | 2.56 ± 0.83 |
| HDL-C (mmol/L) | 1.09 ± 0.26 | 1.30 ± 0.24 | 1.05 ± 0.18 | 0.92 ± 0.18 | <0.001 * | 1.02 ± 0.22 | 0.97 ± 0.21 |
| FPG (mmol/L) | 5.67 (5.18–6.72) | 5.54 (5.08–6.21) | 5.80 (5.26–7.18) | 5.78 (5.18–6.83) | 0.002 * | 5.87 (5.23–7.08) | 5.88 (5.27–7.01) |
| Glycated hemoglobin (%) | 6.0 (5.5–7.1) | 5.8 (5.5–6.6) | 6.3 (5.6–7.6) | 6.0 (5.6–7.1) | 0.004 * | 6.1 (5.6–7.2) | 6.2 (5.6–7.0) |
| Hs-CRP (mg/L) | 1.32 (0.61–3.13) | 0.94 (0.39–2.18) | 1.29 (0.61–2.89) | 1.84 (0.81–4.97) | <0.001 * | 1.30 (0.61–3.39) | 1.71 (0.73–4.31) |
| Variable | BMI < 24 kg/m2 | 24 kg/m2 ≤ BMI < 28 kg/m2 | BMI > 28 kg/m2 | ||||
|---|---|---|---|---|---|---|---|
| Total | T1 | T2 | T3 | p Value | |||
| N = 526 | N = 175 | N = 176 | N = 175 | N = 827 | N = 372 | ||
| Medications at discharge | |||||||
| Aspirin, n (%) | 516 (98.1) | 171 (97.7) | 173 (98.3) | 172 (98.3) | 0.901 | 823 (99.5) | 370 (99.5) |
| P2Y12, n (%) | 525 (99.8) | 175 (100.0) | 175 (99.4) | 175 (100.0) | 0.369 | 826 (99.9) | 372 (100.0) |
| Statins, n (%) | 526 (100.0) | 175 (100.0) | 176 (100.0) | 175 (100.0) | >0.999 | 827 (100.0) | 372 (100.0) |
| ACEIs/ARBs, n (%) | 231 (43.9) | 71 (40.6) | 84 (47.7) | 76 (43.4) | 0.397 | 403 (48.7) | 197 (53.0) |
| β-blockers, n (%) | 356 (67.7) | 111 (63.4) | 131 (74.4) | 114 (65.1) | 0.06 | 588 (71.1) | 267 (71.8) |
| Angiographic findings | |||||||
| One-vessel disease, n (%) | 94 (17.9) | 37 (21.1) | 28 (15.9) | 29 (16.6) | 0.379 | 117 (14.1) | 51 (13.7) |
| Two-vessel disease, n (%) | 145 (27.6) | 51 (29.1) | 42 (23.9) | 52 (29.7) | 0.4 | 232 (28.1) | 111 (29.8) |
| LM/three-vessel disease, n (%) | 287 (54.6) | 87 (49.7) | 106 (60.2) | 94 (53.7) | 0.136 | 478 (57.8) | 210 (56.5) |
| Restenotic lesions, n (%) | 67 (12.7) | 25 (14.3) | 16 (9.1) | 26 (14.9) | 0.203 | 94 (11.4) | 41 (11.0) |
| Chronic total occlusions, n (%) | 107 (20.3) | 35 (20.0) | 33 (18.8) | 39 (22.3) | 0.706 | 171 (20.7) | 87 (23.4) |
| Syntax score | 21.59 ± 11.62 | 19.98 ± 11.04 | 22.63 ± 12.21 | 21.59 ± 11.62 | 0.074 | 21.23 ± 11.90 | 20.78 ± 9.90 |
| Procedural results | |||||||
| DCB | 31 (5.9) | 12 (6.9) | 6 (3.4) | 13 (7.4) | 0.224 | 48 (5.8) | 32 (8.6) |
| DES | 429 (81.6) | 142 (81.1) | 152 (86.4) | 135 (77.1) | 0.082 | 693 (83.8) | 294 (79.0) |
| BRS | 36 (6.8) | 13 (7.4) | 9 (5.1) | 14 (8.0) | 0.525 | 45 (5.4) | 17 (4.6) |
| Complete revascularization, n (%) | 317 (60.3) | 117 (66.9) | 99 (56.3) | 101 (57.7) | 0.089 | 529 (64.0) | 213 (57.3) |
| Variables | Univariate Analysis | Multivariate Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI < 24 kg/m2 | 24 kg/m2 ≤ BMI < 28 kg/m2 | BMI ≥ 28 kg/m2 | BMI < 24 kg/m2 | 24 kg/m2 ≤ BMI < 28 kg/m2 | BMI ≥ 28 kg/m2 | |||||||
| HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | |
| AIP | 3.125 | <0.001 * | 2.431 | 0.002 * | 2.029 | 0.068 | 2.506 | 0.007 * | 1.747 | 0.088 | 2.096 | 0.115 |
| Male sex | 1.029 | 0.877 | 1.377 | 0.219 | 0.927 | 0.791 | 0.819 | 0.378 | 1.289 | 0.356 | 0.662 | 0.221 |
| Age (years) | 1.006 | 0.523 | 1.013 | 0.144 | 1.002 | 0.859 | 0.987 | 0.200 | 0.989 | 0.296 | 0.999 | 0.942 |
| Current smoking | 1.392 | 0.063 | 1.098 | 0.577 | 1.087 | 0.7 | 1.541 | 0.047 * | 0.929 | 0.707 | 1.049 | 0.852 |
| Hypertension | 1.136 | 0.481 | 1.06 | 0.741 | 0.948 | 0.822 | 1.266 | 0.217 | 1.023 | 0.902 | 1.042 | 0.875 |
| Diabetes | 1.386 | 0.065 | 2.369 | <0.001 * | 0.916 | 0.688 | 1.313 | 0.142 | 1.981 | <0.001 * | 0.826 | 0.411 |
| NSTE-ACS | 1.332 | 0.312 | 1.017 | 0.944 | 0.577 | 0.053 | 2.094 | 0.028 * | 1.003 | 0.990 | 0.703 | 0.287 |
| Cardiac failure | 1.502 | 0.139 | 2.373 | <0.001 * | 1.764 | 0.15 | 0.965 | 0.907 | 1.684 | 0.053 | 2.174 | 0.081 |
| CKD | 1.877 | 0.008 * | 3.724 | <0.001 * | 0.472 | 0.455 | 1.627 | 0.089 | 2.324 | 0.008 * | 0.355 | 0.321 |
| Previous MI | 1.894 | <0.001 * | 1.487 | 0.043 * | 1.093 | 0.725 | 1.294 | 0.324 | 0.956 | 0.838 | 0.730 | 0.330 |
| Previous PCI | 1.497 | 0.046 * | 1.752 | 0.002 * | 1.335 | 0.244 | 1.391 | 0.200 | 1.699 | 0.012 * | 1.495 | 0.184 |
| LDL-C | 1.406 | <0.001 * | 0.992 | 0.941 | 1.187 | 0.158 | 1.252 | 0.048 * | 1.047 | 0.678 | 1.214 | 0.159 |
| Hs-CRP | 1.059 | <0.001 * | 1.03 | 0.014 * | 0.998 | 0.904 | 1.061 | <0.001 * | 1.018 | 0.211 | 0.966 | 0.141 |
| STNTAX Score | ||||||||||||
| Score ≤ 22 | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| 22 < Score < 33 | 1.387 | 0.119 | 2.299 | <0.001 * | 1.553 | 0.074 | 0.978 | 0.925 | 1.463 | 0.070 | 1.439 | 0.171 |
| Score ≥ 33 | 2.292 | <0.001 * | 1.98 | 0.003 * | 2.974 | <0.001 * | 1.396 | 0.207 | 1.250 | 0.387 | 2.582 | 0.004 * |
| Complete revascularization | 0.631 | 0.009 * | 0.352 | <0.001 * | 0.376 | <0.001 * | 0.729 | 0.118 | 0.444 | <0.001 * | 0.422 | 0.001 * |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR | p-Value | HR | p-Value | |
| AIP tertiles | ||||
| T1 | Reference | Reference | ||
| T2 | 1.448 (0.900–2.329) | 0.127 | 1.201 (0.729–1.980) | 0.472 |
| T3 | 2.216 (1.419–3.460) | <0.001 * | 1.772 (1.110–2.828) | 0.016 * |
| Male sex | 1.029 (0.715–1.482) | 0.877 | 0.820 (0.525–1.280) | 0.382 |
| Age (years) | 1.006 (0.989–1.023) | 0.523 | 0.986 (0.967–1.006) | 0.160 |
| Current smoking | 1.392 (0.982–1.974) | 0.063 | 1.577 (1.026–2.424) | 0.038 |
| Hypertension | 1.136 (0.797–1.618) | 0.481 | 1.273 (0.877–1.849) | 0.205 |
| Diabetes | 1.386 (0.980–1.961) | 0.065 | 1.339 (0.926–1.935) | 0.121 |
| NSTEACS | 1.332 (0.764–2.321) | 0.312 | 2.076 (1.074–4.010) | 0.030 * |
| Cardiac failure | 1.502 (0.876–2.574) | 0.139 | 0.973 (0.533–1.778) | 0.929 |
| CKD | 1.502 (1.176–2.998) | 0.139 | 1.672 (0.953–2.934) | 0.073 |
| Previous MI | 1.877 (1.296–2.768) | 0.008 | 1.318 (0.791–2.195) | 0.289 |
| Previous PCI | 1.894 (1.007–2.224) | <0.001 * | 1.384 (0.835–2.295) | 0.208 |
| LDL-C | 1.497 (1.150–1.720) | 0.046 * | 1.239 (0.991–1.550) | 0.060 |
| Hs-CRP | 1.406 (1.037–1.082) | <0.001 * | 1.060 (1.033–1.088) | <0.001 * |
| STNTAX Score | ||||
| Score ≤ 22 | Reference | Reference | ||
| 22 < Score < 33 | 1.387 (0.920–2.092) | 0.119 | 1.011 (0.641–1.597) | 0.961 |
| Score ≥ 33 | 2.292 (1.490–3.525) | <0.001 * | 1.402 (0.832–2.362) | 0.204 |
| Complete revascularization | 0.631 (0.446–0.892) | 0.009 * | 0.739 (0.496–1.101) | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, Y.; Sun, Y.; Shen, H.; Liu, X.; Liu, Y.; Shi, D.; Ma, X.; Zhou, Y. Effect of Body Mass Index on the Prognostic Value of Atherogenic Index of Plasma in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2023, 12, 6543. https://doi.org/10.3390/jcm12206543
Kan Y, Sun Y, Shen H, Liu X, Liu Y, Shi D, Ma X, Zhou Y. Effect of Body Mass Index on the Prognostic Value of Atherogenic Index of Plasma in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2023; 12(20):6543. https://doi.org/10.3390/jcm12206543
Chicago/Turabian StyleKan, Yi, Yan Sun, Hua Shen, Xiaoli Liu, Yuyang Liu, Dongmei Shi, Xiaoteng Ma, and Yujie Zhou. 2023. "Effect of Body Mass Index on the Prognostic Value of Atherogenic Index of Plasma in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention" Journal of Clinical Medicine 12, no. 20: 6543. https://doi.org/10.3390/jcm12206543
APA StyleKan, Y., Sun, Y., Shen, H., Liu, X., Liu, Y., Shi, D., Ma, X., & Zhou, Y. (2023). Effect of Body Mass Index on the Prognostic Value of Atherogenic Index of Plasma in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Journal of Clinical Medicine, 12(20), 6543. https://doi.org/10.3390/jcm12206543






