The Use of Regional Anesthesia to Reduce Blood Loss in Isolated Limb Perfusion (ILP)—A Novel Approach
Abstract
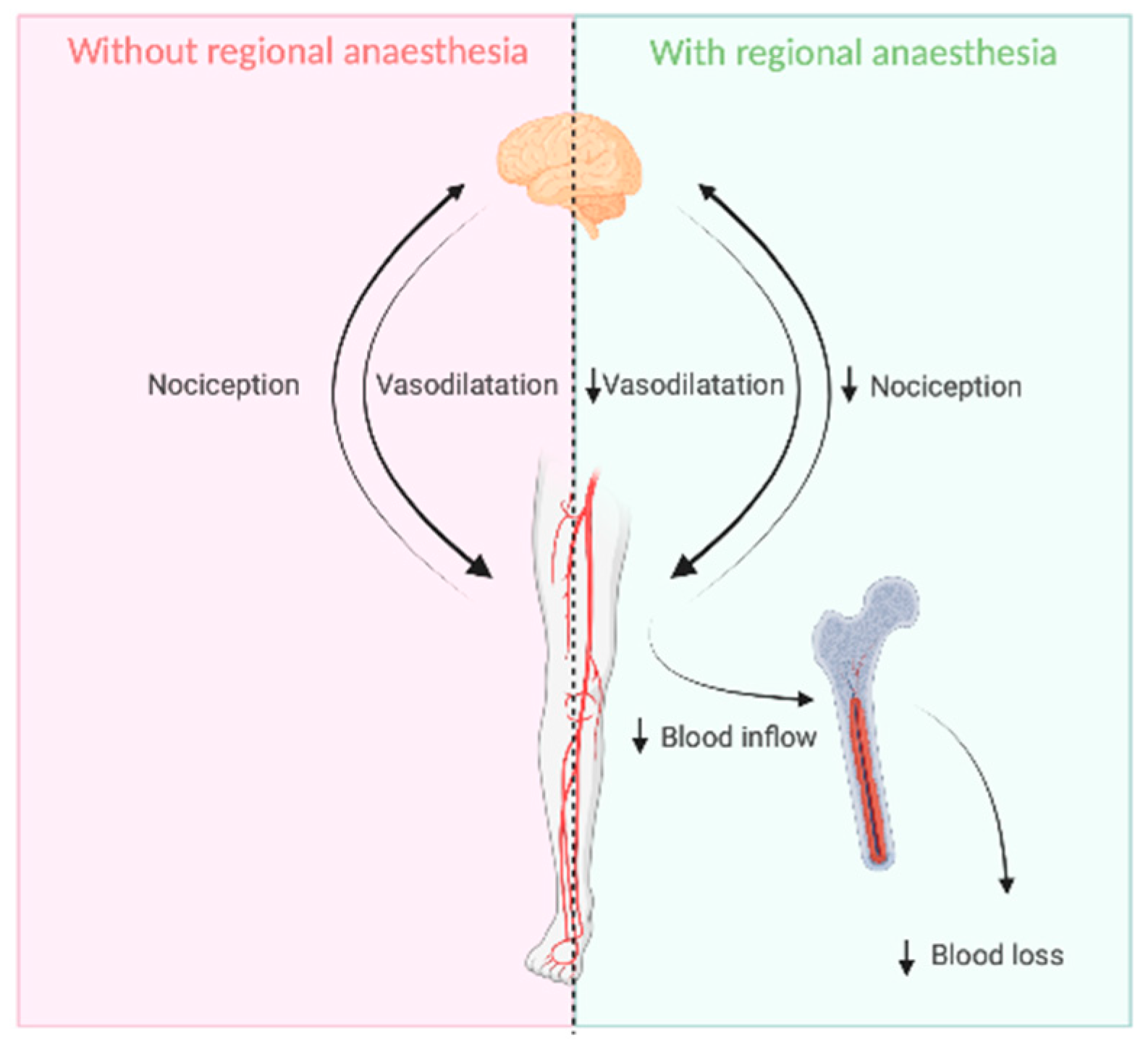
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Treatment
2.3. Anesthetic Care and Monitoring
2.4. Statistical Analysis
3. Results
3.1. Patients
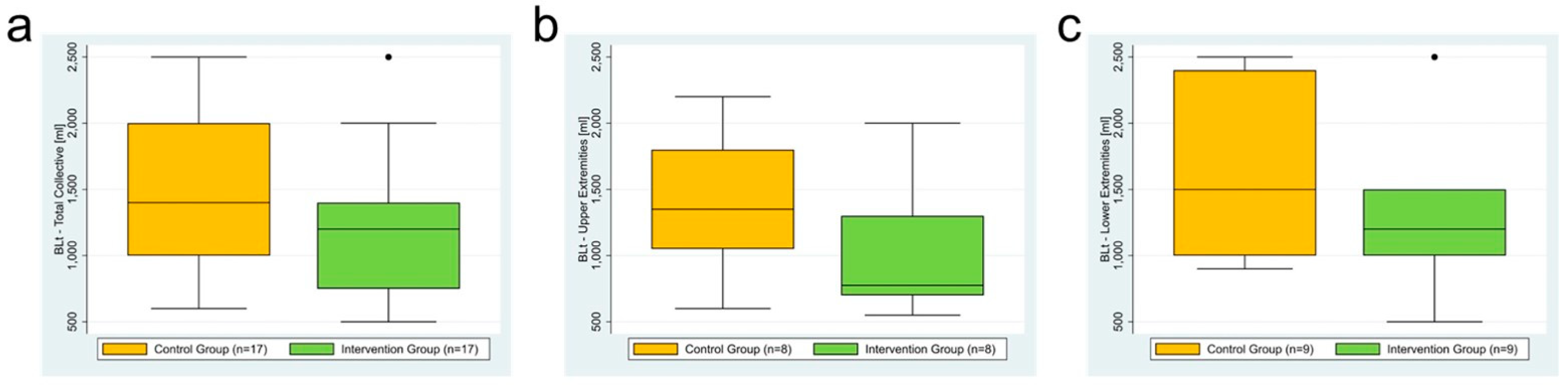
3.2. Total Collective (n = 34)
3.3. Subgroup Analysis: Upper Extremity (n = 16)
3.4. Subgroup Analysis: Lower Extremity (n = 18)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AWMF. S3-Leitlinie Adulte Weichgewebesarkome, Version 1.0; Registernummer 032–044ol. 07.09.2021 ed. 2021. Available online: https://register.awmf.org/de/leitlinien/detail/032-044OL (accessed on 13 March 2023).
- Eggermont, A.M.; Schraffordt Koops, H.; Klausner, J.M.; Kroon, B.B.; Schlag, P.M.; Lienard, D.; van Geel, A.N.; Hoekstra, H.J.; Meller, I.; Nieweg, O.E.; et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter european experience. Ann. Surg. 1996, 224, 756–764, discussion 764–755. [Google Scholar] [CrossRef]
- Reijers, S.J.M.; Davies, E.; Grünhagen, D.J.; Fiore, M.; Honore, C.; Rastrelli, M.; Vassos, N.; Podleska, L.E.; Niethard, M.; Jakob, J.; et al. Variation in response rates to isolated limb perfusion in different soft tissue tumor subtypes—A an international multi-center study. Eur. J. Cancer 2023, 190, 112949. [Google Scholar] [CrossRef]
- Neuwirth, M.G.; Song, Y.; Sinnamon, A.J.; Fraker, D.L.; Zager, J.S.; Karakousis, G.C. Isolated limb perfusion and infusion for extremity soft tissue sarcoma: A contemporary systematic review and meta-analysis. Ann. Surg. Oncol. 2017, 24, 3803–3810. [Google Scholar] [CrossRef]
- Andreou, D.; Fehlberg, S.; Tiedke, C.; Niethard, M.; Tunn, P.U. Revolutionizing the treatment of locally advanced extremity soft tissue sarcomas: A review on tnfα-based isolated limb perfusion. Eur. Surg. 2009, 41, 176–188. [Google Scholar]
- Italiano, A.; Delva, F.; Mathoulin-Pelissier, S.; Le Cesne, A.; Bonvalot, S.; Terrier, P.; Trassard, M.; Michels, J.J.; Blay, J.Y.; Coindre, J.M.; et al. Effect of adjuvant chemotherapy on survival in fnclcc grade 3 soft tissue sarcomas: A multivariate analysis of the french sarcoma group database. Ann. Oncol. 2010, 21, 2436–2441. [Google Scholar] [CrossRef]
- Schnorr, J.; Mischke, W.; Tunn, P.U.; Dresel, S. Kontinuierliche quantitative leckkontrolle mittels radioaktiv markierter blutbestandteile bei isolierter extremitätenperfusion. Nuklearmediziner 2009, 32, 80–83. [Google Scholar] [CrossRef]
- Stam, T.C.; Swaak, A.J.; de Vries, M.R.; ten Hagen, T.L.; Eggermont, A.M. Systemic toxicity and cytokine/acute phase protein levels in patients after isolated limb perfusion with tumor necrosis factor-alpha complicated by high leakage. Ann. Surg. Oncol. 2000, 7, 268–275. [Google Scholar] [CrossRef]
- GmbH, T.P. Fachinformation melphalan, zusammenfassung der merkmale des arzneimittels. Available online: https://tillomed.de/fachinformationen/Fachinfo_Melphalan.pdf (accessed on 5 July 2023).
- Maura, F.; Weinhold, N.; Diamond, B.; Kazandjian, D.; Rasche, L.; Morgan, G.; Landgren, O. The mutagenic impact of melphalan in multiple myeloma. Leukemia 2021, 35, 2145–2150. [Google Scholar] [CrossRef]
- PharmaWiki. Available online: https://www.pharmawiki.ch/wiki/index.php?wiki=melphalan (accessed on 13 March 2023).
- Biopharma, A. Evomela Prescribing Information. Acrotech Biopharma, llc. Available online: https://evomela.com/wp-content/uploads/2022/04/Evomela-Prescribing-Information.pdf (accessed on 13 March 2023).
- Ruschulte, H.; Shi, S.; Tseng, W.W.; Kolodzie, K.; Crawford, P.C.; Schneider, D.B.; Kashani-Sabet, M.; Minor, D.; Apfel, C.; Leong, S.P. Anesthesia management of patients undergoing hyperthermic isolated limb perfusion with melphalan for melanoma treatment: An analysis of 17 cases. BMC Anesthesiol. 2013, 13, 15. [Google Scholar] [CrossRef]
- Goldberg, M.E.; Rosenblum, H.M.; Seltzer, J.L.; Rosato, F.E. Isolated regional perfusion; anaesthetic technique, monitoring and blood replacement. Can. Anaesth. Soc. J. 1984, 31, 552–558. [Google Scholar] [CrossRef]
- Namendys-Silva, S.A.; Ruiz-Beltran, A.M.; Barragan-Dessavre, M.; Bautista-Ocampo, A.R.; Meneses-Garcia, A.; Gonzalez-Chon, O.; Herrera-Gomez, A. Clinical characteristics of critically ill cancer patients who are undergoing isolated limb perfusion. Mol. Clin. Oncol. 2017, 7, 747–750. [Google Scholar] [CrossRef][Green Version]
- Katsarelias, D.; Radbo, E.; Ben-Shabat, I.; Mattsson, J.; Olofsson Bagge, R. The effect of temperature and perfusion time on response, toxicity, and survival in patients with in-transit melanoma metastases treated with isolated limb perfusion. Ann. Surg. Oncol. 2018, 25, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Katsarelias, D.; Bath, J.; Carlson, P.; Mattsson, J.; Olofsson Bagge, R. Leakage through the bone-marrow during isolated limb perfusion in the lower extremity. J. Surg. Case Rep. 2019, 2019, rjz090. [Google Scholar] [CrossRef] [PubMed]
- Grunhagen, D.J.; de Wilt, J.H.; van Geel, A.N.; Graveland, W.J.; Verhoef, C.; Eggermont, A.M. Tnf dose reduction in isolated limb perfusion. Eur. J. Surg. Oncol. 2005, 31, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Deroose, J.P.; Grunhagen, D.J.; de Wilt, J.H.; Eggermont, A.M.; Verhoef, C. Treatment modifications in tumour necrosis factor-alpha (tnf)-based isolated limb perfusion in patients with advanced extremity soft tissue sarcomas. Eur. J. Cancer 2015, 51, 367–373. [Google Scholar] [PubMed]
- Hohenberger, P.; Latz, E.; Kettelhack, C.; Rezaei, A.H.; Schumann, R.; Schlag, P.M. Pentoxifyllin attenuates the systemic inflammatory response induced during isolated limb perfusion with recombinant human tumor necrosis factor-alpha and melphalan. Ann. Surg. Oncol. 2003, 10, 562–568. [Google Scholar]
- Goubran, H.; Sheridan, D.; Radosevic, J.; Burnouf, T.; Seghatchian, J. Transfusion-related immunomodulation and cancer. Transfus. Apher. Sci. 2017, 56, 336–340. [Google Scholar] [PubMed]
- Danilo, J.; Philip, P. Regional Nerve Blocks in Anesthesia and Pain Therapy: Traditional and Ultrasound-Guided Techniques, 4th ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Bendtsen, T.F.; Lopez, A.M.; Vandepitte, C. Ultrasound-Guided Supraclavicular Brachial Plexus Nerve Block. Available online: https://www.nysora.com/topics/regional-anesthesia-for-specific-surgical-procedures/upper-extremity-regional-anesthesia-for-specific-surgical-procedures/anesthesia-and-analgesia-for-elbow-and-forearm-procedures/ultrasound-guided-supraclavicular-brachial-plexus-block/ (accessed on 3 October 2023).
- Armbruster, W.; Eichholz, R.; Notheisen, T. Ultraschall in der Anästhesiologie—Grundlagen, Nadelnavigation, Gefässpunktionen, Nervenblockaden, Atemnotdiagnostik; AEN-Sono: Berlin, Germany, 2016; Volume 2, p. 320. [Google Scholar]
- Steinfeldt, T.; Volk, T.; Kessler, P.; Vicent, O.; Wulf, H.; Gottschalk, A.; Lange, M.; Schwartzkopf, P.; Hüttemann, E.; Tessmann, R.; et al. Peripheral nerve blocks on the upper extremity. Anaesthesist 2015, 64, 846–854. [Google Scholar] [CrossRef]
- Vrouenraets, B.C.; Kroon, B.B.; Ogilvie, A.C.; van Geel, A.N.; Nieweg, O.E.; Swaak, A.J.; Eggermont, A.M. Absence of severe systemic toxicity after leakage-controlled isolated limb perfusion with tumor necrosis factor-alpha and melphalan. Ann. Surg. Oncol. 1999, 6, 405–412. [Google Scholar] [CrossRef]
- Christoforidis, D.; Chassot, P.G.; Mosimann, F.; Lienard, D.; Brunstein, F.; Bejko, D.; Lejeune, F.J.; Chiolero, R. Isolated limb perfusion: Distinct tourniquet and tumor necrosis factor effects on the early hemodynamic response. Arch. Surg. 2003, 138, 17–25. [Google Scholar] [CrossRef]
- Beasley, G.M.; Kahn, L.; Tyler, D.S. Current clinical and research approaches to optimizing regional chemotherapy: Novel strategies generated through a better understanding of drug pharmacokinetics, drug resistance, and the development of clinically relevant animal models. Surg. Oncol. Clin. N. Am. 2008, 17, 731–758. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.F.; Kam, P.C.A.; Ramzan, I.; Yau, D.F. Clinical pharmacokinetics of melphalan in isolated limb perfusion: Compartmental modelling and moment analysis. Reg. Cancer Treat. 1995, 8, 83–87. [Google Scholar]
- Nieweg, O.E.; Kroon, B.B. Isolated limb perfusion with melphalan for melanoma. J. Surg. Oncol. 2014, 109, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Gianakos, A.L.; Saad, B.N.; Haring, R.; Menken, L.G.; Elkattaway, S.; Liporace, F.A.; Yoon, R.S. Tranexamic acid lowers transfusion requirements and hospital length of stay following revision total hip or knee arthroplasty. Patient Saf. Surg. 2021, 15, 21. [Google Scholar] [CrossRef]
- Ye, S.; Chen, M.; Luo, Y.; Zhao, C.; Li, Q.; Kang, P. Comparative study of carbazochrome sodium sulfonate and tranexamic acid in reducing blood loss and inflammatory response following direct anterior total hip arthroplasty: A prospective randomized controlled trial. Int. Orthop. 2023, 47, 2553–2561. [Google Scholar] [CrossRef]
- Lapidus, O.; Baekkevold, M.; Rotzius, P.; Lapidus, L.J.; Eriksson, K. Preoperative administration of local infiltration anaesthesia decreases perioperative blood loss during total knee arthroplasty—A randomised controlled trial. J. Exp. Orthop. 2022, 9, 118. [Google Scholar] [CrossRef]
- Hamawandi, S.A.; Amin, H.I.; Al-Humairi, A.K. Effects of the use of tourniquet in total knee arthroplasty on the clinical and functional outcomes with 5 years of follow-up: A randomized controlled trial. J. Knee Surg. 2023, 36, 222–230. [Google Scholar] [CrossRef]
- D’Souza, R.; Duncan, C.; Whiting, D.; Brown, M.; Warner, M.; Smith, H.; Kremers, H.; Stewart, T. Tranexamic acid is associated with decreased transfusion, hospital length of stay, and hospital cost in simultaneous bilateral total knee arthroplasty. Bosn. J. Basic Med. Sci. 2021, 21, 471–476. [Google Scholar] [CrossRef]
- Baldawi, M.; Awad, M.E.; McKelvey, G.; Pearl, A.D.; Mostafa, G.; Saleh, K.J. Neuraxial anesthesia significantly reduces 30-day venous thromboembolism rate and length of hospital stay in primary total hip arthroplasty: A stratified propensity score-matched cohort analysis. J. Arthroplasty 2023, 38, 108–116. [Google Scholar] [CrossRef]
- Corderfeldt, A.; Nielsen, S.; Katsarelias, D.; Hjarpe, A.; Mattsson, J.; Olofsson Bagge, R. Is blood a necessary component of the perfusate during isolated limb perfusion—A randomized controlled trial. Int. J. Hyperthermia 2019, 36, 794–800. [Google Scholar] [CrossRef]
- Gao, F.Q.; Li, Z.J.; Zhang, K.; Sun, W.; Zhang, H. Four methods for calculating blood-loss after total knee arthroplasty. Chin. Med. J. 2015, 128, 2856–2860. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kapadia, B.H.; Issa, K.; McElroy, M.J.; Khanuja, H.S.; Harwin, S.F.; Mont, M.A. Postoperative blood loss prevention in total knee arthroplasty. J. Knee Surg. 2013, 26, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Stimson, L.N.; Steelman, K.R.; Hamilton, D.A.; Chen, C.; Darwiche, H.F.; Mehaidli, A. Evaluation of blood loss in conventional vs makoplasty total knee arthroplasty. Arthroplast Today 2022, 16, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.M.; Wang, W.; Lin, J.; Weng, X.S.; Qian, W.W.; Wang, W.D. Multimodal peri-articular injection with tranexamic acid can reduce postoperative blood loss versus intravenous tranexamic acid in total knee arthroplasty: A randomized controlled trial. J. Orthop. Surg. Res. 2021, 16, 546. [Google Scholar] [CrossRef]
- Gross, J.B. Estimating allowable blood loss: Corrected for dilution. Anesthesiology 1983, 58, 277–280. [Google Scholar] [CrossRef]
- Nadler, S.B.; Hidalgo, J.H.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar]
| Control Group | Intervention Group | |||
|---|---|---|---|---|
| Variable | Upper Extremity | Lower Extremity | Upper Extremity | Lower Extremity |
| N | 8 | 9 | 8 | 9 |
| Sex (m:f) | 7:1 | 5:4 | 7:1 | 5:4 |
| Age | 60.5 yrs (17–83) | 51 yrs (23–81) | 51.5 yrs (7–82) | 77 yrs (49–83) |
| Surgical approach | brachial n = 7 axillary n = 1 | iliacal n = 4 adductor n = 1 femoral n = 4 | brachial n = 5 femoral (child) n = 3 | iliacal n = 3 femoral n = 6 |
| Extremity volume | 3.22 L (1.89–4.96) | 10.95 L (7.85–14.37) | 3.69 L (2.4–7.06) | 10.3 L (7.83–17.64) |
| Dosage TNF-a | 1 mg | 2 mg | 1 mg | 2 mg |
| Melphalan dosage | 30 mg (20–50) | 100 mg (80–120) | 35 mg (25–60) | 80 mg (60–120) |
| Duration of surgical procedure | 238 min (201–286) | 237 min (211–260) | 227 min (132–247) | 228 min (190–311) |
| Circulation of TNF-a + Melphalan | 90 min (88–97) | 92 min (86–94) | 94 min (69–95) | 93 min (73–97) |
| Leak rate | 1.5% (1–5) | 1% (0.5–8) | 1% (1–5) | 4% (1–10) |
| Total blood loss (BLt) | 1350 mL (600–2200) | 1500 mL (900–2500) | 775 mL (550–2000) | 1200 mL (500–2500) |
| Blood loss after melphalan (BLm) | - | - | 0 mL (n = 3) 150 mL (70–250) (n = 5) | 0 mL (n = 6) 300 mL (100–400) (n = 3) |
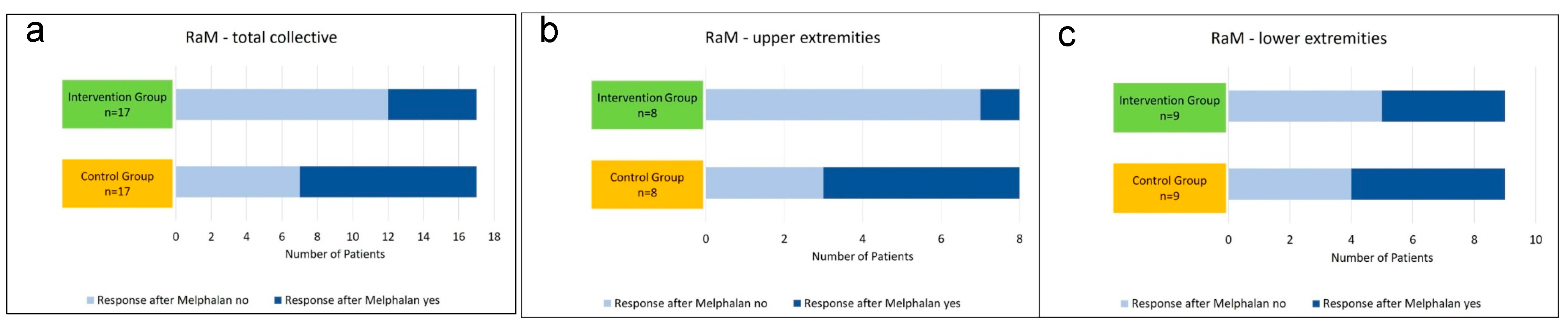
| Response after melphalan (RaM) | Yes = 5 No = 3 | Yes = 5 No = 4 | Yes = 1 No = 7 | Yes = 4 No = 5 |
| Transfusion of RBCs | 1 RBC (n = 0) 2 RBC (n = 1) | 1 RBC (n = 0) 2 RBC (n = 2) 4 RBC (n = 1) | 1 RBC (n = 4) 2 RBC (n = 1) | 1 RBC (n = 2) 2 RBC (n = 1) |
| Intraoperative volume substitution | 5500 mL (3000–7000) | 5000 mL (4000–9000) | 4500 mL (3000–7000) | 5000 mL (3000–8000) |
| Variable | Study Group | N | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|---|
| (a) total collective | |||||||
| BLt [mL] | control | 17 | 600 | 1000 | 1400 | 2000 | 2500 |
| intervention | 17 | 500 | 750 | 1200 | 1400 | 2500 | |
| (b) subgroup analysis: upper extremity | |||||||
| BLt [mL] | control | 8 | 600 | 1050 | 1350 | 1800 | 2200 |
| intervention | 8 | 550 | 700 | 775 | 1300 | 2000 | |
| (c) subgroup analysis: lower extremity | |||||||
| BLt [mL] | control | 9 | 900 | 1000 | 1500 | 2400 | 2500 |
| intervention | 9 | 500 | 1000 | 1200 | 1500 | 2500 | |
| (a) Total Collective | (b) Subgroup Upper Extremity | (c) Subgroup Lower Extremity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control n = 17 | Intervention n = 17 | Control n = 8 | Intervention n = 8 | Control n = 9 | Intervention n = 9 | ||||||||
| Variable | Value | n | % * | n | % * | n | % * | n | % * | n | % * | n | % * |
| Response after | no | 7 | 41.2 | 12 | 70.6 | 3 | 37.5 | 7 | 87.5 | 4 | 44.4 | 5 | 55.6 |
| melphalan | yes | 10 | 58.8 | 5 | 29.4 | 5 | 62.5 | 1 | 12.5 | 5 | 55.6 | 4 | 44.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niethard, M.; Fischer, H.; Gaßmann, B.; Haralambiev, L.; Tipp, A.; Tunn, P.-U. The Use of Regional Anesthesia to Reduce Blood Loss in Isolated Limb Perfusion (ILP)—A Novel Approach. J. Clin. Med. 2023, 12, 6542. https://doi.org/10.3390/jcm12206542
Niethard M, Fischer H, Gaßmann B, Haralambiev L, Tipp A, Tunn P-U. The Use of Regional Anesthesia to Reduce Blood Loss in Isolated Limb Perfusion (ILP)—A Novel Approach. Journal of Clinical Medicine. 2023; 12(20):6542. https://doi.org/10.3390/jcm12206542
Chicago/Turabian StyleNiethard, Maya, Heilwig Fischer, Bernhard Gaßmann, Lyubomir Haralambiev, Alexander Tipp, and Per-Ulf Tunn. 2023. "The Use of Regional Anesthesia to Reduce Blood Loss in Isolated Limb Perfusion (ILP)—A Novel Approach" Journal of Clinical Medicine 12, no. 20: 6542. https://doi.org/10.3390/jcm12206542
APA StyleNiethard, M., Fischer, H., Gaßmann, B., Haralambiev, L., Tipp, A., & Tunn, P.-U. (2023). The Use of Regional Anesthesia to Reduce Blood Loss in Isolated Limb Perfusion (ILP)—A Novel Approach. Journal of Clinical Medicine, 12(20), 6542. https://doi.org/10.3390/jcm12206542






