Screening for Peripheral Artery Disease Using an Automated Four-Limb Blood Pressure Monitor Equipped with Toe–Brachial Index Measurement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Methods
2.2.1. ABI Measurement
Doppler-Assisted ABI Measurement
ABI Measurement with an Automated, Oscillometric Blood Pressure Monitor
2.2.2. TBI Measurement
TBI Measurement Using the MESI mTablet
Toe Pressure Measurement Using LD Flowmetry
Toe Pressure Measurement by the SysToe PPG Device
2.2.3. Vascular Imaging
Colour-Coded Duplex Ultrasound of the Lower Extremity Arteries
Digital Subtraction Angiography
CT Angiography
2.3. Statistical Analysis
3. Results
3.1. ABI Measurement
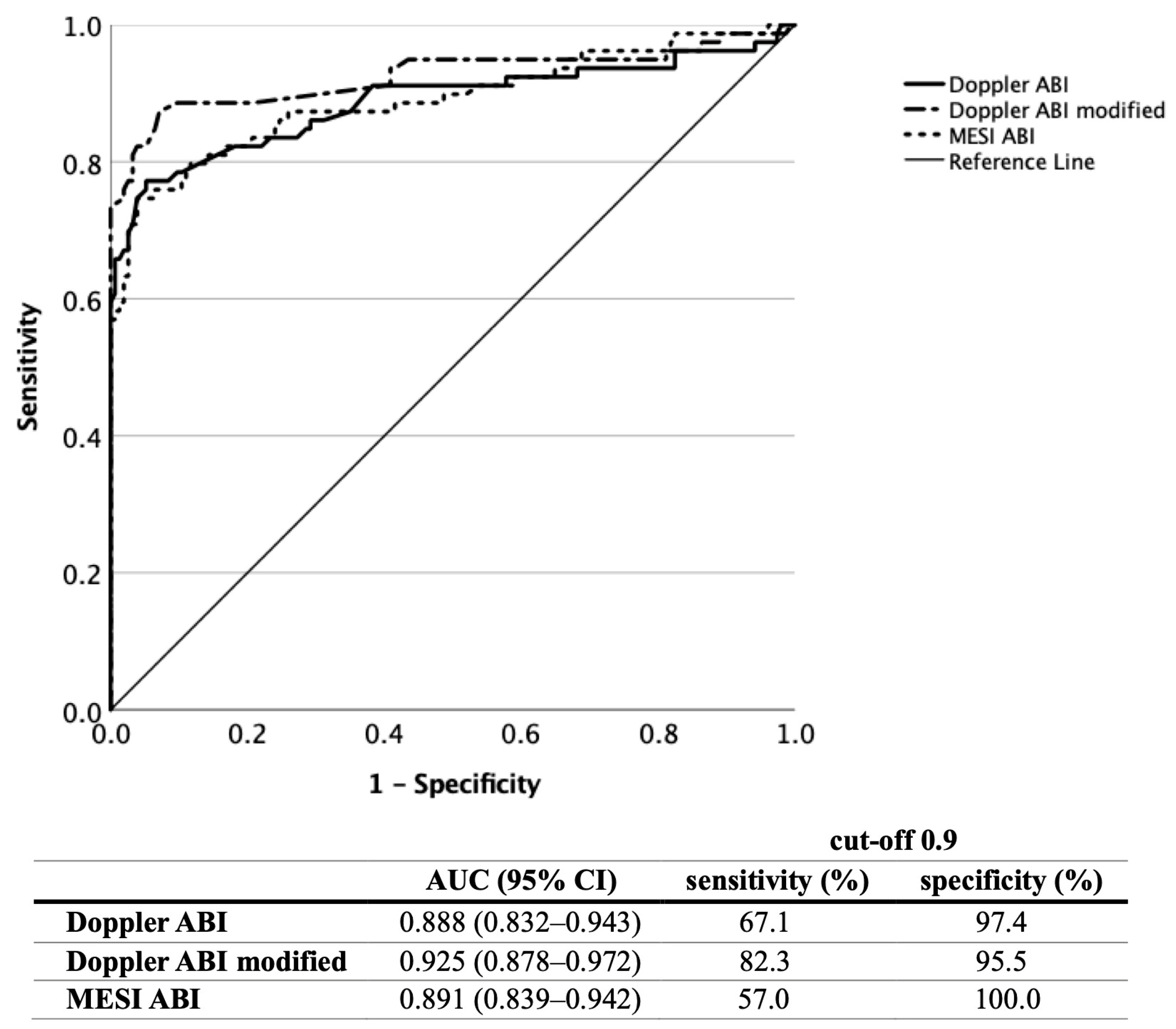
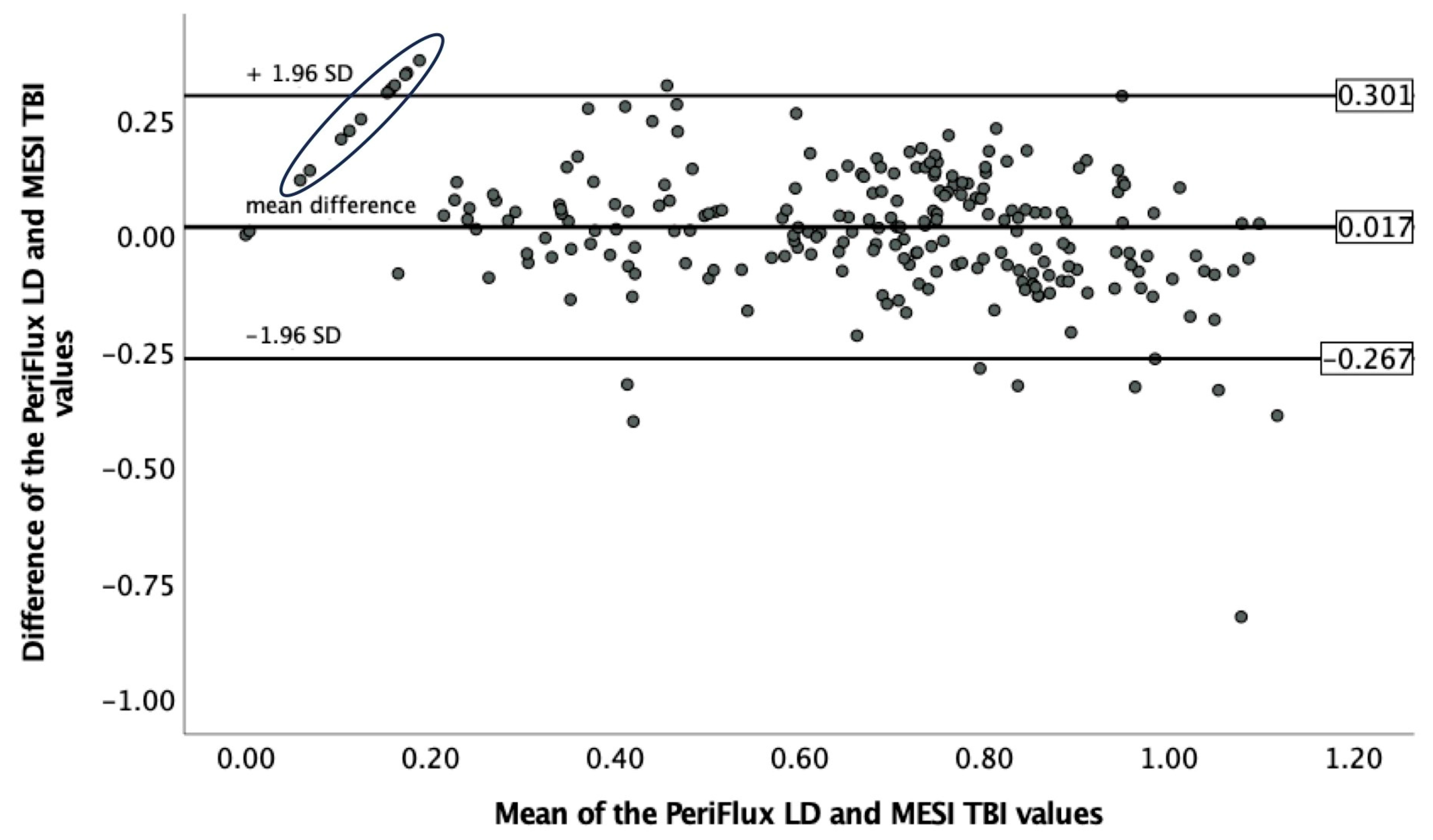
3.2. TBI Measurement
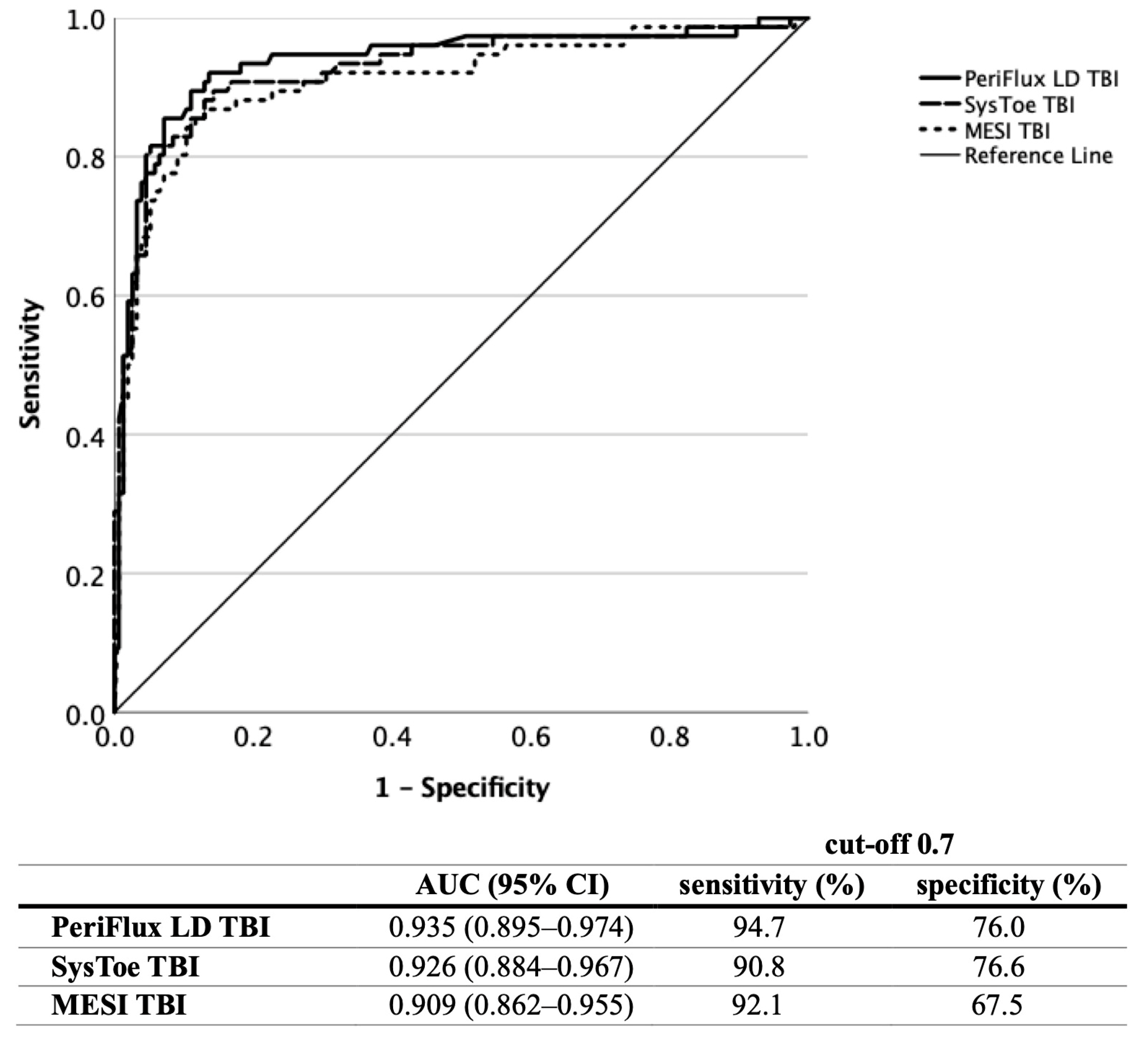
3.3. Screening Using the Various Methods
| Vascular Imaging | ||||||||
|---|---|---|---|---|---|---|---|---|
| Doppler ABI | Doppler ABI Modified | MESI ABI | PeriFlux LD TBI | SysToe TBI | MESI TBI | PAD | Atherosclerosis | |
| Control (n = 18) | 0 | 0 | 0 | 1 | 0 | 3 | 0 (0.0%) | 2 (11.1%) |
| Other CV (n = 12) | 1 | 1 | 1 | 4 | 4 | 4 | 1 (8.3%) | 1 (8.3%) |
| High CV risk (n = 28) | 3 | 3 | 2 | 12 | 13 | 13 | 4 (14.3%) | 19 (67.9%) |
| Very high CV risk (n = 28) | 8 | 11 | 3 | 17 | 16 | 17 | 8 (28.6%) | 26 (92.9%) |
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: The European Stroke Organization (ESO). The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001, 286, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Belch, J.J.F.; Baumgartner, I.; Giovas, P.; Hoffmann, U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis 2020, 293, 94–100. [Google Scholar] [CrossRef]
- Sacks, D.; Bakal, C.W.; Beatty, P.T.; Becker, G.J.; Cardella, J.F.; Raabe, R.D.; Wiener, H.M.; Lewis, C.A. Position statement on the use of the ankle-brachial index in the evaluation of patients with peripheral vascular disease: A consensus statement developed by the standards division of the society of cardiovascular & interventional radiology. J. Vasc. Interv. Radiol. 2002, 13, 353. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Rutherford, R.B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J. Vasc. Surg. 2000, 31 Pt 2, S1–S296. [Google Scholar] [PubMed]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e686–e725. [Google Scholar] [CrossRef]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on peripheral arterial disease. Vasa 2019, 48 (Suppl. S102), 1–79. [Google Scholar] [CrossRef]
- Hageman, D.; Pesser, N.; Gommans, L.N.M.; Willigendael, E.M.; van Sambeek, M.R.H.M.; Huijbers, E.; Snoeijen, A.; Scheltinga, M.R.M.; Teijink, J.A.W. Limited Adherence to Peripheral Arterial Disease Guidelines and Suboptimal Ankle Brachial Index Reliability in Dutch Primary Care. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 867–873. [Google Scholar] [CrossRef]
- Yap Kannan, R.; Dattani, N.; Sayers, R.D.; Bown, M.N. Survey of Ankle-Brachial Pressure Index Use and Its Perceived Barriers by General Practitioners in the UK. Postgrad. Med. J. 2016, 92, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Haigh, K.J.; Bingley, J.; Golledge, J.; Walker, P.J. Barriers to screening and diagnosis of peripheral artery disease by general practitioners. Vasc. Med. 2013, 18, 325–330. [Google Scholar] [CrossRef]
- Herráiz-Adillo, Á.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Martínez-Vizcaíno, V.; Pozuelo-Carrascosa, D.P.; Notario-Pacheco, B. The accuracy of an oscillometric ankle-brachial index in the diagnosis of lower limb peripheral arterial disease: A systematic review and meta-analysis. Int. J. Clin. Pract. 2017, 71, e12994. [Google Scholar] [CrossRef] [PubMed]
- Danieluk, A.; Chlabicz, S. Automated Measurements of Ankle-Brachial Index: A Narrative Review. J. Clin. Med. 2021, 10, 5161. [Google Scholar] [CrossRef]
- Mehlsen, J.; Wiinberg, N.; Bruce, C. Oscillometric blood pressure measurement: A simple method in screening for peripheral arterial disease. Clin. Physiol. Funct. Imaging 2008, 28, 426–429. [Google Scholar] [CrossRef]
- MacDougall, A.M.; Tandon, V.; Wilson, M.P.; Wilson, T.W. Oscillometric measurement of ankle-brachial index. Can. J. Cardiol. 2008, 24, 49–51. [Google Scholar] [CrossRef]
- MacDonald, E.; Froggatt, P.; Lawrence, G.; Blair, S. Are automated blood pressure monitors accurate enough to calculate the ankle brachial pressure index? J. Clin. Monit. Comput. 2008, 22, 381–384. [Google Scholar] [CrossRef]
- Benchimol, A.; Bernard, V.; Pillois, X.; Hong, N.T.; Benchimol, D.; Bonnet, J. Validation of a new method of detecting peripheral artery disease by determination of ankle-brachial index using an automatic blood pressure device. Angiology 2004, 55, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Verberk, W.J.; Kollias, A.; Stergiou, G.S. Automated oscillometric determination of the ankle-brachial index: A systematic review and meta-analysis. Hypertens. Res. 2012, 35, 883–891. [Google Scholar] [CrossRef]
- Stein, R.; Hriljac, I.; Halperin, J.L.; Gustavson, S.M.; Teodorescu, V.; Olin, J.W. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc. Med. 2006, 11, 29–33. [Google Scholar] [CrossRef]
- Taguchi, J.T.; Suwangool, P. “Pipe-stem”: Brachial arteries. A cause of pseudohypertension. JAMA 1974, 228, 733. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, M.A.; Buchanan, B.J.; Abraira, C. Elevated leg systolic pressures and arterial calcification in diabetic occlusive vascular disease. Diabetes Care 1981, 4, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Katz, R.; De Boer, I.H.; Kestenbaum, B.R.; Allison, M.A.; Siscovick, D.S.; Newman, A.B.; Sarnak, M.J.; Shlipak, M.G.; Criqui, M.H. Association of chronic kidney disease with the spectrum of ankle brachial index the CHS (Cardiovascular Health Study). J. Am. Coll. Cardiol. 2009, 54, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Adams, J.E.; Anderson, G.F.; Boulton, A.J.; Cavanagh, P.R. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia 1993, 36, 615–621. [Google Scholar] [CrossRef]
- Yao, J.S.; Flinn, W.R.; Bergan, J.J. Noninvasive vascular diagnostic testing: Techniques and clinical applications. Prog. Cardiovasc. Dis. 1984, 26, 459–494. [Google Scholar] [CrossRef] [PubMed]
- de Graaff, J.C.; Ubbink, D.T.; Legemate, D.A.; de Haan, R.J.; Jacobs, M.J. The usefulness of a laser Doppler in the measurement of toe blood pressures. J. Vasc. Surg. 2000, 32, 1172–1179. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. J. Prev. Cardiol. 2022, 29, 5–115. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef]
- MESI mTablet Instructions for Use. Available online: https://web.archive.org/web/20230718101024/https://support.mesimedical.com/app/uploads/2019/11/mTABLET-SYS_IFU_ENG_2-1_2019-06-17_web.pdf (accessed on 18 July 2023).
- MESI mTablet ABI Module, Instructions for Use. Available online: https://web.archive.org/web/20230718103008/https://support.mesimedical.com/app/uploads/2019/11/mTABLET-ABI_IFU_ENG_2019-09-25-web.pdf (accessed on 18 July 2023).
- MESI mTablet TBI Module, Instructions for Use. Available online: https://support.mesimedical.com/app/uploads/2020/11/mTABLET-TBI_IFU_ENG_v1-2_2020-11-27_web.pdf (accessed on 18 July 2023).
- Perimed Laser Doppler Perfusion Monitoring (LDPM). Available online: https://web.archive.org/web/20230718104048/https://www.perimed-instruments.com/content/laser-doppler-perfusion-monitoring-ldpm/ (accessed on 18 July 2023).
- SYSTOE, Toe Systolic Blood Pressure, Finger Pressure, TBI. Available online: https://web.archive.org/web/20230718104451/https://www.atysmedical.com/our-products/toe-finger-systolic-pressure-measurement/ (accessed on 18 July 2023).
- SysToe—Pocket Guide. Available online: https://web.archive.org/web/20230718104642/https://www.medata.cz/_docs/atys/en_atys_systoe_guide.pdf (accessed on 18 July 2023).
- Salonen, R.; Seppänen, K.; Rauramaa, R.; Salonen, J.T. Prevalence of carotid atherosclerosis and serum cholesterol levels in eastern Finland. Arteriosclerosis 1988, 8, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Robinson, M.L.; Marinelli, D.L.; Perlmutter, G.S. Peripheral arterial Doppler ultrasonography: Diagnostic criteria. J. Ultrasound Med. 1992, 11, 95–103. [Google Scholar] [CrossRef]
- Hodgkiss-Harlow, K.D.; Bandyk, D.F. Interpretation of arterial duplex testing of lower- extremity arteries and interventions. Semin. Vasc. Surg. 2013, 26, 95–104. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assesing agreement between two methods of clinical mea urement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Catillon, F.; Tuffier, S.; Guilcher, A.; Tollenaere, Q.; Métairie, A.; Miossec, A.; Mauger, C.; Laneelle, D.; Mahé, G. Proficiency of Medical Students at Obtaining Pressure Measurement Readings Using Automated Ankle and Toe Measuring Devices for Diagnosis of Lower Extremity Peripheral Artery Disease. Ann. Vasc. Surg. 2020, 65, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Špan, M.; Geršak, G.; Millasseau, S.C.; Meža, M.; Košir, A. Detection of peripheral arterial disease with an improved automated device: Comparison of a new oscillometric device and the standard Doppler method. Vasc. Health Risk Manag. 2016, 12, 305–311. [Google Scholar] [CrossRef]
- Zebari, F.; Amlani, V.; Langenskiöld, M.; Nordanstig, J. Validation of an automated measurement method for determination of the ankle-brachial index. Scand Cardiovasc. J. 2022, 56, 73–78. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Pang, W.; Zhao, M.; Luo, Y.; Sun, Y.; Hu, D. Sensitivity and specificity of ankle-brachial index for detecting angiographic stenosis of peripheral arteries. Circ. J. 2008, 72, 605–610. [Google Scholar] [CrossRef]
- Ichihashi, S.; Hashimoto, T.; Iwakoshi, S.; Kichikawa, K. Validation study of automated oscillometric measurement of the ankle-brachial index for lower arterial occlusive disease by comparison with computed tomography angiography. Hypertens. Res. 2014, 37, 591–594. [Google Scholar] [CrossRef]
- Clairotte, C.; Retout, S.; Potier, L.; Roussel, R.; Escoubet, B. Automated ankle-brachial pressure index measurement by clinical staff for peripheral arterial disease diagnosis in nondiabetic and diabetic patients. Diabetes Care 2009, 32, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Criqui, M.H.; Liu, K.; Guralnik, J.M.; Greenland, P.; Martin, G.J.; Pearce, W. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J. Vasc. Surg. 2000, 32, 1164–1171. [Google Scholar] [CrossRef]
- Schröder, F.; Diehm, N.; Kareem, S.; Ames, M.; Pira, A.; Zwettler, U.; Lawall, H.; Diehm, C. A modified calculation of ankle-brachial pressure index is far more sensitive in the detection of peripheral arterial disease. J. Vasc. Surg. 2006, 44, 531–536. [Google Scholar] [CrossRef]
- Espinola-Klein, C.; Rupprecht, H.J.; Bickel, C.; Lackner, K.; Savvidis, S.; Messow, C.M.; Munzel, T.; Blankenberg, S. Different calculations of ankle-brachial index and their impact on cardiovascular risk prediction. Circulation 2008, 118, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Wahlberg, E.; Gush, R. A new automated toe blood pressure monitor for assessment of limb ischemia. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 304–308. [Google Scholar] [CrossRef]
- Ubbink, D.T. Toe blood pressure measurements in patients suspected of leg ischaemia: A new LD device compared with photoplethysmography. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 629–634. [Google Scholar] [CrossRef]
- Pérez-Martin, A.; Meyer, G.; Demattei, C.; Böge, G.; Laroche, J.P.; Quéré, I.; Dauzat, M. Validation of a fully automatic photoplethysmographic device for toe blood pressure measurement. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 515–520. [Google Scholar] [CrossRef]
- Widmer, L.W.; Vikatmaa, P.; Aho, P.; Lepäntalo, M.; Venermo, M. Reliability and repeatability of toe pressures measured with LD and portable and stationary photoplethysmography devices. Ann. Vasc. Surg. 2012, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Patry, J.; Laurencelle, L.; Bélisle, J.; Beaumier, M. Vascular Assessment in Patients with a Lower Limb Wound: A Correlational Study of Photoplethysmography and LD Flowmetry Toe Pressure Techniques. J. Diabetes Sci. Technol. 2022, 16, 470–477. [Google Scholar] [CrossRef]
- Herraiz-Adillo, Á.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; Solera-Martínez, M. The accuracy of toe brachial index and ankle brachial index in the diagnosis of lower limb peripheral arterial disease: A systematic review and meta-analysis. Atherosclerosis 2020, 315, 81–92. [Google Scholar] [CrossRef]
- Williams, D.T.; Harding, K.G.; Price, P. An Evaluation of the Efficacy of Methods Used in Screening for Lower-Limb Arterial Disease in Diabetes. Diabetes Care 2005, 28, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Vriens, B.; D’Abate, F.; Ozdemir, B.A.; Fenner, C.; Maynard, W.; Budge, J.; Carradice, D.; Hinchliffe, R.J. Clinical examination and non-invasive screening tests in the diagnosis of peripheral artery disease in people with diabetes-related foot ulceration. Diabet. Med. 2018, 35, 895–902. [Google Scholar] [CrossRef]
- Babaei, M.R.; Malek, M.; Rostami, F.T.; Emami, Z.; Madani, N.H.; Khamseh, M.E. Non-invasive vascular assessment in people with type 2 diabetes: Diagnostic performance of Plethysmographic-and-Doppler derived ankle brachial index, toe brachial index, and pulse volume wave analysis for detection of peripheral arterial disease. Prim. Care Diabetes 2020, 14, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, M.; Al Ayed, M.Y.; Robert, A.A.; Al Dawish, M.A. Clinical Utility of the Ankle-Brachial Index and Toe Brachial Index in Patients with Diabetic Foot Ulcers. Curr. Diabetes Rev. 2020, 16, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Tehan, P.; Bray, A.; Keech, R.; Rounsley, R.; Carruthers, A.; Chuter, V.H. Sensitivity and Specificity of the Toe-Brachial Index for Detecting Peripheral Arterial Disease: Initial Findings. J. Ultrasound Med. 2015, 34, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
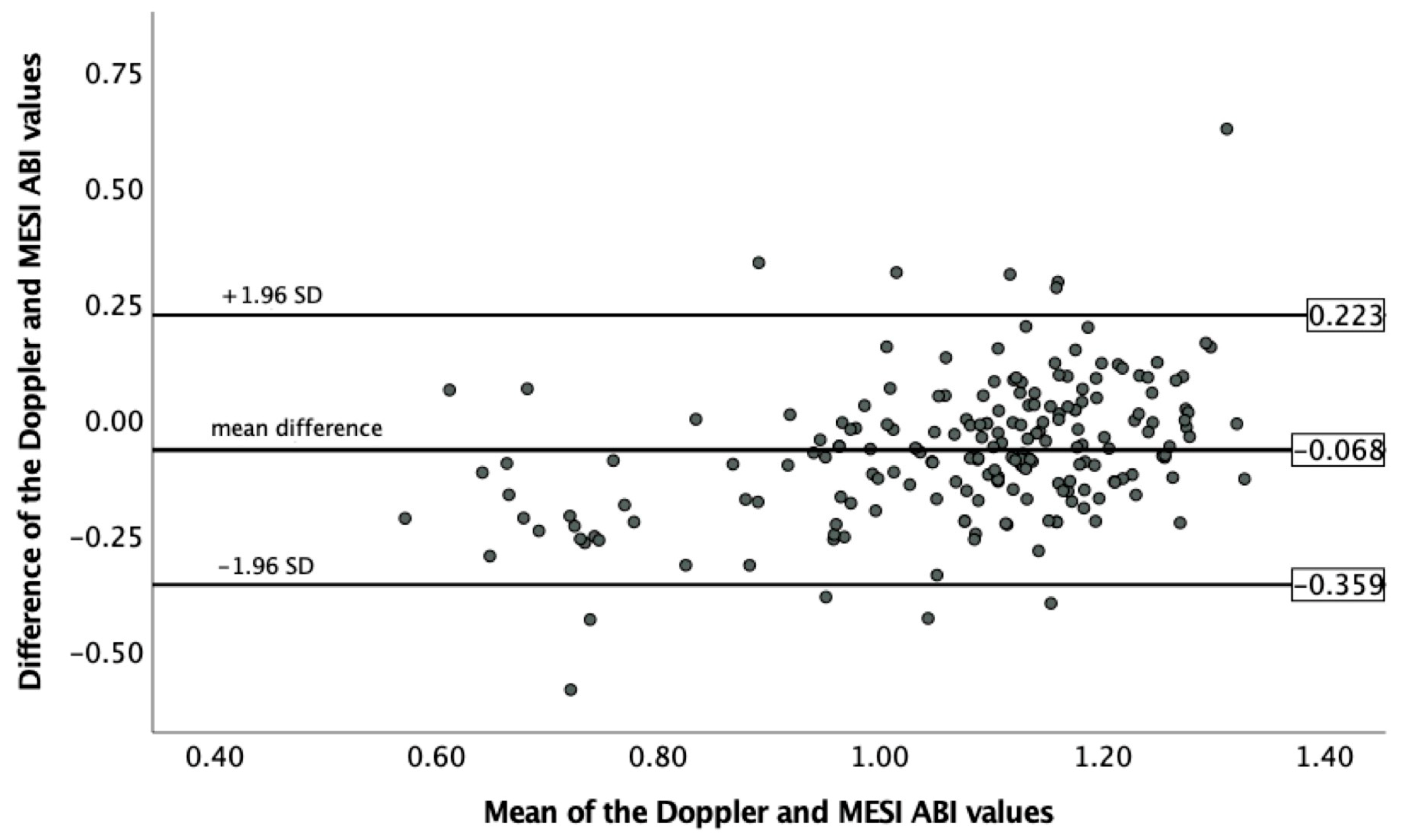
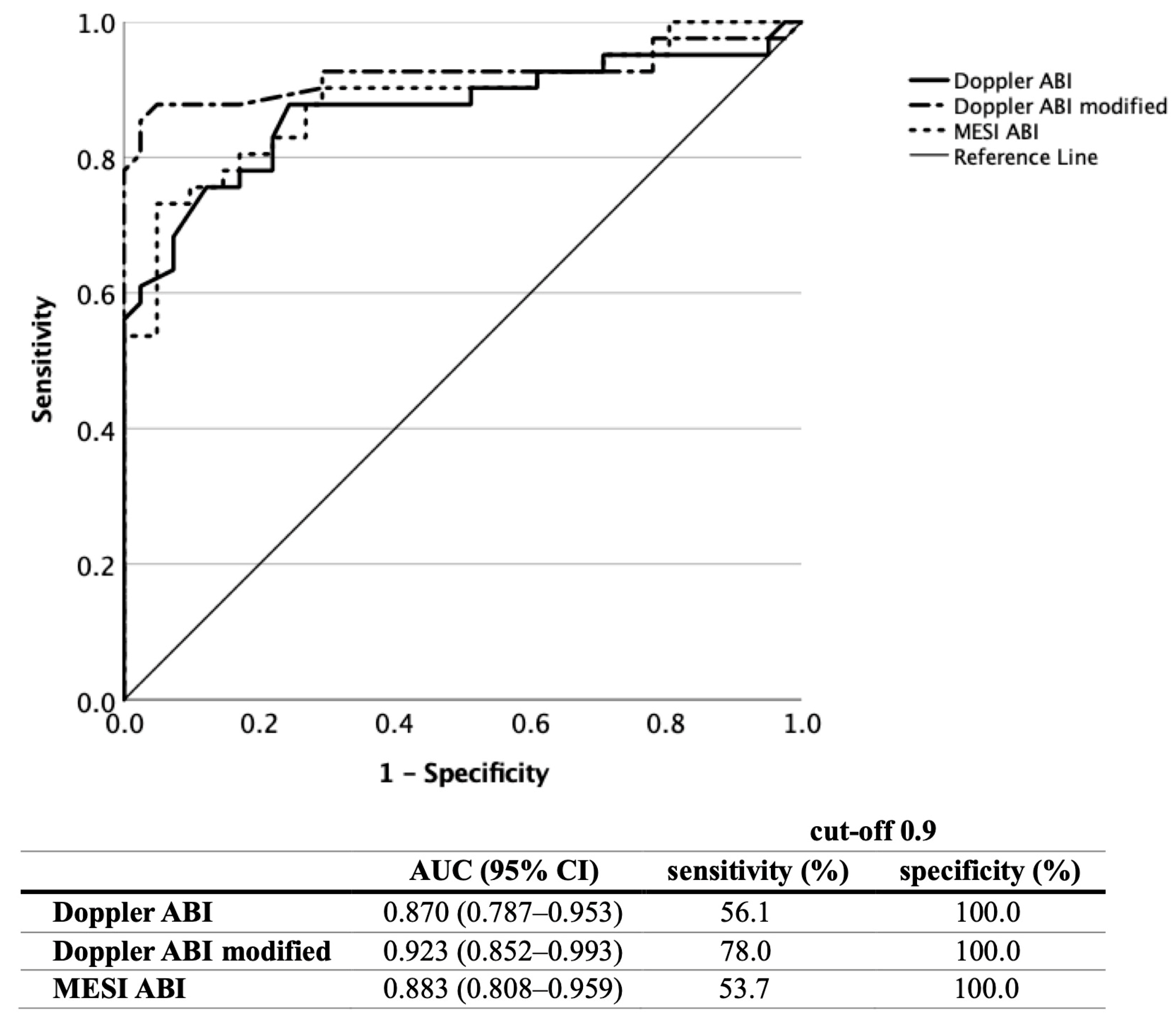

| Control | Other CV | High CV Risk | Very High CV Risk | Confirmed PAD | All Patients | |
|---|---|---|---|---|---|---|
| (n = 18) | (n = 12) | (n = 28) | (n = 28) | (n = 31) | (n = 117) | |
| Age (mean ± SD, years) | 60.4 ± 11.9 | 42.7± 11.9 | 61.6 ± 10.6 | 69.1 ± 10.0 | 68.8 ± 7.9 | 63.2 ± 12.8 |
| Male sex No (%) | 9 (50.0%) | 5 (41.7%) | 7 (25.0%) | 10 (35.7%) | 18 (58.1%) | 49 (41.9%) |
| BMI (mean ± SD, kg/m2) | 24.6 ± 3.6 | 31.5 ± 11.3 | 32.9 ± 7.5 | 30.9 ± 6.9 | 27.7 ± 6.6 | 29.6 ± 7.6 |
| Co-morbidities and risk factors No (%) | ||||||
| Hypertension | 6 (33.3%) | 2 (16.7%) | 27 (96.4%) | 27 (96.4%) | 29 (93.5%) | |
| Diabetes mellitus | 0 (0%) | 0 (0%) | 17 (60.7%) | 8 (28.6%) | 16 (51.6%) | |
| Diabetic polyneuropathy | 0 (0%) | 0 (0%) | 5 (17.9%) | 4 (14.3%) | 5 (16.1%) | |
| Dyslipidaemia | 4 (22.2%) | 0 (0%) | 18 (64.3%) | 25 (89.3%) | 28 (90.3%) | |
| Smoker (current) | 0 (0%) | 3 (25.0%) | 7 (25.0%) | 7 (25.0%) | 11 (35.5%) | |
| Smoker (former) | 4 (22.2%) | 2 (16.7%) | 7 25.0%) | 7 (25.0%) | 16 (51.6%) | |
| Coronary heart disease | 0 (0%) | 0 (0%) | 0 (0%) | 13 (46.4%) | 8 (25.8%) | |
| Carotid artery disease | 0 (0%) | 0 (0%) | 0 (0%) | 8 (28.6%) | 7 (22.6%) | |
| Cerebrovascular events | 0 (0%) | 0 (0%) | 0 (0%) | 6 (21.4%) | 5 (16.1%) | |
| Renal artery disease | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Chronic kidney disease | 0 (0%) | 0 (0%) | 0 (0%) | 2 (7.1%) | 3 (9.7%) | |
| Concomitant medication No (%) | ||||||
| Aspirin | 0 (0%) | 2 (16.7%) | 8 (28.6%) | 7 (25.0%) | 11 (35.5%) | |
| Clopidogrel | 0 (0%) | 0 (0%) | 1 (3.6%) | 6 (21.4%) | 20 (64.5%) | |
| Cilostazol | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3.6%) | 7 (22.6%) | |
| Oral anticoagulants | ||||||
| Vitamin K antagonist | 0 (0%) | 0 (0%) | 0 (0%) | 3 (10.7%) | 0 (0%) | |
| DOAC | 0 (0%) | 4 (19.0%) | 6 (21.4%) | 9 (32.1%) | 6 (19.4%) | |
| ACEI/ARB | 6 (33.3%) | 2 (16.7%) | 24 (85.7%) | 20 (71.4%) | 24 (77.4%) | |
| Beta blocker | 4 (22.2%) | 5 (41.7%) | 12 (42.9%) | 16 (57.1%) | 21 (67.7%) | |
| Calcium channel blocker | 2 (11.1%) | 2 (16.7%) | 14 (50.0%) | 10 (35.7%) | 14 (45.2%) | |
| Statin | 3 (16.7%) | 0 (0%) | 10 ((35.7%) | 20 (71.4%) | 25 (80.1%) | |
| Fibrate | 0 (0%) | 0 (0%) | 2 (7.1%) | 1 (3.6%) | 0 (0%) | |
| Ezetimibe | 0 (0%) | 0 (0%) | 1 (3.6%) | 3 (10.7%) | 3 (9.7%) | |
| Lower limb symptoms No (%) | ||||||
| Leg ulcers | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3.6%) | 5 (16.1%) | |
| Ischaemic rest pain | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (16.1%) | |
| Intermittent claudication | 0 (0%) | 1 (8.3%) | 2 (7.1%) | 6 (21.4%) | 17 (54.8%) | |
| Control (36 Limbs) | Other CV (24 Limbs) | High CV Risk (56 Limbs) | Very High CV Risk (56 Limbs) | Confirmed PAD (61 Limbs) | |
|---|---|---|---|---|---|
| Doppler ABI | 1.158 ± 0.091 | 1.109 ± 0.133 | 1.093 ± 0.164 | 1.006 ± 0.232 | 0.749 ± 2.262 |
| +incompressible arteries (n = limbs) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 5 (8.9%) | 1 (1.6%) |
| Doppler ABI modified | 1.096 ± 0.864 | 1.051 ± 0.137 | 1.007 ± 0.139 | 0.947 ± 0.228 | 0.572 ± 0.320 |
| +incompressible arteries (n = limbs) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (5.4%) | 0 (0.0%) |
| MESI ABI | 1.230 ± 0.659 | 1.144 ± 0.097 | 1.273 ± 0.101 | 1.115 ± 0.100 | 0.945 ± 0.183 |
| +only text data (n = limbs) | 0 (0.0%) | 0 (0.0%) | 1 (1.8%) | 3 (5.4%) | 19 (31.1%) |
| Control (36 Toes) | Other CV (24 Toes) | High CV Risk (56 Toes) | Very High CV Risk (56 Toes) | Confirmed PAD (58 Toes) | |
|---|---|---|---|---|---|
| PeriFlux LD TBI | 0.822 ± 0.068 | 0.801 ± 0.206 | 0.751 ± 0.193 | 0.698 ± 0.189 | 0.399 ± 0.181 |
| SysToe TBI | 0.846 ± 0.076 | 0.781 ± 0.189 | 0.768 ± 0.188 | 0.708 ± 0.176 | 0.439 ± 0.198 |
| MESI TBI | 0.838 ± 0.125 | 0.765 ± 0.347 | 0.766 ± 0.211 | 0.703 ± 0.252 | 0.344 ± 0.239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fendrik, K.; Biró, K.; Endrei, D.; Koltai, K.; Sándor, B.; Tóth, K.; Késmárky, G. Screening for Peripheral Artery Disease Using an Automated Four-Limb Blood Pressure Monitor Equipped with Toe–Brachial Index Measurement. J. Clin. Med. 2023, 12, 6539. https://doi.org/10.3390/jcm12206539
Fendrik K, Biró K, Endrei D, Koltai K, Sándor B, Tóth K, Késmárky G. Screening for Peripheral Artery Disease Using an Automated Four-Limb Blood Pressure Monitor Equipped with Toe–Brachial Index Measurement. Journal of Clinical Medicine. 2023; 12(20):6539. https://doi.org/10.3390/jcm12206539
Chicago/Turabian StyleFendrik, Krisztina, Katalin Biró, Dóra Endrei, Katalin Koltai, Barbara Sándor, Kálmán Tóth, and Gábor Késmárky. 2023. "Screening for Peripheral Artery Disease Using an Automated Four-Limb Blood Pressure Monitor Equipped with Toe–Brachial Index Measurement" Journal of Clinical Medicine 12, no. 20: 6539. https://doi.org/10.3390/jcm12206539
APA StyleFendrik, K., Biró, K., Endrei, D., Koltai, K., Sándor, B., Tóth, K., & Késmárky, G. (2023). Screening for Peripheral Artery Disease Using an Automated Four-Limb Blood Pressure Monitor Equipped with Toe–Brachial Index Measurement. Journal of Clinical Medicine, 12(20), 6539. https://doi.org/10.3390/jcm12206539







