Ten Issues to Update in Nosocomial or Hospital-Acquired Pneumonia: An Expert Review
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. Etiologic Update of Hospital-Acquired Pneumonia
3.2. Importance of Respiratory Sample Quality for the Diagnosis of Ventilator-Associated Pneumonia
3.3. Implementation of Imaging Techniques (CT and Lung Ultrasound) in Diagnosis of NP-HAP
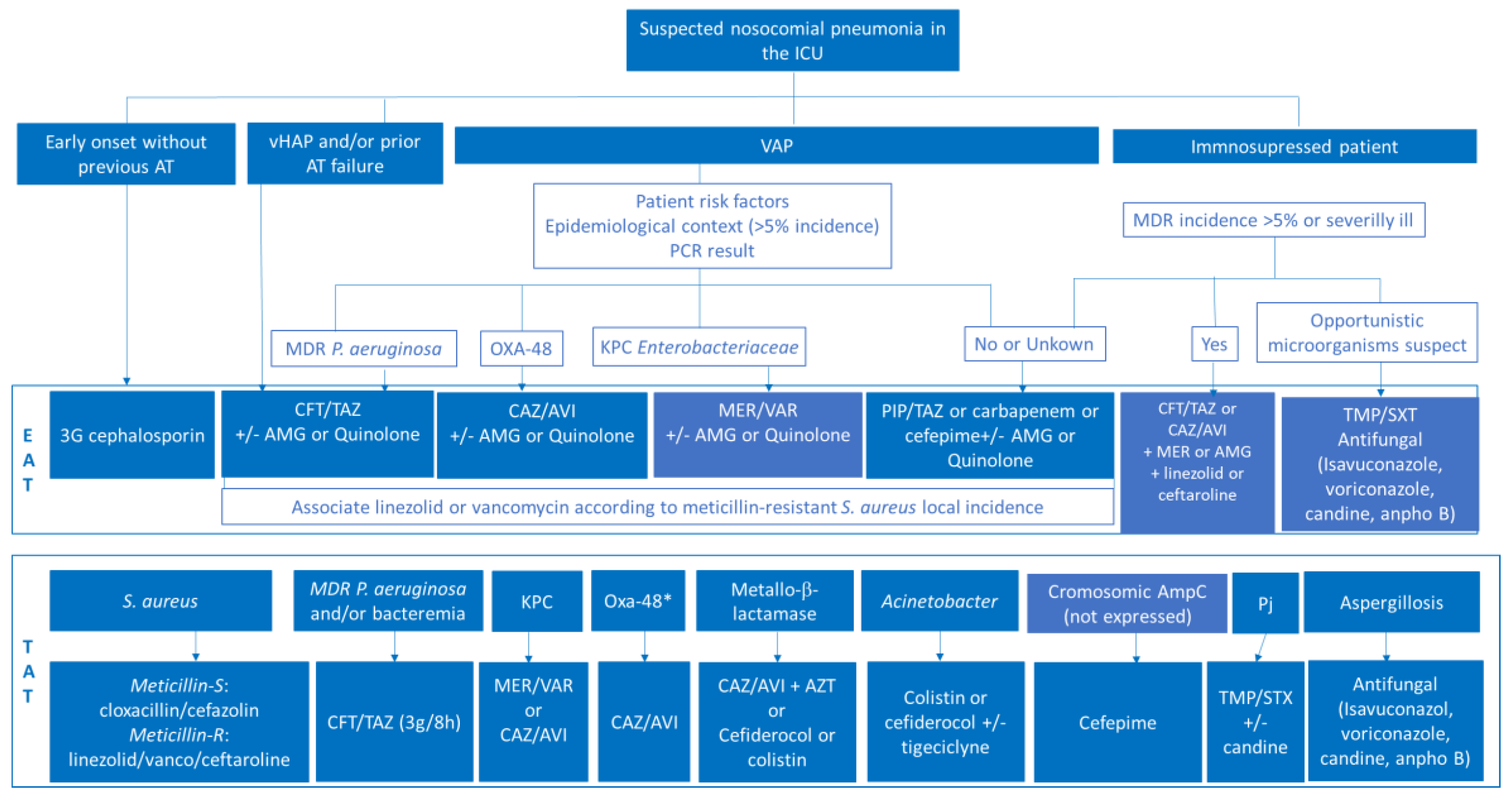
3.4. Update on Antimicrobial Treatment in HAP-NP and VAP-New Evidence
3.5. Nebulized Treatment in NP-HAP
3.6. Approach to the Management of NP-HAP in the Immunosuppressed Patient
3.7. Management of Nosocomial Pneumonia and Health-Associated Pneumonia at Home
3.8. Management of Healthcare-Associated Pneumonia Presenting and Attending at the Emergency Department
3.9. Therapeutic Failure and Rescue in HAP-NP
3.10. Measures for Prevention and Prophylaxis of HAP-NN: Controversies to Be Resolved
- Semi-recumbent position (30–45%). Compared with supine position, it seems that the semi-recumbent position reduces VAP incidence, mainly in patients receiving enteral nutrition [136,137]. The evidence is limited, and other positions have been proposed, such as lateral Trendelemburg, which has shown efficacy but increased adverse events [138].
- Strict hand hygiene before and after handling the airway and single-use sterile gloves. Hand washing with alcohol-based solutions should be performed before and after manipulating the airway. The use of gloves does not prevent hand hygiene. Although usually included in bundles, the application of hand hygiene programs have been shown to reduce the incidence of VAP by themselves [139,140].
- Education and training of all staff involved in airway management
- Encourage early extubation. Duration of mechanical ventilation is one of the main risk factors for VAP. Application of weaning protocols and daily extubation trials have shown a reduction in VAP incidence [141]. The use of non-invasive ventilation in weaning from mechanical ventilation shortens mechanical ventilation time and, therefore, reduces the risk of VAP [142].
- Continuous aspiration of subglottic secretions. The most common route by which bacteria reach the lower respiratory tract is through aspiration of accumulated secretions above the cuff of the endotracheal tube. Continuous or intermittent aspiration of subglottic secretions has been proposed as a way to reduce the incidence of VAP. Clinical trials have consistently shown a reduction in the incidence of VAP related with subglottic aspiration [146,147] and even a reduction in mortality [148].
- No scheduled ventilator circuit changes, unless soiled or malfunctioning. Ventilator circuits should be changed only if visibly soiled or malfunctioning (or following the manufacturer’s instructions). Scheduled changes do not reduce the incidence of VAP and increase healthcare costs [149].
- Administration of antibiotics for 24 h after intubation of comatose patients. Several cohort studies and randomized trials have shown a significant reduction in the incidence of early-onset VAP through the use of systemic antibiotics in the first 24 h after intubation [150,151]. The effect of this measure, by itself, seems to be restricted to the reduction in early-onset VAP in comatose patients, without affecting mortality or duration of mechanical ventilation but decreasing ICU length of stay [152].
- Oral care with clorhexidine 0.12–0.2%. The effect of oral care with chlorhexidine is controversial. It has been related to a reduction in VAP incidence following cardiac surgery [153], while other studies have shown no benefits in non-cardiac surgery patients, and it may be even harmful in some patients [137,154,155,156]. Oral care with chlorhexidine is not recommended by the SHEA/IDSA/APIC guidelines [157].
- Selective digestive decontamination (SDD). The goal of SDD is to reduce the incidence of VAP caused by endogenous microorganisms. SDD includes the administration of systemic antibiotics and the administration of non-absorbable topical antibiotics in the oropharynx (oral paste) and through the nasogastric tube (solution). The most frequently used combination is polymyxin E, tobramycin, and amphotericin B. Dozens of clinical trials have shown a significant reduction in the incidence of VAP without adverse effects (mainly related to the appearance of antimicrobial resistance) [157]. Recent meta-analyses found that the reduction in the incidence of VAP is accompanied by a significant decrease in mortality when complete digestive decontamination is applied (oropharyngeal, digestive, and short systemic antibiotic therapy [158,159,160].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
- GEIPC-SEIMC: Study group of infection in the critically ill patient—Spanish Society of Clinical Microbiology and Infectious Diseases.
- SEQ: Spanish Society of Chemotherapy
- InfurgSemes-SEMES: Emergency Department Infection Study Group. Spanish Society of Emergency Medicine.
- GTEIS- SEMICYUC: Working Group on Infectious Diseases and Sepsis- Spanish Society of Intensive Care Medicine, Critical Care and Coronary Units.
- SEPAR: Spanish Society of Pneumology and thoracic surgery
- GESITRA-SEIMC: Transplant Infections and Immunosuppressed Patients Study Group. Spanish Society of Clinical Microbiology and Infectious Diseases.
- SEHAD: Spanish Society of Hospital at Home.
- GEMARA-SEIMC: Task Force on Mechanisms of Action and Antimicrobial Resistance. Spanish Society of Clinical Microbiology and Infectious Diseases.
- GEIRAS-SEIMC: Healthcare-associated Infection Study Group. Spanish Society of Clinical Microbiology and Infectious Diseases.
Conflicts of Interest
References
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Executive Summary: Management of adults with hospital acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Barberán, J.; Ceccato, A.; Martin-Loeches, I.; Ferrer, M.; Menéndez, M.; Rigau, D. Hospital-Acquired Pneumonia. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Guidelines. 2019 Update. Arch. Bronconeumol. 2020, 56 (Suppl. S1), 11–19. [Google Scholar] [CrossRef]
- Erb, C.T.; Patel, B.; Orr, J.E.; Bice, T.; Richards, J.B.; Metersky, M.L.; Wilson, K.C.; Thomson, C.C. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia. Ann. Am. Thorac. Soc. 2016, 12, 2258–2260. [Google Scholar] [CrossRef] [PubMed]
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1999–2006. [Google Scholar] [CrossRef]
- Metersky, M.L.; Wang, Y.; Klompas, M.; Eckenrode, S.; Bakullari, A.; Eldridge, N. Trend in ventilator-associated pneumonia rates between 2005 and 2013. JAMA 2016, 316, 2427–2429. [Google Scholar] [CrossRef]
- Cillóniz, C.; Dominedò, C.; Torres, A. An overview of guidelines for the management of hospital-acquired and ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria. Curr. Opin. Infect. Dis. 2019, 32, 656–662. [Google Scholar] [CrossRef]
- Dominedò, C.; Ceccato, A.; Niederman, M.; Cillóniz, C.; Gabarrús, A.; Martin-Loeches, I.; Ferrer, M.; Antonelli, M.; Torres, A. Predictive Performance of Risk Factors for Multidrug-Resistant Pathogens in Nosocomial Pneumonia. Ann. Am. Thorac. Soc. 2021, 18, 807–814. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospitalacquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Vena, A.; Graziano, E.; Russo, A.; Peghin, M. Risk stratification and treatment of ICU acquired pneumonia caused by multidrug-resistant/extensively drug-resistant/pandrug-resistant bacteria. Curr. Opin. Crit. Care 2018, 24, 385–393. [Google Scholar] [CrossRef]
- Barbier, F.; Andremont, A.; Wolff, M.; Bouadma, L. Hospital-acquired pneumonia and ventilator-associated pneumonia: Recent advances in epidemiology and management. Curr. Opin. Pulm. Med. 2013, 19, 216–228. [Google Scholar] [CrossRef]
- Vacas-Córdoba, M.; Cardozo-Espinola, C.; Puerta-Alcalde, P.; Cilloniz, C.; Torres, A.; García-Vidal, C. Empirical treatment of adults with hospital-acquired pneumonia: Lights and shadows of the 2016 Clinical Practice ATS/IDSA Guidelines. Rev. Esp. Quimioter. 2017, 30 (Suppl. S1), 30–33. [Google Scholar]
- Vallés, J.; Martin-Loeches, I.; Torres, A.; Diaz, E.; Seijas, I.; López, M.J.; Garro, P.; Castillo, C.; Garnacho-Montero, J.; Martin Mdel, M.; et al. Epidemiology, antibiotic therapy and clinical outcomes of healthcare-associated pneumonia in critically ill patients: A Spanish cohort study. Intensive Care Med. 2014, 40, 572–581. [Google Scholar] [CrossRef]
- Grupo de Trabajo EPINE. Encuesta de Prevalencia de Infecciones Relacionadas con la Asistencia Sanitaria y uso de Antimicrobianos en Hospitales de Agudos en España 2012–2021. Madrid: SEMPSPGS, 2022. Available online: https://www.sempspgs.es (accessed on 2 February 2023).
- Zilberberg, M.D.; Nathanson, B.H.; Puzniak, L.A.; Shorr, A.F. Microbiology, empiric therapy and its impact on the outcomes of nonventilated hospital-acquired, ventilated hospital-acquired, and ventilator-associated bacterial pneumonia in the United States, 2014–2019. Infect. Control Hosp. Epidemiol. 2022, 43, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Estudio Nacional de Vigilancia de Infección Nosocomial en Servicios de Medicina Intensiva. Sociedad Española de Medicina Intensiva Crítica y Unidades Coronarias (SEMICYUC). Informe 2022. Available online: https://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI%202022.pdf (accessed on 2 February 2023).
- Sader, H.S.; Streit, J.M.; Carvalhaes, C.G.; Huband, M.D.; Shortridge, D.; Mendes, R.E.; Castanheira, M. Frequency of occurrence and antimicrobial susceptibility of bacteria isolated from respiratory samples of patients hospitalized with pneumonia in Western Europe, Eastern Europe and the USA: Results from the SENTRY Antimicrobial Surveillance Program (2016–2019). JAC Antimicrob. Resist. 2021, 3, dlab117. [Google Scholar] [CrossRef] [PubMed]
- Waterer, G.W. Health Care-Associated Pneumonia: Is It Still a Useful Concept? Clin. Chest Med. 2018, 39, 765–773. [Google Scholar] [CrossRef]
- Luyt, C.E.; Hékimian, G.; Bréchot, N.; Chastre, J. Viral Ventilator-Associated Pneumonia/Hospital-Acquired Pneumonia. Semin. Respir. Crit. Care Med. 2022, 43, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, R.; Vidal-Cortés, P.; Aguilar, G.; Borges, M.; Diaz, E.; Ferrer, R.; Maseda, E.; Nieto, M.; Nuvials, F.X.; Ramirez, P.; et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit. Care 2020, 24, 383. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers. 2021, 7, 25. [Google Scholar] [CrossRef]
- Chen, J.H.; Lam, H.Y.; Yip, C.C.; Cheng, V.C.; Chan, J.F.; Leung, T.H.; Sridhar, S.; Chan, K.H.; Tang, B.S.; Yuen, K.Y. Evaluation of the molecular Xpert Xpress Flu/RSV assay vs. Alere i Influenza A & B assay for rapid detection of influenza viruses. Diagn. Microbiol. Infect Dis. 2018, 90, 177–180. [Google Scholar] [CrossRef]
- Parčina, M.; Schneider, U.V.; Visseaux, B.; Jozić, R.; Hannet, I.; Lisby, J.G. Multicenter evaluation of the QIAstat Respiratory Panel-A new rapid highly multiplexed PCR based assay for diagnosis of acute respiratory tract infections. PLoS ONE 2020, 15, e0230183. [Google Scholar] [CrossRef]
- Babady, N.E.; England, M.R.; Jurcic Smith, K.L.; He, T.; Wijetunge, D.S.; Tang, Y.W.; Chamberland, R.R.; Menegus, M.; Swierkosz, E.M.; Jerris, R.C.; et al. Multicenter Evaluation of the ePlex Respiratory Pathogen Panel for the Detection of Viral and Bacterial Respiratory Tract Pathogens in Nasopharyngeal Swabs. J. Clin. Microbiol. 2018, 56, e01658-17. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L.; Everhart, K.; Daly, J.A.; Hopper, A.; Harrington, A.; Schreckenberger, P.; McKinley, K.; Jones, M.; Holmberg, K.; Kensinger, B. Multicenter Evaluation of BioFire FilmArray Respiratory Panel 2 for Detection of Viruses and Bacteria in Nasopharyngeal Swab Samples. J. Clin. Microbiol. 2018, 56, e01945-17. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ruan, S.Y.; Pan, S.C.; Lee, T.F.; Chien, J.Y.; Hsueh, P.R. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J. Microbiol. Immunol. Infect. 2019, 52, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Schechter-Perkins, E.M.; Mitchell, P.; Mace, S.; Tian, Y.; Williams, K.; Luo, R.; Yen-Lieberman, B. Multi-center evaluation of the cobas® Liat® Influenza A/B & RSV assay for rapid point of care diagnosis. J. Clin. Virol. 2017, 95, 5–9. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Pulido, M.R.; Moreno-Martínez, P.; González-Galán, V.; Fernández Cuenca, F.; Pascual, Á.; Garnacho-Montero, J.; Antonelli, M.; Dimopoulos, G.; Lepe, J.A.; McConnell, M.J.; et al. Application of BioFire FilmArray Blood Culture Identification panel for rapid identification of the causative agents of ventilator-associated pneumonia. Clin. Microbiol. Infect. 2018, 24, 1213.e1–1213.e4. [Google Scholar] [CrossRef]
- Stafylaki, D.; Maraki, S.; Vaporidi, K.; Georgopoulos, D.; Kontoyiannis, D.P.; Kofteridis, D.P.; Chamilos, G. Impact of Molecular Syndromic Diagnosis of Severe Pneumonia in the Management of Critically Ill Patients. Microbiol. Spectr. 2022, 10, e0161622. [Google Scholar] [CrossRef]
- Leone, M.; Malavieille, F.; Papazian, L.; Meyssignac, B.; Cassir, N.; Textoris, J.; Antonini, F.; La Scola, B.; Martin, C.; Allaouchiche, B.; et al. Routine use ofStaphylococcus aureus rapid diagnostic test inpatients with suspected ventilator-associated pneumonia. Crit. Care 2013, 17, R170. [Google Scholar] [CrossRef]
- Fagon, J.Y.; Chastre, J.; Wolff, M.; Gervais, C.; Parer-Aubas, S.; Stéphan, F.; Similowski, T.; Mercat, A.; Diehl, J.L.; Sollet, J.P.; et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Intern. Med. Ann. Intern. Med. 2000, 132, 621–630. [Google Scholar] [CrossRef]
- The Canadian Critical Care Trial Group. A randomized trial of diagnostic techniques for ventilator associated pneumonia. N. Engl. J. Med. 2006, 355, 2619–2630. [Google Scholar] [CrossRef]
- Estella, A. Análisis de 208 fibrobroncoscopias realizadas en una unidad de cuidados intensivos [Analysis of 208 flexible bronchoscopies performed in an intensive care unit]. Med. Intensiv. 2012, 36, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Estella, A.; Gracia Romero, M.; Perez Ruiz, M.; Fernadez Ruiz, L.; Diez Del Corral, B.; Moreno Cano, S.; Gimenez Beltrán, B.; Recuerda Nuñez, M. Bronchoscopy bronchoalveolar lavage in mechanically ventilated patients in the prone position with acute respiratory distress syndrome. Intensive Care Med. Exp. 2019, 7 (Suppl. S3), 55. [Google Scholar]
- Fujitani, S.; Yu, V.L. Diagnosis of ventilator-associated pneumonia: Focus on nonbronchoscopic techniques (nonbronchoscopic bronchoalveolar lavage, including mini-BAL, blinded protected specimen brush, and blinded bronchial sampling) and endotracheal aspirates. J. Intensive Care Med. 2006, 21, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.C.; Kefala, K.; Simpson, A.J.; Wilkinson, T.S.; Everingham, K.; Kerslake, D.; Raby, S.; Laurenson, I.F.; Swann, D.G.; Walsh, T.S. Evaluation of the effect of diagnostic methodology on the reported incidence of ventilator-associated pneumonia. Thorax 2009, 64, 516–522. [Google Scholar] [CrossRef]
- Flanagan, P.G.; Findlay, G.P.; Magee, J.T.; Ionescu, A.; Barnes, R.A.; Smithies, M. The diagnosis of ventilator-associated pneumonia using non-bronchoscopic, non-directed lung lavages. Intensive Care Med. 2000, 26, 20–30. [Google Scholar] [CrossRef]
- Kunihiro, Y.; Tanaka, N.; Kawano, R.; Yujiri, T.; Kubo, M.; Ueda, K.; Gondo, T.; Kobayashi, T.; Matsumoto, T. Differential diagnosis of pulmonary infections in immunocompromised patients using high-resolution computed tomography. Eur. Radiol. 2019, 29, 6089–6099. [Google Scholar] [CrossRef]
- Haga, T.; Fukuoka, M.; Morita, M.; Cho, K.; Tatsumi, K. Computed Tomography for the Diagnosis and Evaluation of the Severity of Community-acquired Pneumonia in the Elderly. Intern. Med. 2016, 55, 437–441. [Google Scholar] [CrossRef]
- Prendki, V.; Scheffler, M.; Huttner, B.; Garin, N.; Herrmann, F.; Janssens, J.P.; Marti, C.; Carballo, S.; Roux, X.; Serratrice, C.; et al. Low-dose computed tomography for the diagnosis of pneumonia in elderly patients: A prospective, interventional cohort study. Eur. Respir. J. 2018, 51, 1702375. [Google Scholar] [CrossRef]
- Claessens, Y.E.; Debray, M.P.; Tubach, F.; Brun, A.L.; Rammaert, B.; Hausfater, P.; Naccache, J.M.; Ray, P.; Choquet, C.; Carette, M.F.; et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 2015, 192, 974–982. [Google Scholar] [CrossRef]
- Miyashita, N.; Kawai, Y.; Tanaka, T.; Akaike, H.; Teranishi, H.; Wakabayashi, T.; Nakano, T.; Ouchi, K.; Okimoto, N. Detection failure rate of chest radiography for the identification of nursing and healthcare-associated pneumonia. J. Infect. Chemother. 2015, 21, 492–496. [Google Scholar] [CrossRef]
- Douglas, A.P.; Smibert, O.C.; Bajel, A.; Halliday, C.L.; Lavee, O.; McMullan, B.; Yong, M.K.; van Hal, S.J.; Chen, S.C. Consensus guidelines for the diagnosis and management of invasive aspergillosis. Intern. Med. J. 2021, 51 (Suppl. S7), 143–176. [Google Scholar] [CrossRef] [PubMed]
- Jenks, J.D.; Nam, H.H.; Hoenigl, M. Invasive aspergillosis in critically ill patients: Review of definitions and diagnostic approaches. Mycoses 2021, 64, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Karhu, J.M.; Ala-Kokko, T.I.; Ahvenjärvi, L.K.; Rauvala, E.; Ohtonen, P.; Syrjälä, H.P. Early chest computed tomography in adult acute severe community-acquired pneumonia patients treated in the intensive care unit. Acta Anaesthesiol. Scand. 2016, 60, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, C.P.; Grijalva, C.G.; Wunderink, R.G.; Williams, D.J.; Waterer, G.W.; Anderson, E.J.; Zhu, Y.; Hart, E.M.; Carroll, F.; Bramley, A.M.; et al. Community-Acquired Pneumonia Visualized on CT Scans but Not Chest Radiographs: Pathogens, Severity, and Clinical Outcomes. Chest 2018, 153, 601–610. [Google Scholar] [CrossRef]
- Llamas-Alvarez, A.M.; Tenza-Lozano, E.M.; Latour-Perez, J. Accuracy of lung ultrasonography in the diagnosis of pneumonia in adults: Systematic review and meta-analysis. Cofre 2017, 151, 374–382. [Google Scholar]
- Xia, Y.; Ying, Y.; Wang, S.; Li, W.; Shen, H. Effectiveness of lung ultrasound for the diagnosis of pneumonia in adults: A systematic review and meta-analysis. J. Thorac. Dis. 2016, 8, 2822–2831. [Google Scholar] [CrossRef]
- Liu, X.L.; Lian, R.; Tao, Y.K.; Gu, C.D.; Zhang, G.Q. Lung ultrasonography: An effective way to diagnose community-acquired pneumonia. Emerg. Med. J. 2015, 32, 433–438. [Google Scholar] [CrossRef]
- Nazerian, P.; Volpicelli, G.; Vanni, S.; Gigli, C.; Betti, L.; Bartolucci, M.; Zanobetti, M.; Ermini, F.R.; Iannello, C.; Grifoni, S. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am. J. Emerg. Med. 2015, 33, 620–625. [Google Scholar] [CrossRef]
- Amatya, Y.; Rupp, J.; Russell, F.M.; Saunders, J.; Bales, B.; House, D.R. Diagnostic use of lung ultrasound compared to chest radiograph for suspected pneumonia in a resource-limited setting. Int. J. Emerg. Med. 2018, 11, 8. [Google Scholar] [CrossRef]
- Ellington, L.E.; Gilman, R.H.; Chavez, M.A.; Pervaiz, F.; Marin-Concha, J.; Compen-Chang, P.; Riedel, S.; Rodriguez, S.J.; Gaydos, C.; Hardick, J.; et al. Lung ultrasound as a diagnostic tool for radiographically confirmed pneumonia in low resource settings. Respir. Med. 2017, 128, 57–64. [Google Scholar] [CrossRef]
- Prendki, V.; Garin, N.; Stirnemann, J.; Combescure, C.; Platon, A.; Bernasconi, E.; Sauter, T.; Hautz, W.; OCTOPLUS study group. Low-dose CT Or Lung UltraSonography versus standard of care based-strategies for the diagnosis of pneumonia in the elderly: Protocol for a multicentre randomised controlled trial (OCTOPLUS). BMJ Open 2022, 12, e055869. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Bonine, N.G.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Lodise, T. Impact of Delayed Appropriate Antibiotic Therapy on Patient Outcomes by Antibiotic Resistance Status from Serious Gram-negative Bacterial Infections. Am. J. Med. Sci. 2019, 357, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zasowski, E.J.; Bassetti, M.; Blasi, F.; Goossens, H.; Rello, J.; Sotgiu, G.; Tavoschi, L.; Arber, M.R.; McCool, R.; Patterson, J.V.; et al. A Systematic Review of the Effect of Delayed Appropriate Antibiotic Treatment on the Outcomes of Patients with Severe Bacterial Infections. Chest 2020, 158, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Moreno, G.; Bodi, M.; Martín-Loeches, I. Antibiotics in development for multiresistant gram-negative bacilli. Med. Intensiv. 2022, 46, 630–640. [Google Scholar] [CrossRef]
- Millot, G.; Voisin, B.; Loiez, C.; Wallet, F.; Nseir, S. The next generation of rapid point-of-care testing identification tools for ventilator-associated pneumonia. Ann. Transl. Med. 2017, 5, 451. [Google Scholar] [CrossRef]
- Clavel, M.; Barraud, O.; Moucadel, V.; Meynier, F.; Karam, E.; Ploy, M.C.; François, B.; VALIBI study group. Molecular quantification of bacteria from respiratory samples in patients with suspected ventilator-associated pneumonia. Clin. Microbiol. Infect. 2016, 22, 812.e1–812.e7. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Pintado, V.; Ruiz-Garbajosa, P.; Aguilera-Alonso, D.; Baquero-Artigao, F.; Bou, G.; Cantón, R.; Grau, S.; Gutiérrez-Gutiérrez, B.; Larrosa, N.; Machuca, I.; et al. Executive summary of the consensus document of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) on the diagnosis and antimicrobial treatment of infections due to carbapenem-resistant Gram-negative bacteria. Enferm. Infecc. Microbiol. Clin. 2023, 41, 360–370. [Google Scholar] [CrossRef]
- Goodlet, K.J.; Nicolau, D.P.; Nailor, M.D. In Vitro Comparison of Ceftolozane-Tazobactam to Traditional Beta-Lactams and Ceftolozane-Tazobactam as an Alternative to Combination Antimicrobial Therapy for Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Mensa, J.; Barberán, J.; Soriano, A.; Llinares, P.; Marco, F.; Cantón, R.; Bou, G.; González Del Castillo, J.; Maseda, E.; Azanza, J.R.; et al. Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa: Guidelines by the Spanish Society of Chemotherapy. Rev. Española Quimioter. 2018, 31, 78–100. [Google Scholar]
- Kollef, M.H.; Nováček, M.; Kivistik, Ü.; Réa-Neto, Á.; Shime, N.; Martin-Loeches, I.; Timsit, J.F.; Wunderink, R.G.; Bruno, C.J.; Huntington, J.A.; et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): A randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2019, 19, 1299–1311. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; Corona, A.; De Rosa, F.G.; Bassetti, M.; Mussini, C.; Menichetti, F.; Viscoli, C.; Campoli, C.; Venditti, M.; et al. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients With Infections Caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin. Infect. Dis. 2019, 68, 355–364. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Potoski, B.A.; Marini, R.V.; Doi, Y.; Kreiswirth, B.N.; Clancy, C.J. Ceftazidime-Avibactam Is Superior to Other Treatment Regimens against Carbapenem-Resistant Klebsiella pneumoniae Bacteremia. Antimicrob. Agents Chemother. 2017, 61, e00883-17. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Cortés, P.; Martin-Loeches, I.; Rodríguez, A.; Bou, G.; Cantón, R.; Diaz, E.; De la Fuente, C.; Torre-Cisneros, J.; Nuvials, F.X.; Salavert, M.; et al. Current Positioning against Severe Infections Due to Klebsiella pneumoniae in Hospitalized Adults. Antibiotics 2022, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Giamarellos-Bourboulis, E.J.; Rahav, G.; Mathers, A.J.; Bassetti, M.; Vazquez, J.; Cornely, O.A.; Solomkin, J.; Bhowmick, T.; Bishara, J.; et al. Effect and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections: The TANGO II Randomized Clinical Trial. Infect. Dis. Ther. 2018, 7, 439–455. [Google Scholar] [CrossRef]
- Motsch, J.; Murta de Oliveira, C.; Stus, V.; Köksal, I.; Lyulko, O.; Boucher, H.W.; Kaye, K.S.; File, T.M.; Brown, M.L.; Khan, I.; et al. RESTORE-IMI 1: A Multicenter, Ran-domized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin. Infect. Dis. 2020, 70, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Simmonds, A.; Stump, S.; Giddins, M.J.; Annavajhala, M.K.; Uhlemann, A.C. Clonal Background, Resistance Gene Profile, and Porin Gene Mutations Modulate In Vitro Susceptibility to Imipenem-Relebactam in Diverse Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00573-18. [Google Scholar] [CrossRef]
- Lombardo, D.; Ambretti, S.; Lazzarotto, T.; Gaibani, P. In vitro activity of imipenem-relebactam against KPC-producing Klebsiella pneumoniae resistant to ceftazidime-avibactam and/or meropenem-vaborbactam. Clin. Microbiol. Infect. 2022, 28, 749–751. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Barnes, M.D.; Alsop, J.; Taracila, M.A.; Bethel, C.R.; Becka, S.A.; van Duin, D.; Kreiswirth, B.N.; Kaye, K.S.; Bonomo, R.A. Relebactam Is a Potent Inhibitor of the KPC-2 β-Lactamase and Restores Imipenem Susceptibility in KPC-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00174-18. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients with Escherichia coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef]
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): A randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect. Dis. 2018, 18, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Maseda, E.; Suárez de la Rica, A. The role of cefiderocol in clinical practice. Rev. Esp. Quimioter. 2022, 35 (Suppl. S2), 39–44. [Google Scholar] [CrossRef] [PubMed]
- Golden, A.R.; Adam, H.J.; Baxter, M.; Walkty, A.; Lagacé-Wiens, P.; Karlowsky, J.A.; Zhanel, G.G. In Vitro Activity of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacilli Isolated from Patients in Canadian Intensive Care Units. Diagn. Microbiol. Infect Dis. 2020, 97, 115012. [Google Scholar] [CrossRef]
- Shaw, E.; Rombauts, A.; Tubau, F.; Padullés, A.; Càmara, J.; Lozano, T.; Cobo-Sacristán, S.; Sabe, N.; Grau, I.; Rigo-Bonnin, R.; et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J. Antimicrob. Chemother. 2018, 73, 1104–1106. [Google Scholar] [CrossRef]
- Falcone, M.; Daikos, G.L.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of ceftazidime-avibactam plus aztreonam in patients with bloodstream infections caused by metallo-β-lactamase-producing Enterobacterales. Clin. Infect. Dis. 2021, 72, 1871–1878. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Leonildi, A.; Della Sala, L.; Vecchione, A.; Barnini, S.; Farcomeni, A.; Menichetti, F. Cefiderocol-Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2022, 66, e0214221. [Google Scholar] [CrossRef]
- Rello, J.; Solé-Lleonart, C.; Rouby, J.J.; Chastre, J.; Blot, S.; Poulakou, G.; Luyt, C.E.; Riera, J.; Palmer, L.B.; Pereira, J.M.; et al. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: A position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2017, 23, 629–639. [Google Scholar] [CrossRef]
- Alves, J.; Alp, E.; Koulenti, D.; Zhang, Z.; Ehrmann, S.; Blot, S.; Bassetti, M.; Conway-Morris, A.; Reina, R.; Teran, E.; et al. Nebulization of antimicrobial agents in mechanically ventilated adults in 2017: An international cross-sectional survey. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 785–794. [Google Scholar] [CrossRef]
- Póvoa, F.C.C.; Cardinal-Fernandez, P.; Maia, I.S.; Reboredo, M.M.; Pinheiro, B.V. Effect of antibiotics administered via the respiratory tract in the prevention of ventilator-associated pneumonia: A systematic review and meta-analysis. J. Crit. Care 2018, 43, 240–245. [Google Scholar] [CrossRef]
- Tang, R.; Luo, R.; Wu, B.; Wang, F.; Song, H.; Chen, X. Effectiveness, and safety of adjunctive inhaled antibiotics for ventilator-associated pneumonia: A systematic review and meta-analysis of randomized controlled trials. J. Crit. Care 2021, 65, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Szychowiak, P.; Desgrouas, M.; Ehrmann, S. Inhaled antibiotics in critical care: State of the art and future perspectives. Infect. Dis. Now 2022, 52, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Rouby, J.J.; Monsel, A. Nebulized Antibiotics: Epithelial Lining Fluid Concentrations Overestimate Lung Tissue Concentrations. Anesthesiology 2019, 131, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Amin, M.M.; Palmer, L.B.; Shah, A.R.; Smaldone, G.C. Aerosol delivery and modern mechanical ventilation: In vitro/in vivo evaluation. Am. J. Respir. Crit. Care Med. 2003, 168, 1205–1209. [Google Scholar] [CrossRef]
- Monsel, A.; Torres, A.; Zhu, Y.; Pugin, J.; Rello, J.; Rouby, J.J.; on behalf of the European Investigators Network for Nebulized Antibiotics in Ventilator-associated Pneumonia (ENAVAP). Nebulized antibiotics for ventilator-associated pneumonia: Methodological framework for future multicenter randomized controlled trials. Curr. Opin. Infect. Dis. 2021, 34, 156–168. [Google Scholar] [CrossRef]
- Rouby, J.J.; Monsel, A.; Leone, M.; Mimoz, O.; Laterre, P.F.; Pugin, J. The IASIS, INHALE and VAPORISE trials. Reasons for a triple failure: Study design, aminoglycosides dosing and technique of nebulisation. Anaesth. Crit. Care Pain. Med. 2020, 39, 179–183. [Google Scholar] [CrossRef]
- Karaiskos, I.; Gkoufa, A.; Polyzou, E.; Schinas, G.; Athanassa, Z.; Akinosoglou, K. High-Dose Nebulized Colistin Methanesulfonate and the Role in Hospital-Acquired Pneumonia Caused by Gram-Negative Bacteria with Difficult-to-Treat Resistance: A Review. Microorganisms 2023, 11, 1459. [Google Scholar] [CrossRef]
- Rouby, J.J.; Bouhemad, B.; Monsel, A.; Brisson, H.; Arbelot, C.; Lu, Q. Nebulized Antibiotics Study Group. Aerosolized antibiotics for ventilator-associated pneumonia: Lessons from experimental studies. Anesthesiology 2012, 117, 1364–1380. [Google Scholar] [CrossRef]
- Poulakou, G.; Siakallis, G.; Tsiodras, S.; Arfaras-Melainis, A.; Dimopoulos, G. Nebulized antibiotics in mechanically ventilated patients: Roadmap and challenges. Expert. Rev. Anti Infect. Ther. 2017, 15, 211–229. [Google Scholar] [CrossRef]
- Aguilar-Guisado, M.; Jiménez-Jambrina, M.; Espigado, I.; Rovira, M.; Martino, R.; Oriol, A.; Borrell, N.; Ruiz, I.; Martín-Dávila, P.; de la Cámara, R.; et al. Pneumonia in allogeneic stem cell transplantation recipients: A multicenter prospective study. Clin. Transplant. 2011, 25, E629–E638. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guisado, M.; Givaldá, J.; Ussetti, P.; Ramos, A.; Morales, P.; Blanes, M.; Bou, G.; de la Torre-Cisneros, J.; Román, A.; Borro, J.M.; et al. Pneumonia after lung transplantation in the RESITRA cohort: A multicenter prospective study. Am. J. Transplant. 2007, 7, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.T.; Mickus, T.J.; Barbosa, E.; Mullin, C.; Van Deerlin, V.M.; Shiley, K.T. CT of Viral Lower Respiratory Tract Infections in Adults: Comparison among Viral Organisms and between Viral and Bacterial Infections. AJR Am. J. Roentgenol. 2011, 197, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Choo, R.; Anantham, D. Role of bronchoalveolar lavage in the management of immunocompromised patients with pulmonary infiltrates. Ann. Transl. Med. 2019, 7, 49. [Google Scholar] [CrossRef]
- Wohlfarth, P.; Turki, A.T.; Steinmann, J.; Fiedler, M.; Steckel, N.K.; Beelen, D.W.; Liebregts, T. Microbiological diagnostic work-up of acute respiratory failure with pulmonary infiltrates following allogeneic hematopoietic stem cell transplantation: Findings in the era of molecular and biomarker-based assays. Biol. Blood Marrow Transplant. 2018, 24, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.K.; Klassen, C.L.; Bollin, K.B.; Grys, T.E.; Slack, J.L.; Wesselius, L.J.; Vikram, H.R. Microbiologic yield of bronchoalveolar lavage specimens from stem cell transplant recipients. Transpl. Infect. Dis. 2017, 19, e12684. [Google Scholar] [CrossRef]
- Vasala, A.; Hytönen, V.P.; Laitinen, O.H. Modern Tools for Rapid Diagnostics of Antimicrobial Resistance. Front. Cell Infect. Microbiol. 2020, 10, 308. [Google Scholar] [CrossRef]
- Gross, A.E.; van Schooneveld, T.C.; Olsen, K.M.; Rupp, M.E.; Bui, T.H.; Forsung, E.; Kalil, A.C. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob. Agents Chemother. 2014, 58, 5262–5268. [Google Scholar] [CrossRef]
- Cavallazzi, R.; Ramírez, J.A. How and when to manage respiratory infections out of hospital. Eur. Respir. Rev. 2022, 31, 220092. [Google Scholar] [CrossRef]
- Xu, E.; Pérez-Torres, D.; Fragkou, P.C.; Zahar, J.R.; Koulenti, D. Nosocomial pneumonia in the era of multidrug-resistance: Updates in diagnosis and management. Microorganisms 2021, 9, 534. [Google Scholar] [CrossRef]
- Plata-Menchaca, E.P.; Ferrer, R. Current treatment of nosocomial pneumonia and ventilator-associated pneumonia. Rev. Esp. Quimioter. 2022, 35 (Suppl. S3), 25–29. [Google Scholar] [CrossRef] [PubMed]
- Micek, S.T.; Chew, B.; Hampton, N.; Kollef, M.H. A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient outcomes. Chest 2016, 150, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.S.; Ryoo, S.R.; Byun, S.J.; Jeong, Y.J.; Oh, J.Y.; Yoon, Y.S. Antimicrobial resistance and clinical outcomes in Nursing Home-Acquired Pneumonia, compared to Community-Acquired Pneumonia. Yonsei Med. J. 2017, 58, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Picciarella, A.; Russo, R.; Sabetta, F. Clinical features, therapy and outcome of patients hospitalized or not for nursing-home acquired pneumonia. J. Infect. Chemother. 2020, 26, 807–812. [Google Scholar] [CrossRef]
- Montalto, M.; Chu, M.Y.; Ratnam, I.; Spelman, T.; Thursky, K. The treatment of nursing home-acquired pneumonia using a medically intensive Hospital in the Home service. Med. J. Aust. 2015, 203, 441–442. [Google Scholar] [CrossRef]
- Mirón-Rubio, M.; González-Ramallo, V.; Estrada-Cuxart, O.; Sanroma, P.; Segado, A.; Mujal, A.; Del Río-Vizoso, M.; García-Lezcano, M.; Martín-Blanco, N.; Florit-Serra, L.; et al. Intravenous antimicrobial therapy in the hospital-at-home setting: Data from the Spanish Outpatient Parenteral Antimicrobial Therapy Registry. Future Microbiol. 2016, 11, 375–390. [Google Scholar] [CrossRef]
- González-Ramallo, V.J.; Mirón-Rubio, M.; Mujal, A.; Estrada, O.; Forné, C.; Aragón, B.; Rivera, A.J. Costs of outpatient parenteral antimicrobial therapy (OPAT) administered by Hospital at Home units in Spain. Int. J. Antimicrob. Agents 2017, 50, 114–118. [Google Scholar] [CrossRef]
- González Ramallo, V.J.; Mirón Rubio, M.; Estrada Cuxart, O.; García Leoni, M.E. Usefulness of Hospital at Home in nosocomial infections: Advantages and limitations. Rev. Esp. Quimioter. 2017, 30 (Suppl. S1), 61–65. [Google Scholar]
- Mujal, A.; Solá, J.; Hernández, M.; Villarino, M.A.; Machado, M.L.; Baylina, M.; Taján, J.; Oristrell, J. Safety and effectiveness of home intravenous antibiotic therapy for multidrug-resistant bacterial infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1125–1133. [Google Scholar] [CrossRef]
- Esteban-Cartelle, B.; Vicente-Oliveros, N.; Pérez Menéndez-Conde, C.; Serrano, D.R.; Martín-Dávila, P.; Fortún-Abete, J.; León-Gil, L.A.; Álvarez-Díaz, A. Antibiotic stability in portable elastomeric infusion devices: A systematic review. Am. J. Health Syst. Pharm. 2022, 79, 1355–1368. [Google Scholar] [CrossRef]
- Lal, A.; Jaoude, P.; El-Solh, A.A. Prolonged versus Intermittent Infusion of β-Lactams for the Treatment of Nosocomial Pneumonia: A Meta-Analysis. Infect. Chemother. 2016, 48, 81–90. [Google Scholar] [CrossRef]
- Martínez Ortiz de Zárate, M.; González Del Castillo, J.; Julián Jiménez, A.; Piñera Salmerón, P.; Llopis Roca, F.; Guardiola Tey, J.M.; Chanovas Borràs, M.R.; Grinspan, M.R.; García Lamberechts, E.J.; Esparza, C.I.; et al. INFURG-SEMES study: Epidemiology of infections treated in hospital emergency departments and evolution during the last decade. Emergencias 2013, 25, 368–378. [Google Scholar]
- Julián-Jiménez, A.; Adán Valero, I.; Beteta López, A.; Cano Martín, L.M.; Fernández Rodríguez, O.; Rubio Díaz, R.; Sepúlveda Berrocal, M.A.; González Del Castillo, J.; Candel González, F.J.; CAP group (community-acquired pneumonia) from the Infections in Emergencies—Sepsis Code working group. Recomendaciones para la atención del paciente con neumonía adquirida en la comunidad en los Servicios de Urgencias [Recommendations for the care of patients with community-acquired pneumonia in the Emergency Department]. Rev. Esp. Quimioter. 2018, 31, 186–202. [Google Scholar] [PubMed]
- Julián Jiménez, A.; González del Castillo, J.; Martínez Ortiz de Zárate, M.; Candel González, F.J.; Piñera Salmerón, P.; Moya Mir, M.S.; on behalf of the group INFURG-SEMES. Characteristics and epidemiological changes for patients with community-acquired pneumonia in hospital emergency departments. An. Sist. Sanit. Navar. 2013, 36, 387–395. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Rothberg, M.B.; Zilberberg, M.D.; Pekow, P.S.; Priya, A.; Haessler, S.; Belforti, R.; Skiest, D.; Lagu, T.; Higgins, T.L.; Lindenauer, P.K. Association of guideline-based antimicrobial therapy and outcomes in healthcare-associated pneumonia. J. Antimicrob. Chemother. 2015, 70, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Ewig, S.; Kolditz, M.; Pletz, M.W.; Chalmers, J. Healthcare-associated pneumonia: Is there any reason to continue to utilize this label in 2019? Clin. Microbiol. Infect. 2019, 25, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Zilberberg, M.D.; Reichley, R.; Kan, J.; Hoban, A.; Hoffman, J.; Micek, S.T.; Kollef, M.H. Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clin. Infect. Dis. 2012, 54, 193–198. [Google Scholar] [CrossRef]
- Ryan, K.; Karve, S.; Peeters, P.; Baelen, E.; Potter, D.; Rojas-Farreras, S.; Pascual, E.; Rodríguez-Baño, J. The impact of initial antibiotic treatment failure: Real-world insights in healthcare-associated or nosocomial pneumonia. J. Infect. 2018, 77, 9–17. [Google Scholar] [CrossRef]
- Webb, B.J.; Dascomb, K.; Stenehjem, E.; Vikram, H.R.; Agrwal, N.; Sakata, K.; Williams, K.; Bockorny, B.; Bagavathy, K.; Mirza, S.; et al. Derivation and Multicenter Validation of the Drug Resistance in Pneumonia Clinical Prediction Score. Antimicrob. Agents Chemother. 2016, 60, 2652–2663. [Google Scholar] [CrossRef]
- González Del Castillo, J.; Julián-Jiménez, A.; Gamazo-Del Rio, J.J.; García-Lamberechts, E.J.; Llopis-Roca, F.; Guardiola Tey, J.M.; Martínez-Ortiz de Zarate, M.; Navarro Bustos, C.; Piñera Salmerón, P.; Álvarez-Manzanares, J.; et al. INFURG-SEMES investigators (see addedum). A multidrug-resistant microorganism infection risk prediction model: Development and validation in an emergency medicine population. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 309–323. [Google Scholar] [CrossRef]
- Yiang, G.T.; Tzeng, I.S.; Shui, H.A.; Wu, M.Y.; Peng, M.Y.; Chan, C.Y.; Chan, E.D.; Wu, Y.K.; Lan, C.C.; Yang, M.C.; et al. Early Screening of Risk for Multidrug-Resistant Organisms in the Emergency Department in Patients with Pneumonia and Early Septic Shock: Single-Center, Retrospective Cohort Study. Shock 2021, 55, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ishida, T.; Tokumasu, H.; Yamazaki, A.; Washio, Y. Evaluation of pneumonia severity scoring systems in nursing and healthcare-associated pneumonia for predicting prognosis: A prospective, cohort study. J. Infect. Chemother. 2020, 26, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Senussi, T.; Idone, F.; Ceccato, A.; Li Bassi, G.; Ferrer, M.; Torres, A. Invasive and non-invasive diagnostic approaches for microbiological diagnosis of hospital-acquired pneumonia. Crit. Care 2019, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Jitmuang, A.; Puttinad, S.; Hemvimol, S.; Pansasiri, S.; Horthongkham, N. A multiplex pneumonia panel for diagnosis of hospital-acquired and ventilator-associated pneumonia in the era of emerging antimicrobial resistance. Front. Cell. Infect. Microbiol. 2022, 12, 977320. [Google Scholar] [CrossRef]
- Dennesen, P.J.; van der Ven, A.J.; Kessels, A.G.; Ramsay, G.; Bonten, M.J. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2001, 163, 1371–1375. [Google Scholar] [CrossRef]
- Carrie, C.; Chadefaux, G.; Sauvage, N.; de Courson, H.; Petit, L.; Nouette-Gaulain, K.; Pereira, B.; Biais, M. Increased beta-Lactams dosing regimens improve clinical outcome in critically ill patients with augmented renal clearance treated for a first episode of hospital or ventilator-acquired pneumonia: A before and after study. Crit. Care 2019, 23, 379. [Google Scholar] [CrossRef]
- Kollef, M.H. Treatment of ventilator-associated pneumonia: Get it right from the start. Crit. Care Med. 2003, 31, 969–970. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F.; Micek, S.T.; Vazquez-Guillamet, C.; Kollef, M.H. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit. Care 2014, 18, 596. [Google Scholar] [CrossRef]
- ENVIN—HELICS. Available online: http://hws.vhebron.net/envin-helics/ (accessed on 2 February 2023).
- Ranzani, O.T.; Ferrer, M.; Esperatti, M.; Giunta, V.; Bassi, G.L.; Carvalho, C.R.; Torres, A. Association between systemic corticosteroids and outcomes of intensive care unit-acquired pneumonia. Crit. Care Med. 2012, 40, 2552–2561. [Google Scholar] [CrossRef]
- Esperatti, M.; Ferrer, M.; Giunta, V.; Ranzani, O.T.; Saucedo, L.M.; Li Bassi, G.; Blasi, F.; Rello, J.; Niederman, M.S.; Torres, A. Validation of predictors of adverse outcomes in hospital-acquired pneumonia in the ICU. Crit. Care Med. 2013, 41, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Montravers, P.; Bassetti, M. The ideal patient profile for new beta-lactam/beta-lactamase inhibitors. Curr. Opin. Infect. Dis. 2018, 31, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Hujer, A.M.; Rojas, L.J.; Papp-Wallace, K.M.; Humphries, R.M.; Spellberg, B.; Hujer, K.M.; Marshall, E.K.; Rudin, S.D.; Perez, F.; et al. Can Ceftazidime-Avibactam and Aztreonam Overcome beta-Lactam Resistance Conferred by Metallo-beta-Lactamases in Enterobacteriaceae? Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Yang, Z.; Tang, X.; Yuan, Q.; Deng, L.; Sun, X. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst. Rev. 2016, 2016, CD009946. [Google Scholar] [CrossRef]
- Klompas, M.; Li, L.; Kleinman, K.; Szumita, P.M.; Massaro, A.F. Associations Between Ventilator Bundle Components and Outcomes. JAMA Intern. Med. 2016, 176, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Li Bassi, G.; Panigada, M.; Ranzani, O.T.; Zanella, A.; Berra, L.; Cressoni, M.; Parrini, V.; Kandil, H.; Salati, G.; Selvaggi, P.; et al. Randomized, multicenter trial of lateral Trendelenburg versus semirecumbent body position for the prevention of ventilator-associated pneumonia. Intensive Care Med. 2017, 43, 1572–1584. [Google Scholar] [CrossRef]
- Ma, S.; Liu, S.; Huang, L.; Xu, C.; Liu, W.; Huang, Y. A meta analysis of the effect of enhanced hand hygiene on the morbidity of ventilator-associated pneumonia. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014, 26, 304–308. [Google Scholar] [CrossRef]
- Koff, M.D.; Corwin, H.L.; Beach, M.L.; Surgenor, S.D.; Loftus, R.W. Reduction in ventilator associated pneumonia in a mixed intensive care unit after initiation of a novel hand hygiene program. J. Crit. Care 2011, 26, 489–495. [Google Scholar] [CrossRef]
- Weinberger, J.; Cocoros, N.; Klompas, M. Ventilator-Associated Events: Epidemiology, Risk Factors, and Prevention. Infect. Dis. Clin. N. Am. 2021, 35, 871–899. [Google Scholar] [CrossRef]
- Yeung, J.; Couper, K.; Ryan, E.G.; Gates, S.; Hart, N.; Perkins, G.D. Non-invasive ventilation as a strategy for weaning from invasive mechanical ventilation: A systematic review and Bayesian meta-analysis. Intensive Care Med. 2018, 44, 2192–2204. [Google Scholar] [CrossRef]
- Dauvergne, J.E.; Geffray, A.L.; Asehnoune, K.; Rozec, B.; Lakhal, K. Automatic regulation of the endotracheal tube cuff pressure with a portable elastomeric device. A randomised controlled study. Anaesth. Crit. Care Pain. Med. 2020, 39, 435–441. [Google Scholar] [CrossRef]
- Dat, V.Q.; Minh Yen, L.; Thi Loan, H.; Dinh Phu, V.; Thien Binh, N.; Geskus, R.B.; Khanh Trinh, D.H.; Hoang Mai, N.T.; Hoan Phu, N.; Huong Lan, N.P.; et al. Effectiveness of Continuous Endotracheal Cuff Pressure Control for the Prevention of Ventilator-Associated Respiratory Infections: An Open-Label Randomized, Controlled Trial. Clin. Infect. Dis. 2022, 74, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, N.; Boisson, M.; Asehnoune, K.; Foucrier, A.; Lasocki, S.; Ichai, C.; Leone, M.; Pottecher, J.; Lefrant, J.Y.; Falcon, D.; et al. Continuous Pneumatic Regulation of Tracheal Cuff Pressure to Decrease Ventilator-associated Pneumonia in Trauma Patients Who Were Mechanically Ventilated: The AGATE Multicenter Randomized Controlled Study. Chest 2021, 160, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Damas, P.; Frippiat, F.; Ancion, A.; Canivet, J.L.; Lambermont, B.; Layios, N.; Massion, P.; Morimont, P.; Nys, M.; Piret, S.; et al. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: A randomized controlled trial with subglottic secretion suctioning. Crit. Care Med. 2015, 43, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Hamishehkar, H.; Hamidi, M.; Shadvar, K.; Sanaie, S.; Golzari, S.E.; Khan, Z.H.; Nader, N.D. A prospective randomized trial of tapered-cuff endotracheal tubes with intermittent subglottic suctioning in preventing ventilator-associated pneumonia in critically ill patients. J. Crit. Care 2017, 38, 152–156. [Google Scholar] [CrossRef]
- Pozuelo-Carrascosa, D.P.; Herráiz-Adillo, Á.; Alvarez-Bueno, C.; Añón, J.M.; Martínez-Vizcaíno, V.; Cavero-Redondo, I. Subglottic secretion drainage for preventing ventilator-associated pneumonia: An overview of systematic reviews and an updated meta-analysis. Eur. Respir. Rev. 2020, 29, 190107. [Google Scholar] [CrossRef]
- Lorente, L.; Lecuona, M.; Galván, R.; Ramos, M.J.; Mora, M.L.; Sierra, A. Periodically changing ventilator circuits is not necessary to prevent ventilator-associated pneumonia when a heat and moisture exchanger is used. Infect. Control Hosp. Epidemiol. 2004, 25, 1077–1082. [Google Scholar] [CrossRef]
- Mirtalaei, N.; Farazi, A.; Ebrahimi Monfared, M.; Jokar, A. Efficacy of antibiotic prophylaxis against ventilator-associated pneumonia. J. Hosp. Infect. 2019, 101, 272–275. [Google Scholar] [CrossRef]
- Vallés, J.; Peredo, R.; Burgueño, M.J.; Rodrigues de Freitas, A.P.; Millán, S.; Espasa, M.; Martín-Loeches, I.; Ferrer, R.; Suarez, D.; Artigas, A. Efficacy of single-dose antibiotic against early-onset pneumonia in comatose patients who are ventilated. Chest 2013, 143, 1219–1225. [Google Scholar] [CrossRef]
- Righy, C.; do Brasil, P.E.A.; Vallés, J.; Bozza, F.A.; Martin-Loeches, I. Systemic antibiotics for preventing ventilator-associated pneumonia in comatose patients: A systematic review and meta-analysis. Ann. Intensive Care 2017, 7, 67. [Google Scholar] [CrossRef]
- Klompas, M.; Speck, K.; Howell, M.D.; Greene, L.R.; Berenholtz, S.M. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: Systematic review and meta-analysis. JAMA Intern. Med. 2014, 174, 751–761. [Google Scholar] [CrossRef]
- Price, R.; MacLennan, G.; Glen, J.; SuDDICU Collaboration. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: Systematic review and network meta-analysis. BMJ 2014, 348, g2197. [Google Scholar] [CrossRef] [PubMed]
- Deschepper, M.; Waegeman, W.; Eeckloo, K.; Vogelaers, D.; Blot, S. Effects of chlorhexidine gluconate oral care on hospital mortality: A hospital-wide, observational cohort study. Intensive Care Med. 2018, 44, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Branson, R.; Cawcutt, K.; Crist, M.; Eichenwald, E.C.; Greene, L.R.; Lee, G.; Maragakis, L.L.; Powell, K.; Priebe, G.P.; et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2022, 43, 687–713. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Seppelt, I.M.; Goodman, F.; Billot, L.; Correa, M.; Davis, J.S.; Gordon, A.C.; Hammond, N.E.; Iredell, J.; Li, Q.; et al. SuDDICU Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group. “Effect of Selective Decontamination of the Digestive Tract on Hospital Mortality in Critically Ill Patients Receiving Mechanical Ventilation: A Randomized Clinical Trial. JAMA 2022, 328, 1911–1921. [Google Scholar]
- Plantinga, N.L.; de Smet, A.M.G.A.; Oostdijk, E.A.N.; de Jonge, E.; Camus, C.; Krueger, W.A.; Bergmans, D.; Reitsma, J.B.; Bonten, M.J.M. Selective digestive and oropharyngeal decontamination in medical and surgical ICU patients: Individual patient data meta-analysis. Clin. Microbiol. Infect. 2018, 24, 505–513. [Google Scholar] [CrossRef]
- Scheufele, F.; Schirren, R.; Friess, H.; Reim, D. Selective decontamination of the digestive tract in upper gastrointestinal surgery: Systematic review with meta-analysis of randomized clinical trials. BJS Open 2020, 4, 1015–1021. [Google Scholar] [CrossRef]
- Minozzi, S.; Pifferi, S.; Brazzi, L.; Pecoraro, V.; Montrucchio, G.; D’Amico, R. Topical antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving mechanical ventilation. Cochrane Database Syst. Rev. 2021, 1, CD000022. [Google Scholar]
- Mastrogianni, M.; Katsoulas, T.; Galanis, P.; Korompeli, A.; Myrianthefs, P. The Impact of Care Bundles on Ventilator-Associated Pneumonia (VAP) Prevention in Adult ICUs: A Systematic Review. Antibiotics 2023, 12, 227. [Google Scholar] [CrossRef]
- Álvarez-Lerma, F.; Palomar-Martínez, M.; Sánchez-García, M.; Martínez-Alonso, M.; Álvarez-Rodríguez, J.; Lorente, L.; Arias-Rivera, S.; García, R.; Gordo, F.; Añón, J.M.; et al. Prevention of Ventilator-Associated Pneumonia: The Multimodal Approach of the Spanish ICU «Pneumonia Zero» Program. Crit. Care Med. 2018, 46, 181–188. [Google Scholar] [CrossRef]
| Molecular Technique | Methodology | Target | Time to Response |
|---|---|---|---|
| VERIGENE® Respiratory Pathogens Flex Test (RP Flex) (Luminex) | Multiplex RT-PCR/Solid-phase microarray with gold nanoparticles | Inf (A, H1, H3, H1 2009, B), AdV, VRS (A, B), MpV, PiV (1, 2, 3, 4), RnV, BPer, BPar, BHol | 2 h |
| Film Array Respiratory 2 plus Panel (bioMerieux, Marcy-l’Étoile, France) | Nested multiplex RT-PCR/Melting analysis | Inf (A, H1, H3, H1 2009, B), VRS, AdV, CoV (229E, OC43, NL63, HKU1, MERS), MpV, PiV (1, 2, 3, 4), RnV/EV, BPer, BPar, MPne, CPne | 45 min |
| BiofireFilmArray Pneumonia Plus Panel (bioMerieux, Marcy-l’Étoile, France) | Nested multiplex RT-PCR/Melting analysis | ABau, EClo, ECol, HInf, KAer, KOxy, KPne, MCat, Prot, PAer, SMar, SAur, SAga, SPne, SPyo, CPne, LPne, MPne, Inf (A, B), VRS, AdV, CoV, MERS, MpV, PiV, RnV/EV, mecA, mecC, MERJ, KPC, NDM, OXA48, VIM, IMP, CTXM | 1 h 15 min |
| Xpert® XpressFlu/RSV (Cepheid, Sunnyvale, CA, USA) | Real-time RT-PCR | Inf (A, B), VRS | 20 min |
| QIAstat-Dx Respiratory SARS-CoV- 2 Panel (QIAGEN, Hilden, Germany) | Real-time RT-PCR | Inf (A, H1, H1 2009, H3, B), VRS (A, B), AdV, CoV (229E, OC43, NL63, HKU1), SARS-CoV-2, MpV, PiV (1, 2, 3, 4), RnV/EV, BPer, MPne, CPne, LPne | 1 h 10 min |
| cobas® Liat® (Roche, Basel, Switzerland) | Real-time PCR | Inf (A, B), VRS | 20 min |
| ePlexRespiratory Pathogen (RP) Panel (GenMark, Carlsbad, CA, USA) | Electrowetting/Microarray Solid phase/Detection electrochemistry | Inf (A, H1, H3, H1 2009, B), VRS (A, B), AdV, PiV (1, 2, 3, 4), MpV, CoV (229E, OC43, NL63, HKU1), RnV, MPne, CPne | 1 h 30 min |
| Non-Invasive Strategy Tracheal Aspiration | Invasive Strategy Bronchoscopy and Bronchoalveolar Samples | |
|---|---|---|
| Advantages | Quick Easy to perform Safe Inexpensive | Lower respiratory tract guided sample obtained High specificity Distinguish between infection and colonization Noninfectious diagnosis by direct visualization Safe |
| Disadvantages | Upper respiratory tract Difficult to differentiate from colonization Risk of overuse of antibiotics | Need for trained staff |
| Technique | Microorganisms | Advantages | Disadvantages | |
|---|---|---|---|---|
| Respiratory sample (BAL) | Gram stain | Bacteria, yeast | Immediate results | False negatives. Observer dependent |
| Ziehl-Nielsen stain, modified Ziehl-Nielsen stain | Mycobacterium tuberculosis, non-tuberculous mycobacteria, Nocardia spp. | |||
| Fungal morphology (KOH, calcofluor, papanicolau, H&E, GMS or PAS staining, ink staining) | Fungus | |||
| Culture | Bacteria, fungus, virus | Time dependent. False negatives | ||
| Galactomannan (ELISA, lateral-flow) | Aspergillus spp. | Immediate results | Discrepancy among techniques | |
| Direct fluorescent antibodies | Aspergillus spp. Mycobacterium tuberculosis | |||
| PCR (simplex or multiple) | Aspergillus spp. Pneumocystis jirovecii, Mycobacteria, Virus (respiratory virus, CMV, VHS), Bacteria | Immediate results. High sensitivity | Positivity does not always imply infection. Microorganisms not included in multiple test | |
| Nasopharyngeal swab | PCR (simplex or multiple) | Mainly respiratory virus | Immediate results | Positivity does not always imply infection |
| Serum sample | Galactomannan | Aspergillus spp. | Rapid results | False negative in non-neutropenic patients |
| (1-3)-β_D-glucan | Fungus (except mucorales and Crypctococcus spp.) | High negative predictive value. Treatment evaluation | False negatives | |
| Cryptococcal antigen | ||||
| Urine sample | Soluble antigen tests | Histoplasma spp., Cryptococcus, S. pneumoniae, L. pneumophila | Immediate results | |
| Blood | Culture | Bacteria, fungus | Time dependent. False negatives. | |
| PCR (simplex or multiple) | CMV, VHS, VEB, Adenovirus. Bacteria | Rapid results | Microorganisms not included in multiple test |
| Antibiotic | Standard Dose | Microbiological Target | Stability at 25 °C | Home iv Infusion Device/Modality | |
|---|---|---|---|---|---|
| Electronic Pump | Elastomeric Pump/Gravity | ||||
| Piperacillin-tazobactam | 4/0.5 g every 6–8 h | Pseudomonas aeruginosa, Enterobacteriaceae | >24 h | Yes | Optional (self-administration) b |
| Ceftazidime | 1–2 g every 8 h | Pseudomonas aeruginosa, Enterobacteriaceae | >24 h | Yes | Optional (self-administration) b |
| Cefepime | 2 g every 8–12 h | Pseudomonas aeruginosa, Enterobacteriaceae | >24 h | Yes | Optional (self-administration) b |
| Meropenem | 1–2 g every 8 h | Pseudomonas aeruginosa, Enterobacteriaceae ESBL | <24 h | No a | Yes (self-administration) c |
| Ertapenem | 1 g every 24 h | Enterobacteriaceae ESBL | <24 h | No needed | |
| Ceftolozane-tazobactam | 2/1 g every 8 h | Pseudomonas aeruginosa | Up to 24 h | Yes | Optional (self-administration) b |
| Ceftazidime-avibactam | 2/0.5 g every 8 h | Pseudomonas aeruginosa, other resistant Enterobacteriaceae | <24 h | No a | Yes (self-administration) |
| Amikacin * | 15–20 mg/kg/d | Pseudomonas aeruginosa | >24 h | No needed | Yes |
| Tobramycin * | 5–7 mg/kg/d | Pseudomonas aeruginosa | >24 h | No needed | Yes |
| Gentamicin * | 5–7 mg/kg/d | Pseudomonas aeruginosa | >24 h | No needed | Yes |
| Aztreonam * | 1–2 g every 8 h | Pseudomonas aeruginosa, Enterobacteriaceae | >24 h | Yes | Optional (self-administration) b |
| Levofloxacin * | 500 mg every 24 h | Pseudomonas aeruginosa, Enterobacteriaceae | >24 h | No needed | Gravity (presentation as 100 mL ready-to-use containers) |
| Linezolid | 600 mg every 12 h | MRSA | >24 h | No | Gravity (presentation as 300 mL ready-to-use containers) |
| Vancomycin | 15–20 mg/kg every 12 h | MRSA | >24 h | Yes | Consider two nursing visits. Optional: self-administration b,d |
| Ceftaroline | 600 mg every 12 h | MRSA | Up to 24 h (6 mg/mL in sodium chloride 0.9%, protected from light) | Yes | Consider two nursing visits. Optional: self-administration b |
| Ceftobiprole | 500 mg every 8 h | MRSA | Up to 24 h (2 mg/mL in sodium chloride 0.9%, protected from light) | Yes | Gravity (2-h infusion) |
| Cause | Recommendation |
|---|---|
| Inadequate antibiotic treatment | Escalate based on microbiological results. |
| Sub-therapeutic antibiotic concentrations | Increase antimicrobial dosing. Use extended or continuous antibiotic infusions to optimize PK/PD parameters |
| New pathogens isolated | Antimicrobial treatment according to microbiological data |
| Undrained pyogenic focus (i.e., empyema) | Therapeutic drainage |
| Drug fever | Change antibiotic treatment |
| A non-infectious illness presenting as NP-HAP | Management as appropriate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candel, F.J.; Salavert, M.; Estella, A.; Ferrer, M.; Ferrer, R.; Gamazo, J.J.; García-Vidal, C.; del Castillo, J.G.; González-Ramallo, V.J.; Gordo, F.; et al. Ten Issues to Update in Nosocomial or Hospital-Acquired Pneumonia: An Expert Review. J. Clin. Med. 2023, 12, 6526. https://doi.org/10.3390/jcm12206526
Candel FJ, Salavert M, Estella A, Ferrer M, Ferrer R, Gamazo JJ, García-Vidal C, del Castillo JG, González-Ramallo VJ, Gordo F, et al. Ten Issues to Update in Nosocomial or Hospital-Acquired Pneumonia: An Expert Review. Journal of Clinical Medicine. 2023; 12(20):6526. https://doi.org/10.3390/jcm12206526
Chicago/Turabian StyleCandel, Francisco Javier, Miguel Salavert, Angel Estella, Miquel Ferrer, Ricard Ferrer, Julio Javier Gamazo, Carolina García-Vidal, Juan González del Castillo, Víctor José González-Ramallo, Federico Gordo, and et al. 2023. "Ten Issues to Update in Nosocomial or Hospital-Acquired Pneumonia: An Expert Review" Journal of Clinical Medicine 12, no. 20: 6526. https://doi.org/10.3390/jcm12206526
APA StyleCandel, F. J., Salavert, M., Estella, A., Ferrer, M., Ferrer, R., Gamazo, J. J., García-Vidal, C., del Castillo, J. G., González-Ramallo, V. J., Gordo, F., Mirón-Rubio, M., Pérez-Pallarés, J., Pitart, C., del Pozo, J. L., Ramírez, P., Rascado, P., Reyes, S., Ruiz-Garbajosa, P., Suberviola, B., ... Zaragoza, R. (2023). Ten Issues to Update in Nosocomial or Hospital-Acquired Pneumonia: An Expert Review. Journal of Clinical Medicine, 12(20), 6526. https://doi.org/10.3390/jcm12206526






