Efficacy of Granulocyte Colony-Stimulating Factor in Acute on Chronic Liver Failure: A Systematic Review and Survival Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Data Collection and Extraction
2.3. Quality Assessment
2.4. Outcome Measurements
2.5. Statistical Analysis
3. Results
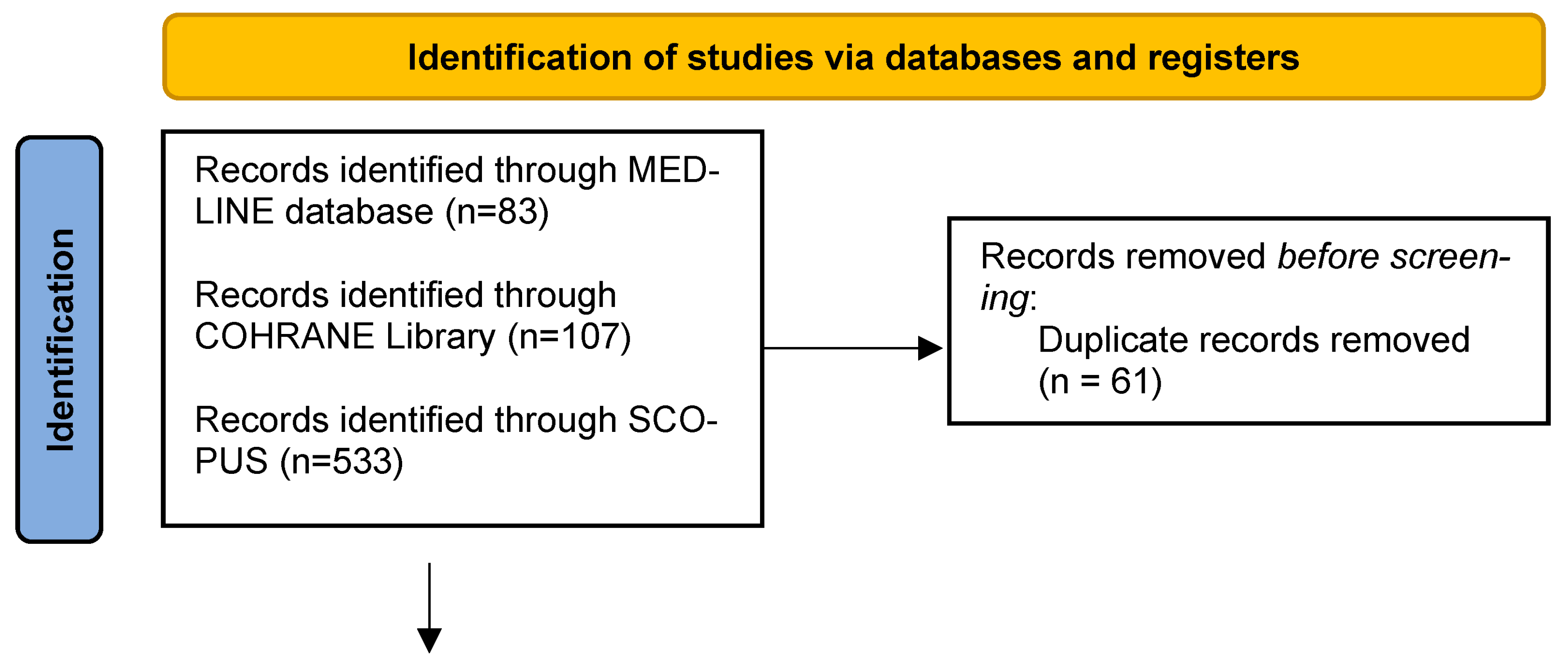
3.1. Search Results
3.2. Baseline Characteristics
3.3. Risk of Bias in the Included Studies
3.4. Analysis of Primary Outcomes
3.4.1. Overall Survival
3.4.2. Sensitivity Analyses
3.5. Analysis of Secondary Outcomes
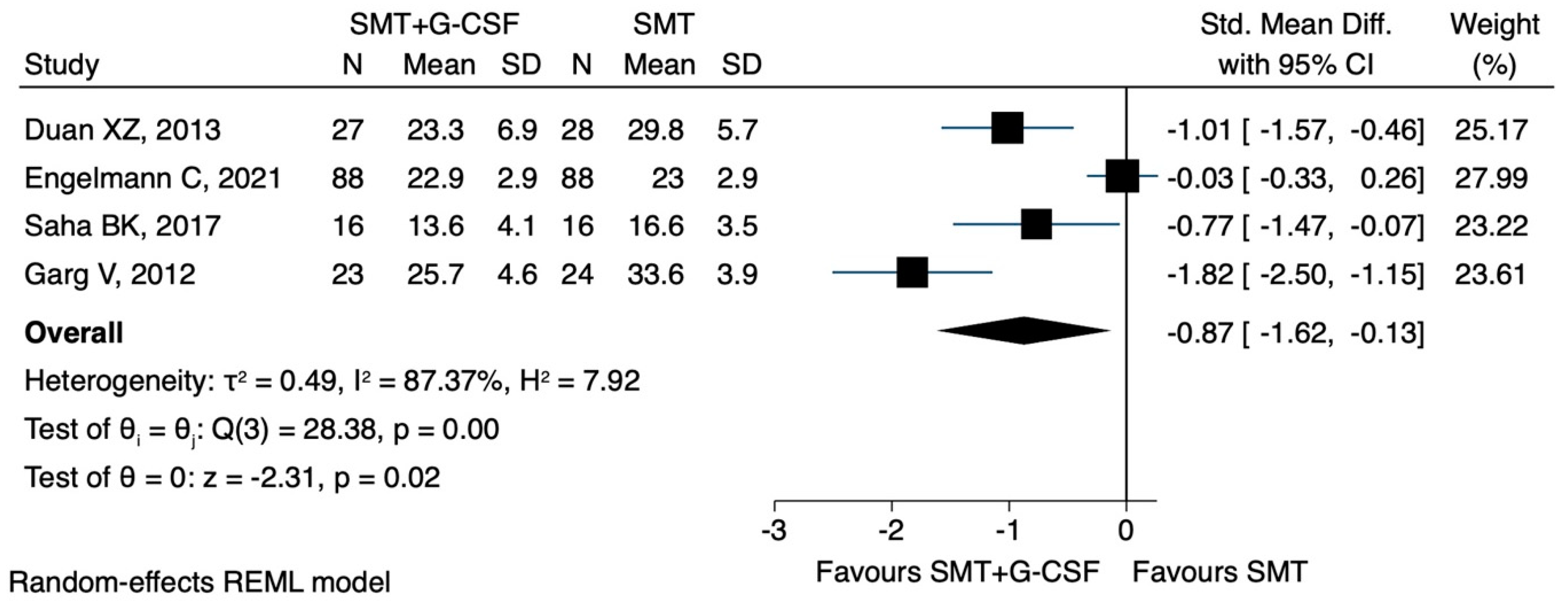
3.5.1. Change in Liver Disease Severity Scores
MELD Score
Sensitivity Analyses
Post hoc Analysis
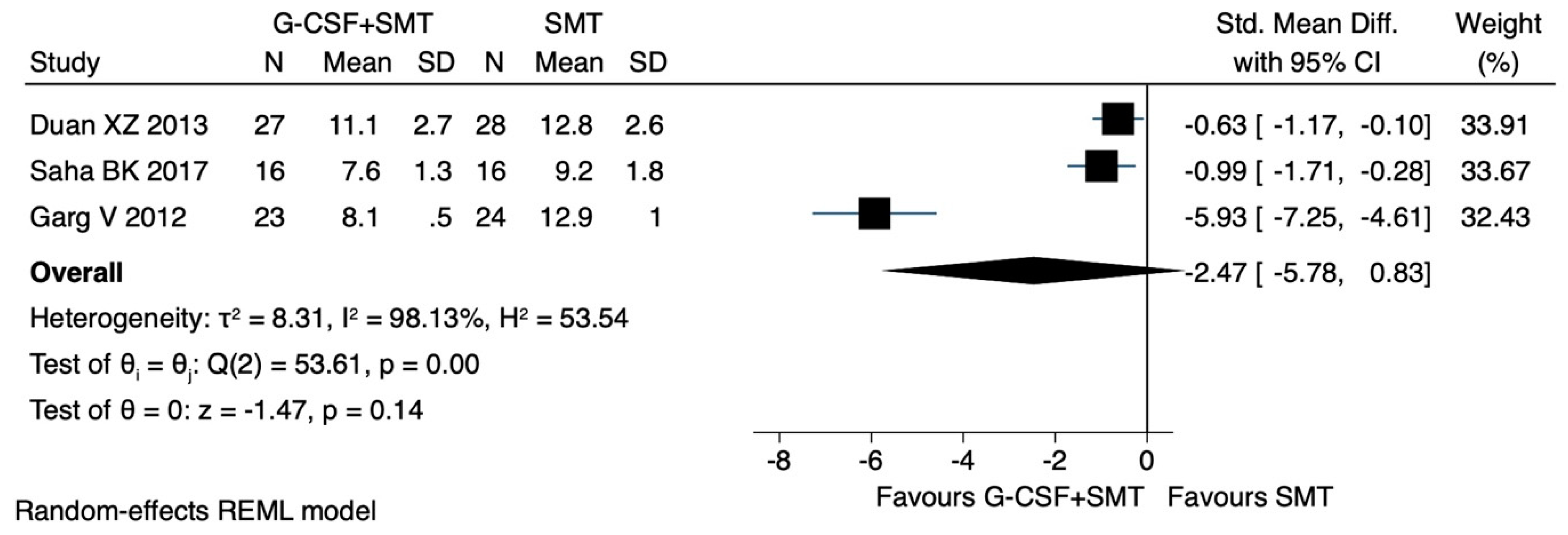
Child–Pugh Score
Sensitivity Analyses
Post hoc
3.6. Mortality
3.7. Sensitivity Analyses
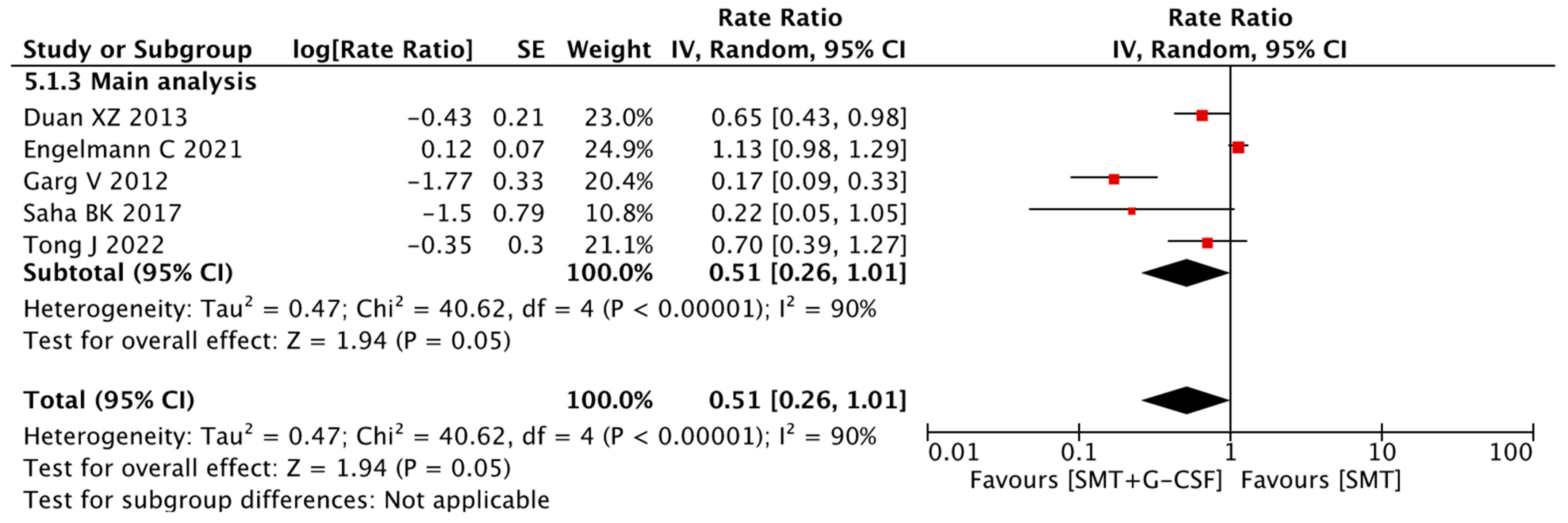
3.8. Complications of Cirrhosis
3.9. Sensitivity Analysis
3.10. G-CSF-Related Adverse Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACLF | Acute on chronic liver failure |
| APASL | Asian Pacific Association for the Study of the Liver |
| BMSC | Bone-marrow-derived stem cells |
| CARS | Compensated anti-inflammatory response |
| CTP | Child–Pugh score |
| DAMPs | Damage-associated molecular patterns |
| EASL | European Association for the Study of the Liver |
| We confEPO | Erythropoietin |
| G-CSF | Granulocyte colony-stimulating factor |
| HR | Hazard ratio |
| HSC | Hematopoietic stem cells |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| MARS | Mixed anti-inflammatory response |
| MELD | Model for End-Stage Liver Disease |
| PAMPs | Pathogen-associated molecular patterns |
| RCT | Randomized controlled trial |
| RR | Rate ratio |
| ROB | Risk of bias |
| SIRS | Systemic inflammatory response syndrome |
| SMD | Standardized mean difference |
| SMT | Standard medical treatment |
| TLR | Toll-like receptor |
| TNF-a | Tumor Necrosis Factor Alpha |
References
- Jalan, R.; Yurdaydin, C.; Bajaj, J.S.; Acharya, S.K.; Arroyo, V.; Lin, H.-C.; Gines, P.; Kim, W.R.; Kamath, P.S.; World Gastroenterology Organization Working Party. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology 2014, 147, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Defining acute-on-chronic liver failure: Will East and West ever meet? Gastroenterology 2013, 144, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, A.; Almeida, J.A.; Chawla, Y.K.; Fan, S.T.; Garg, H.; de Silva, H.J.; Hamid, S.S.; Jalan, R.; Komolmit, P. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol. Int. 2009, 3, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Linderoth, G.; Jepsen, P.; Schønheyder, H.C.; Johnsen, S.P.; Sørensen, H.T. Short-term prognosis of community-acquired bacteremia in patients with liver cirrhosis or alcoholism: A population-based cohort study. Alcohol. Clin. Exp. Res. 2006, 30, 636–641. [Google Scholar] [CrossRef]
- Gustot, T. Multiple organ failure in sepsis: Prognosis and role of systemic inflammatory response. Curr. Opin. Crit. Care 2011, 17, 153–159. [Google Scholar] [CrossRef]
- Sen, S.; Davies, N.A.; Mookerjee, R.P.; Cheshire, L.M.; Hodges, S.J.; Williams, R.; Jalan, R. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: A randomized controlled study. Liver Transplant. 2004, 10, 1109–1119. [Google Scholar] [CrossRef]
- Wasmuth, H.E.; Kunz, D.; Yagmur, E.; Timmer-Stranghöner, A.; Vidacek, D.; Siewert, E.; Bach, J.; Geier, A.; Purucker, E.A.; Gressner, A.M. Patients with acute on chronic liver failure display ‘sepsis-like’immune paralysis. J. Hepatol. 2005, 42, 195–201. [Google Scholar] [CrossRef]
- Maiwal, R.; Kumar, A.; Sarin, S.K. Liver regeneration during acute-on-chronic liver failure using growth factors: In vivo or ex vivo indulgence of bone marrow? Gastroenterology 2013, 145, 901–904. [Google Scholar] [CrossRef]
- Liu, W.-H.; Ren, L.-N.; Wang, T.; Navarro-Alvarez, N.; Tang, L.-J. The involving roles of intrahepatic and extrahepatic stem/progenitor cells (SPCs) to liver regeneration. Int. J. Biol. Sci. 2016, 12, 954. [Google Scholar] [CrossRef]
- Bird, T.; Lorenzini, S.; Forbes, S. Activation of stem cells in hepatic diseases. Cell Tissue Res. 2008, 331, 283–300. [Google Scholar] [CrossRef][Green Version]
- Grompe, M. The role of bone marrow stem cells in liver regeneration. Semin Liver Dis. 2003, 23, 363–372. [Google Scholar] [PubMed]
- Nakamura, T.; Torimura, T.; Iwamoto, H.; Kurogi, J.; Inoue, H.; Hori, Y.; Sumie, S.; Fukushima, N.; Sakata, M.; Koga, H. CD34+ cell therapy is safe and effective in slowing the decline of hepatic reserve function in patients with decompensated liver cirrhosis. J. Gastroenterol. Hepatol. 2014, 29, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Zacharoulis, D.; Milicevic, M.N.; Helmy, S.; Jiao, L.R.; Levicar, N.; Tait, P.; Scott, M.; Marley, S.B.; Jestice, K. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Off. J. Am. Coll. Gastroenterol. ACG 2008, 103, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.M.; Mohamed, S.A.; Fares, K.M.; Abdelemam, R.M.; Elmasry, H.M.; Mansour, S. Immunosuppressive Effect of Intrathecal Morphine, Dexmedetomidine, or Both in Combination with Bupivacaine on Patients Undergoing Major Abdominal Cancer Surgery. Pain Physician 2022, 25, 555–567. [Google Scholar]
- Khanam, A.; Trehanpati, N.; Garg, V.; Kumar, C.; Garg, H.; Sharma, B.C.; Sarin, S.K. Altered frequencies of dendritic cells and IFN-γ-secreting T cells with granulocyte colony-stimulating factor (G-CSF) therapy in acute-on-chronic liver failure. Liver Int. 2014, 34, 505–513. [Google Scholar] [CrossRef]
- Qujeq, D.; Abassi, R.; Faeizi, F.; Parsian, H.; Faraji, A.S.; Taheri, H.; Tatar, M.; Elmi, M.M.; Halalkhor, S. Effect of granulocyte colony-stimulating factor administration on tissue regeneration due to carbon tetrachloride–induced liver damage in experimental model. Toxicol. Ind. Health 2013, 29, 498–503. [Google Scholar] [CrossRef]
- Theocharis, S.E.; Papadimitriou, L.J.; Retsou, Z.P.; Margeli, A.P.; Ninos, S.S.; Papadimitriou, J.D. Granulocyte-colony stimulating factor administration ameliorates liver regeneration in animal model of fulminant hepatic failure and encephalopathy. Dig. Dis. Sci. 2003, 48, 1797–1803. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, W.; Lei, Y.; Han, Q.; Zhang, G.; Lv, Y.; Li, Z.; Lou, S.; Liu, Z. Granulocyte colony-stimulating factor treatment ameliorates liver injury and improves survival in rats with D-galactosamine-induced acute liver failure. Toxicol. Lett. 2011, 204, 92–99. [Google Scholar] [CrossRef]
- Engelmann, C.; Herber, A.; Franke, A.; Bruns, T.; Reuken, P.; Schiefke, I.; Zipprich, A.; Zeuzem, S.; Goeser, T.; Canbay, A. Granulocyte-colony stimulating factor (G-CSF) to treat acute-on-chronic liver failure: A multicenter randomized trial (GRAFT study). J. Hepatol. 2021, 75, 1346–1354. [Google Scholar] [CrossRef]
- Moreau, R.; Tonon, M.; Krag, A.; Angeli, P.; Berenguer, M.; Berzigotti, A.; Fernandez, J.; Francoz, C.; Gustot, T.; Jalan, R.; et al. EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J. Hepatol. 2023, 79, 461–491. [Google Scholar] [CrossRef]
- Huang, W.; Ma, Y.; Du, L.; Kang, S.; Liu, C.-H.; Bai, L.; Lei, X.; Tang, H. Effectiveness of granulocyte colony-stimulating factor for patients with acute-on-chronic liver failure: A meta-analysis. Ann. Saudi Med. 2021, 41, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Colli, A.; Fraquelli, M.; Prati, D.; Casazza, G. Granulocyte colony-stimulating factor with or without stem or progenitor cell or growth factors infusion for people with compensated or decompensated advanced chronic liver disease. Cochrane Database Syst. Rev. 2023, 2023, CD013532. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 144, 1426–1437.e29. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Duan, X.-Z.; Liu, F.-F.; Tong, J.-J.; Yang, H.-Z.; Chen, J.; Liu, X.-Y.; Mao, Y.-L.; Xin, S.-J.; Hu, J.-H. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J. Gastroenterol. WJG 2013, 19, 1104. [Google Scholar] [CrossRef]
- Garg, V.; Garg, H.; Khan, A.; Trehanpati, N.; Kumar, A.; Sharma, B.C.; Sakhuja, P.; Sarin, S.K. Granulocyte colony–stimulating factor mobilizes CD34+ cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 2012, 142, 505–512.e501. [Google Scholar] [CrossRef]
- Saha, B.K.; Mahtab, M.A.; Akbar, S.M.F.; Noor-E-Alam, S.M.; Mamun, A.A.; Hossain, S.M.S.; Alam, M.A.; Moben, A.L.; Khondaker, F.A.; Chowdhury, F.I. Therapeutic implications of granulocyte colony stimulating factor in patients with acute-on-chronic liver failure: Increased survival and containment of liver damage. Hepatol. Int. 2017, 11, 540–546. [Google Scholar] [CrossRef]
- Tong, J.; Wang, H.; Xu, X.; Wan, Z.; Fang, H.; Chen, J.; Mu, X.; Liu, Z.; Su, H.; Liu, X. Granulocyte Colony-Stimulating Factor Accelerates the Recovery of Hepatitis B Virus-Related Acute-on-Chronic Liver Failure by Promoting M2-Like Transition of Monocytes. Front. Immunol. 2022, 13, 885829. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Li, Y.; Yuan, H.; Cai, J.; Liu, R.; Li, J.; Zhu, C. Therapeutic effect and safety of granulocyte colony-stimulating factor therapy for acute-on-chronic liver failure: A systematic review and meta-analysis of randomized controlled trials. Front. Med. 2021, 8, 784240. [Google Scholar] [CrossRef]
- Martin-Mateos, R.; González-Alonso, R.; Álvarez-Díaz, N.; Muriel, A.; Gaetano-Gil, A.; Ortega, J.D.; López-Jerez, A.; Tubio, A.F.; Albillos, A. Granulocyte-Colony Stimulating Factor For Acute-On-Chronic Liver Failure: Systematic Review And Meta-Analysis Of Randomized Controlled Trials. Gastroenterol. Y Hepatol. 2023, 46, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Infante-Rivard, C.; Esnaola, S.; Villeneuve, J.P. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology 1987, 7, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.C.; Kamath, P.S. Acute-on-chronic liver failure: Concept, natural history, and prognosis. Curr. Opin. Crit. Care 2011, 17, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, K.; Rüschenbaum, S.; Barat, S.; Cai, C.; Mücke, M.M.; Fitting, D.; Weigert, A.; Brüne, B.; Zeuzem, S.; Welsch, C. IL-22 and IL-22-binding protein are associated with development of and mortality from acute-on-chronic liver failure. Hepatol. Commun. 2019, 3, 392–405. [Google Scholar] [CrossRef]
- Du, X.X.; Shi, Y.; Yang, Y.; Yu, Y.; Lou, H.G.; Lv, F.F.; Chen, Z.; Yang, Q. DAMP molecular IL-33 augments monocytic inflammatory storm in hepatitis B-precipitated acute-on-chronic liver failure. Liver Int. 2018, 38, 229–238. [Google Scholar] [CrossRef]
- Fernández, J.; Acevedo, J.; Wiest, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martínez, J.; Saliba, F.; Jalan, R. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2018, 67, 1870–1880. [Google Scholar] [CrossRef]
- Moreau, R. The Pathogenesis of ACLF: The Inflammatory Response and Immune Function. Semin Liver Dis. 2016, 36, 133–140. [Google Scholar] [CrossRef]
- Langer, M.M.; Sichelschmidt, S.; Bauschen, A.; Bornemann, L.; Guckenbiehl, S.; Gunzer, M.; Lange, C.M. Pathological neutrophil migration predicts adverse outcomes in hospitalized patients with liver cirrhosis. Liver Int. 2023, 43, 896–905. [Google Scholar] [CrossRef]
- Mücke, M.M.; Rumyantseva, T.; Mücke, V.T.; Schwarzkopf, K.; Joshi, S.; Kempf, V.A.; Welsch, C.; Zeuzem, S.; Lange, C.M. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018, 38, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Mookerjee, R.P.; Jalan, R. Infection and inflammation in liver failure: Two sides of the same coin. J. Hepatol. 2009, 51, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.M.; Moreau, R. Immunodysfunction in acute-on-chronic liver failure. Visc. Med. 2018, 34, 276–282. [Google Scholar] [CrossRef]
- Rueschenbaum, S.; Ciesek, S.; Queck, A.; Widera, M.; Schwarzkopf, K.; Brüne, B.; Welsch, C.; Wedemeyer, H.; Zeuzem, S.; Weigert, A. Dysregulated adaptive immunity is an early event in liver cirrhosis preceding acute-on-chronic liver failure. Front. Immunol. 2021, 11, 3565. [Google Scholar] [CrossRef]
- Gustot, T. Beneficial role of G-CSF in acute-on-chronic liver failure: Effects on liver regeneration, inflammation/immunoparalysis or both? Liver Int. Off. J. Int. Assoc. Study Liver 2014, 34, 484–486. [Google Scholar] [CrossRef]
- Piscaglia, A.C.; Shupe, T.D.; Oh, S.H.; Gasbarrini, A.; Petersen, B.E. Granulocyte–Colony Stimulating Factor Promotes Liver Repair and Induces Oval Cell Migration and Proliferation in Rats. Gastroenterology 2007, 133, 619–631. [Google Scholar] [CrossRef]
- Yannaki, E.; Athanasiou, E.; Xagorari, A.; Constantinou, V.; Batsis, I.; Kaloyannidis, P.; Proya, E.; Anagnostopoulos, A.; Fassas, A. G-CSF–primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp. Hematol. 2005, 33, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Habtesion, A.; Hassan, M.; Kerbert, A.J.; Hammerich, L.; Novelli, S.; Fidaleo, M.; Philips, A.; Davies, N.; Ferreira-Gonzalez, S.; et al. Combination of G-CSF and a TLR4 inhibitor reduce inflammation and promote regeneration in a mouse model of ACLF. J. Hepatol. 2022, 77, 1325–1338. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Chisari, F.V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 23–61. [Google Scholar] [CrossRef]
- Cohen, J.I.; Nagy, L.E. Pathogenesis of alcoholic liver disease: Interactions between parenchymal and non-parenchymal cells. J. Dig. Dis. 2011, 12, 3–9. [Google Scholar] [CrossRef]
- Plumlee, C.R.; Lazaro, C.A.; Fausto, N.; Polyak, S.J. Effect of ethanol on innate antiviral pathways and HCV replication in human liver cells. Virol. J. 2005, 2, 89. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Berg, T. G-CSF treatment in decompensated liver disease: A double-edged sword? Hepatol. Int. 2022, 16, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Bihari, C.; Anand, L.; Rooge, S.; Kumar, D.; Saxena, P.; Shubham, S.; Trehanpati, N.; Kumar, G.; Pamecha, V.; Sharma, S. Bone marrow stem cells and their niche components are adversely affected in advanced cirrhosis of the liver. Hepatology 2016, 64, 1273–1288. [Google Scholar] [CrossRef]
- Geiger, H.; De Haan, G.; Florian, M.C. The ageing haematopoietic stem cell compartment. Nat. Rev. Immunol. 2013, 13, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, M.T.; King, K.Y.; Boles, N.C.; Weksberg, D.C.; Goodell, M.A. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature 2010, 465, 793–797. [Google Scholar] [CrossRef]
- King, K.Y.; Goodell, M.A. Inflammatory modulation of HSCs: Viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol. 2011, 11, 685–692. [Google Scholar] [CrossRef]
- Anand, L.; Bihari, C.; Kedarisetty, C.K.; Rooge, S.B.; Kumar, D.; Shubham, S.; Kumar, G.; Sahney, A.; Sharma, M.K.; Maiwall, R. Early cirrhosis and a preserved bone marrow niche favour regenerative response to growth factors in decompensated cirrhosis. Liver Int. 2019, 39, 115–126. [Google Scholar] [CrossRef]
- Pontes Pereira, T.T.; Fideles Duarte-Andrade, F.; Gardone Vitório, J.; do Espírito Santo Pereira, T.; Braga Martins, F.R.; Marques Souza, J.A.; Malacco, N.L.; Mathias Melo, E.; Costa Picossi, C.R.; Pinto, E. Chronic alcohol administration alters metabolomic profile of murine bone marrow. Front. Immunol. 2023, 14, 1128352. [Google Scholar] [CrossRef]
- Shi, X.; DeLucia, A.L.; Bao, J.; Zhang, P. Alcohol abuse and disorder of granulopoiesis. Pharmacol. Ther. 2019, 198, 206–219. [Google Scholar] [CrossRef]
- Rubin, D.B. Inference and missing data. Biometrika 1976, 63, 581–592. [Google Scholar] [CrossRef]
- Wood, A.M.; White, I.R.; Thompson, S.G. Are missing outcome data adequately handled? A review of published randomized controlled trials in major medical journals. Clin. Trials 2004, 1, 368–376. [Google Scholar] [CrossRef]



| Author (Year), Country | Duration of Study | Number of Patients (Male %) | Age of Patients in SMT + G-CSF Group | Age of Patients in SMT Group | Cause of Cirrhosis | MELD Score in SMT+ G-CSF Group | MELD Score in SMT Group |
|---|---|---|---|---|---|---|---|
| Engelmann C [19] (2021), Germany | 360 days | 111 (63%) | 54.4 ± 10.2 *1 | 57.1 ± 9.6 *1 | Not reported | 24.4 ± 6.3 | 24.5 ± 6.1 |
| Garg V [30] (2012), India | 60 days | 41 (87%) | 40 (30–65) *2 | 40 (19–55) *2 | Alcohol-related cirrhosis: 62% Viral-related cirrhosis: 23% Other causes of cirrhosis: 15% | 29.7 ± 4.9 | 30.7 ± 5.1 |
| Saha BK [31] (2017), India | 90 days | 28 (87.5%) | 39 (18–55) *2 | 48 (22–62) *2 | Viral-related cirrhosis: 91% Autoimmune disease-related cirrhosis: 3% Other causes of cirrhosis: 6% | 25.3 ± 3.3 | 26.4 ± 4.6 |
| Duan XZ [29] (2013), China | 90 days | 44 (80%) | 43.5 (29–63) *2 | 45.9 (22–65) *2 | Viral-related cirrhosis: 100% | 25.11 ± 3.3 | 26.3 ± 4.1 |
| Tong J [32] (2022), China | 180 days | 91 (82%) | 42.5 ± 10.2 *1 | 45.3 ± 10.6 *1 | Viral-related cirrhosis: 100% | 22.8 (20.7–26) *2 | 24.1 (21.6–27.1) *2 |
| Author (Year), Country | G-CSF Doses | Control | Standard Medical Treatment | Administration Scheme | Administration Form | Total Doses |
|---|---|---|---|---|---|---|
| Saha BK [31] (2017) India | 5 μg/kg | Standard medical treatment | Furosemide, spironolactone, lactulose, Rifaximin, antibiotics | Administration for 6 consecutive days | Subcutaneous injection | 6 |
| Engelmann C [19] (2021) Germany | 5 μg/kg | Standard medical treatment | Lactulose, L-ornithine-L-aspartate, albumin, vasopressors, antibiotics, N-acetylcysteine, antiviral therapy * | Once a day for the first 5 days. After that, every third day until completing 12 total doses | Subcutaneous injection | 12 |
| Duan XZ [29] (2013) China | 5 μg/kg | Standard medical treatment | Entecavir, albumin, glutathione, glycyrrhizin, ademetionine, polyene phosphatidylcholine, alprostadil, antiviral therapy as needed | Administration for 6 consecutive days | Subcutaneous injection | 6 |
| Garg V [30] (2012) India | 5 μg/kg | Standard medical treatment | Lactulose, bowel wash, albumin, fresh frozen plasma, terlipressin, antibiotics, mechanical ventilation, vasopressors, renal replacement therapy, tenofovir pentoxifylline as needed | Once a day for the first 5 days. After that, every third day until completing 12 total doses | Subcutaneous injection | 12 |
| Tong J [32] (2022) China | 5 μg/kg | Standard medical treatment | Intensive care monitoring, antiviral therapy, antibiotics albumin, terlipressin as needed | Once a day for the first 6 days. After that, every other day until day 18 | Subcutaneous injection | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantis, G.; Tsaousi, G.; Pourzitaki, C.; Kitsikidou, E.; Magouliotis, D.E.; Wiener, S.; Zeller, A.C.; Willuweit, K.; Schmidt, H.H.; Rashidi-Alavijeh, J. Efficacy of Granulocyte Colony-Stimulating Factor in Acute on Chronic Liver Failure: A Systematic Review and Survival Meta-Analysis. J. Clin. Med. 2023, 12, 6541. https://doi.org/10.3390/jcm12206541
Konstantis G, Tsaousi G, Pourzitaki C, Kitsikidou E, Magouliotis DE, Wiener S, Zeller AC, Willuweit K, Schmidt HH, Rashidi-Alavijeh J. Efficacy of Granulocyte Colony-Stimulating Factor in Acute on Chronic Liver Failure: A Systematic Review and Survival Meta-Analysis. Journal of Clinical Medicine. 2023; 12(20):6541. https://doi.org/10.3390/jcm12206541
Chicago/Turabian StyleKonstantis, Georgios, Georgia Tsaousi, Chryssa Pourzitaki, Elisavet Kitsikidou, Dimitrios E. Magouliotis, Sebastian Wiener, Amos Cornelius Zeller, Katharina Willuweit, Hartmut H. Schmidt, and Jassin Rashidi-Alavijeh. 2023. "Efficacy of Granulocyte Colony-Stimulating Factor in Acute on Chronic Liver Failure: A Systematic Review and Survival Meta-Analysis" Journal of Clinical Medicine 12, no. 20: 6541. https://doi.org/10.3390/jcm12206541
APA StyleKonstantis, G., Tsaousi, G., Pourzitaki, C., Kitsikidou, E., Magouliotis, D. E., Wiener, S., Zeller, A. C., Willuweit, K., Schmidt, H. H., & Rashidi-Alavijeh, J. (2023). Efficacy of Granulocyte Colony-Stimulating Factor in Acute on Chronic Liver Failure: A Systematic Review and Survival Meta-Analysis. Journal of Clinical Medicine, 12(20), 6541. https://doi.org/10.3390/jcm12206541