Cardiovascular Risk Associated with Alpha-1 Antitrypsin Deficiency (AATD) Genotypes: A Meta-Analysis with Meta-Regressions
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Extraction of Data and Quality Assessment
2.3. Meta-Analysis and Publication Bias
2.4. Sensitivity Analyses
2.5. Subgroup Analyses
2.6. Meta-Regression Analyses
3. Results
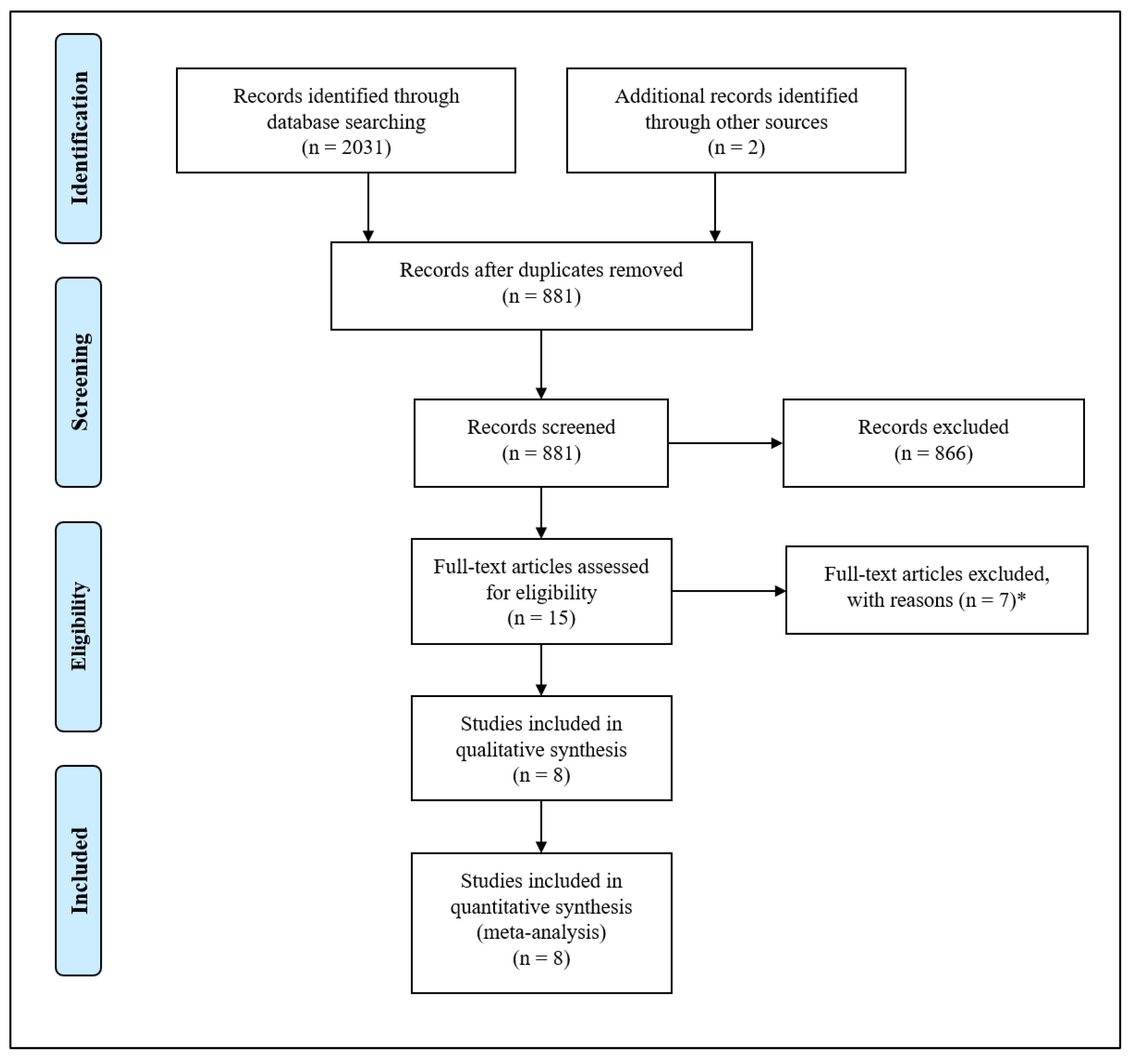
3.1. Study Characteristics
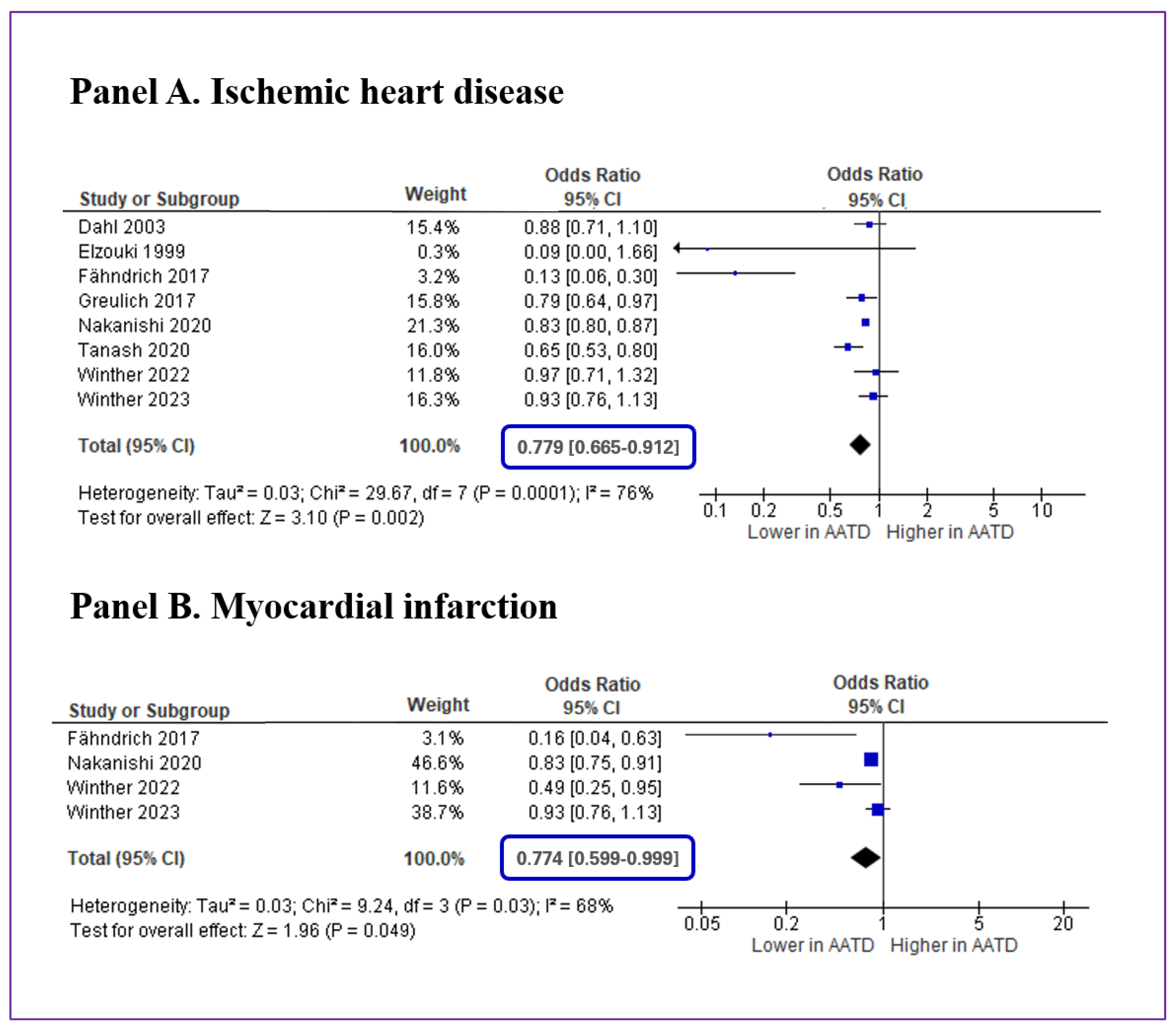
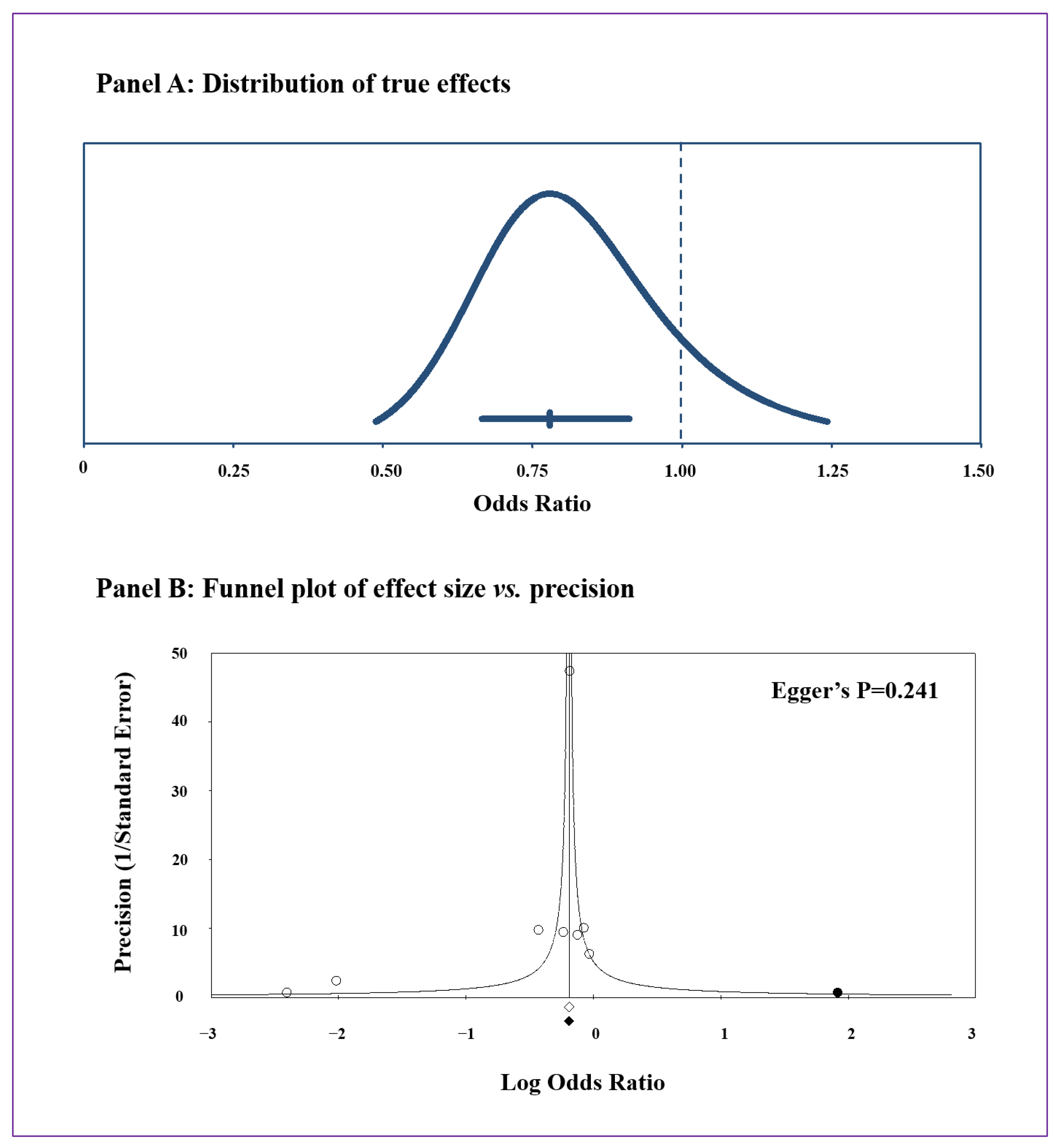
3.2. Meta-Analysis and Publication Bias
3.3. Sensitivity Analyses
3.4. Subgroup Analyses
3.5. Meta-Regression Analyses
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoller, J.K.; Aboussouan, L.S. A review of alpha1-antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 2012, 185, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Fregonese, L.; Stolk, J. Hereditary alpha-1-antitrypsin deficiency and its clinical consequences. Orphanet J. Rare Dis. 2008, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Belmonte, I.; Nunez, A.; Farago, G.; Barrecheguren, M.; Pons, M.; Orriols, G.; Gabriel-Medina, P.; Rodriguez-Frias, F.; Miravitlles, M.; et al. New variants of alpha-1-antitrypsin: Structural simulations and clinical expression. Respir. Res. 2022, 23, 339. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, A.; Ferrarotti, I.; Coppola, A.; Lanza, M.; Imitazione, P.; Spinelli, S.; Micco, P.D.; Fiorentino, G. Alpha-1 Antitrypsin Screening in a Selected Cohort of Patients Affected by Chronic Pulmonary Diseases in Naples, Italy. J. Clin. Med. 2021, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Diego, I.; Bueno, P.; Perez-Holanda, S.; Casas-Maldonado, F.; Miravitlles, M. Prevalence of alpha(1)-antitrypsin PiZZ genotypes in patients with COPD in Europe: A systematic review. Eur. Respir. Rev. 2020, 29, 200014. [Google Scholar] [CrossRef]
- Brantly, M.L.; Wittes, J.T.; Vogelmeier, C.F.; Hubbard, R.C.; Fells, G.A.; Crystal, R.G. Use of a highly purified alpha 1-antitrypsin standard to establish ranges for the common normal and deficient alpha 1-antitrypsin phenotypes. Chest 1991, 100, 703–708. [Google Scholar] [CrossRef]
- Miravitlles, M.; Dirksen, A.; Ferrarotti, I.; Koblizek, V.; Lange, P.; Mahadeva, R.; McElvaney, N.G.; Parr, D.; Piitulainen, E.; Roche, N.; et al. European Respiratory Society statement: Diagnosis and treatment of pulmonary disease in alpha(1)-antitrypsin deficiency. Eur. Respir. J. 2017, 50, 1700610. [Google Scholar] [CrossRef]
- de Serres, F.J.; Blanco, I.; Fernandez-Bustillo, E. Estimated numbers and prevalence of PI*S and PI*Z deficiency alleles of alpha1-antitrypsin deficiency in Asia. Eur. Respir. J. 2006, 28, 1091–1099. [Google Scholar] [CrossRef]
- Jonigk, D.; Al-Omari, M.; Maegel, L.; Muller, M.; Izykowski, N.; Hong, J.; Hong, K.; Kim, S.H.; Dorsch, M.; Mahadeva, R.; et al. Anti-inflammatory and immunomodulatory properties of alpha1-antitrypsin without inhibition of elastase. Proc. Natl. Acad. Sci. USA 2013, 110, 15007–15012. [Google Scholar] [CrossRef]
- Stoller, J.K. Alpha1-antitrypsin deficiency. Thorax 2004, 59, 92–93. [Google Scholar] [CrossRef][Green Version]
- Pons, M.; Nunez, A.; Esquinas, C.; Torres-Duran, M.; Rodriguez-Hermosa, J.L.; Calle, M.; Tubio-Perez, R.; Belmonte, I.; Rodriguez-Frias, F.; Rodriguez, E.; et al. Utility of Transient Elastography for the Screening of Liver Disease in Patients with Alpha1-Antitrypsin Deficiency. J. Clin. Med. 2021, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Mucke, V.T.; Fischer, J.; Mucke, M.M.; Teumer, A.; Koch, A.; Vermehren, J.; Fromme, M.; Zeuzem, S.; Trautwein, C.; Sarrazin, C.; et al. Association of Alpha-1 Antitrypsin Pi*Z Allele Frequency and Progressive Liver Fibrosis in Two Chronic Hepatitis C Cohorts. J. Clin. Med. 2022, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E.K.; Sandhaus, R.A. Clinical practice. Alpha1-antitrypsin deficiency. N. Engl. J. Med. 2009, 360, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Edwards, L.D.; Agusti, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Crim, C.; Lomas, D.A.; Miller, B.E.; et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir. Med. 2013, 107, 1376–1384. [Google Scholar] [CrossRef]
- Needham, M.; Stockley, R.A. Alpha 1-antitrypsin deficiency. 3: Clinical manifestations and natural history. Thorax 2004, 59, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Pini, L.; Giordani, J.; Ciarfaglia, M.; Pini, A.; Arici, M.; Tantucci, C. Alpha1-antitrypsin deficiency and cardiovascular disease: Questions and issues of a debated relation. J. Cardiovasc. Med. 2022, 23, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.; Tybjaerg-Hansen, A.; Sillesen, H.; Jensen, G.; Steffensen, R.; Nordestgaard, B.G. Blood pressure, risk of ischemic cerebrovascular and ischemic heart disease, and longevity in alpha(1)-antitrypsin deficiency: The Copenhagen City Heart Study. Circulation 2003, 107, 747–752. [Google Scholar] [CrossRef]
- Winther, S.V.; Ahmed, D.; Al-Shuweli, S.; Landt, E.M.; Nordestgaard, B.G.; Seersholm, N.; Dahl, M. Severe alpha(1)-antitrypsin deficiency associated with lower blood pressure and reduced risk of ischemic heart disease: A cohort study of 91,540 individuals and a meta-analysis. Respir. Res. 2022, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Curjuric, I.; Imboden, M.; Bettschart, R.; Caviezel, S.; Dratva, J.; Pons, M.; Rothe, T.; Schmidt-Trucksass, A.; Stolz, D.; Thun, G.A.; et al. Alpha-1 antitrypsin deficiency: From the lung to the heart? Atherosclerosis 2018, 270, 166–172. [Google Scholar] [CrossRef]
- Hamesch, K.; Mandorfer, M.; Pereira, V.M.; Moeller, L.S.; Pons, M.; Dolman, G.E.; Reichert, M.C.; Schneider, C.V.; Woditsch, V.; Voss, J.; et al. Liver Fibrosis and Metabolic Alterations in Adults With alpha-1-antitrypsin Deficiency Caused by the Pi*ZZ Mutation. Gastroenterology 2019, 157, 705–719. [Google Scholar] [CrossRef]
- Turhan Caglar, F.N.; Ksanski, V.; Polat, V.; Ungan, I.; Kural, A.; Ciftci, S.; Demir, B.; Ugurlucan, M.; Akturk, F.; Karakaya, O. The Association Between alpha(1)-Antitrypsin and Coronary Artery Ectasia. Angiology 2016, 67, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Van Tassell, B.W.; Christopher, S.; Abouzaki, N.A.; Sonnino, C.; Oddi, C.; Carbone, S.; Melchior, R.D.; Gambill, M.L.; Roberts, C.S.; et al. Effects of Prolastin C (Plasma-Derived Alpha-1 Antitrypsin) on the acute inflammatory response in patients with ST-segment elevation myocardial infarction (from the VCU-alpha 1-RT pilot study). Am. J. Cardiol. 2015, 115, 8–12. [Google Scholar] [CrossRef]
- Talmud, P.J.; Martin, S.; Steiner, G.; Flavell, D.M.; Whitehouse, D.B.; Nagl, S.; Jackson, R.; Taskinen, M.R.; Frick, M.H.; Nieminen, M.S.; et al. Progression of atherosclerosis is associated with variation in the alpha1-antitrypsin gene. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 644–649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ambrosino, P.; Lupoli, R.; Cafaro, G.; Iervolino, S.; Carone, M.; Pappone, N.; Di Minno, M.N.D. Subclinical carotid atherosclerosis in patients with chronic obstructive pulmonary disease: A meta-analysis of literature studies. Ann. Med. 2017, 49, 513–524. [Google Scholar] [CrossRef]
- Ambrosino, P.; Lupoli, R.; Iervolino, S.; De Felice, A.; Pappone, N.; Storino, A.; Di Minno, M.N.D. Clinical assessment of endothelial function in patients with chronic obstructive pulmonary disease: A systematic review with meta-analysis. Intern. Emerg. Med. 2017, 12, 877–885. [Google Scholar] [CrossRef]
- Tanash, H.; Ekstrom, M.; Basil, N.; Ronmark, E.; Lindberg, A.; Piitulainen, E. Decreased Risk of Ischemic Heart Disease in Individuals with Severe Alpha 1-Antitrypsin Deficiency (PiZZ) in Comparison with the General Population. Int. J. Chron. Obstruct Pulmon Dis. 2020, 15, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Fahndrich, S.; Biertz, F.; Karch, A.; Kleibrink, B.; Koch, A.; Teschler, H.; Welte, T.; Kauczor, H.U.; Janciauskiene, S.; Jorres, R.A.; et al. Cardiovascular risk in patients with alpha-1-antitrypsin deficiency. Respir. Res. 2017, 18, 171. [Google Scholar] [CrossRef]
- Winther, S.V.; Landt, E.M.; Nordestgaard, B.G.; Seersholm, N.; Dahl, M. α1-Antitrypsin deficiency associated with increased risk of heart failure. ERJ Open Res. 2023, 9, 00319–2023. [Google Scholar] [CrossRef]
- Elzouki, A.N.; Ryden Ahlgren, A.; Lanne, T.; Sonesson, B.; Eriksson, S. Is there a relationship between abdominal aortic aneurysms and alpha1-antitrypsin deficiency (PiZ)? Eur. J. Vasc. Endovasc. Surg. 1999, 17, 149–154. [Google Scholar] [CrossRef][Green Version]
- Greulich, T.; Nell, C.; Hohmann, D.; Grebe, M.; Janciauskiene, S.; Koczulla, A.R.; Vogelmeier, C.F. The prevalence of diagnosed alpha1-antitrypsin deficiency and its comorbidities: Results from a large population-based database. Eur. Respir. J. 2017, 49, 1600154. [Google Scholar] [CrossRef]
- Nakanishi, T.; Forgetta, V.; Handa, T.; Hirai, T.; Mooser, V.; Lathrop, G.M.; Cookson, W.; Richards, J.B. The undiagnosed disease burden associated with alpha-1 antitrypsin deficiency genotypes. Eur. Respir. J. 2020, 56, 2001441. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pertenson, J.; Welch, V.; Losos, M.; Tugwell, P.; Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 June 2023).
- Miettinen, O.S. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am. J. Epidemiol. 1974, 99, 325–332. [Google Scholar] [CrossRef] [PubMed]
- IntHout, J.; Ioannidis, J.P.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Johnston, R.; Jones, K.; Manley, D. Confounding and collinearity in regression analysis: A cautionary tale and an alternative procedure, illustrated by studies of British voting behaviour. Qual. Quant. 2018, 52, 1957–1976. [Google Scholar] [CrossRef]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha(1)-Antitrypsin Deficiency. N. Engl. J. Med. 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- Brantly, M.; Campos, M.; Davis, A.M.; D’Armiento, J.; Goodman, K.; Hanna, K.; O’Day, M.; Queenan, J.; Sandhaus, R.; Stoller, J.; et al. Detection of alpha-1 antitrypsin deficiency: The past, present and future. Orphanet J. Rare Dis. 2020, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Duque, B.; Banuls, L.; Reinoso-Arija, R.; Carrasco-Hernandez, L.; Caballero-Eraso, C.; Dasi, F.; Lopez-Campos, J.L. Methodologies for the Determination of Blood Alpha1 Antitrypsin Levels: A Systematic Review. J. Clin. Med. 2021, 10, 5132. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; McAllister, S.L.; Teckman, J.H. Alpha-1 antitrypsin deficiency liver disease. Transl. Gastroenterol. Hepatol. 2021, 6, 23. [Google Scholar] [CrossRef]
- Topic, A.; Prokic, D.; Stankovic, I. Alpha-1-antitrypsin deficiency in early childhood. Fetal Pediatr. Pathol. 2011, 30, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Stakisaitis, D.; Basys, V.; Benetis, R. Does alpha-1-proteinase inhibitor play a protective role in coronary atherosclerosis? Med. Sci. Monit. 2001, 7, 701–711. [Google Scholar] [PubMed]
- Engstrom, G.; Stavenow, L.; Hedblad, B.; Lind, P.; Tyden, P.; Janzon, L.; Lindgarde, F. Inflammation-sensitive plasma proteins and incidence of myocardial infarction in men with low cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2247–2251. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, L.; Xu, Q.; Yuan, H.; Ba, L.; He, Y.; Che, H. Cytoprotective Role of Alpha-1 Antitrypsin in Vascular Endothelial Cell Under Hypoxia/Reoxygenation Condition. J. Cardiovasc. Pharmacol. 2015, 66, 96–107. [Google Scholar] [CrossRef]
- Toldo, S.; Mauro, A.G.; Marchetti, C.; Rose, S.W.; Mezzaroma, E.; Van Tassell, B.W.; Kim, S.; Dinarello, C.A.; Abbate, A. Recombinant Human Alpha-1 Antitrypsin-Fc Fusion Protein Reduces Mouse Myocardial Inflammatory Injury After Ischemia-Reperfusion Independent of Elastase Inhibition. J. Cardiovasc. Pharmacol. 2016, 68, 27–32. [Google Scholar] [CrossRef]
- Boomsma, D.I.; Orbeleke, J.F.; Martin, N.G.; Frants, R.R.; Clark, P. Alpha-1-antitrypsin and blood pressure. Lancet 1991, 337, 1547. [Google Scholar] [CrossRef]
- Huggard, P.R.; West, M.J.; Summers, K.M. Alpha 1-antitrypsin deficiency alleles and blood pressure in an Australian population. Clin. Exp. Pharmacol. Physiol. 1996, 23, 600–601. [Google Scholar] [CrossRef]
- Duckers, J.M.; Shale, D.J.; Stockley, R.A.; Gale, N.S.; Evans, B.A.; Cockcroft, J.R.; Bolton, C.E. Cardiovascular and musculskeletal co-morbidities in patients with alpha 1 antitrypsin deficiency. Respir. Res. 2010, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, A.R.; Piitulainen, E.; Sonesson, B.; Lanne, T. Changes in aortic wall stiffness in men with alpha 1-antitrypsin deficiency. Eur. J. Vasc. Endovasc. Surg. 1997, 14, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Corda, L.; Vizzardi, E.; De Cicco, G.; D’Aloia, A.; Farina, D.; Morone, M.; Faggiano, P.; Gelsomino, S.; Lorusso, R. Left ventricular pseudoaneurysm and alpha1-antitrypsin enzyme deficiency: Another pathological correlation. Int. J. Cardiol. 2010, 145, 384–386. [Google Scholar] [CrossRef]
- Vizzardi, E.; Corda, L.; Sciatti, E.; Roca, E.; Redolfi, S.; Arici, M.; Pini, L.; Bonadei, I.; Metra, M.; Tantucci, C. Echocardiographic evaluation in subjects with alpha1-Antitrypsin deficiency. Eur. J. Clin. Investig. 2015, 45, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Schachner, T.; Golderer, G.; Sarg, B.; Lindner, H.H.; Bonaros, N.; Mikuz, G.; Laufer, G.; Werner, E.R. The amounts of alpha 1 antitrypsin protein are reduced in the vascular wall of the acutely dissected human ascending aorta. Eur. J. Cardiothorac. Surg. 2010, 37, 684–690. [Google Scholar] [CrossRef]
- Pini, L.; Peroni, M.; Zanotti, C.; Pini, A.; Bossoni, E.; Giordani, J.; Bargagli, E.; Perger, E.; Ferrarotti, I.; Vizzardi, E.; et al. Investigating the Link between Alpha-1 Antitrypsin Deficiency and Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2021, 77, 195–201. [Google Scholar] [CrossRef]
- Schievink, W.I.; Katzmann, J.A.; Piepgras, D.G.; Schaid, D.J. Alpha-1-antitrypsin phenotypes among patients with intracranial aneurysms. J. Neurosurg. 1996, 84, 781–784. [Google Scholar] [CrossRef]
- Martin Davila, F.; Delgado Portela, M.; Garcia Rojo, M.; Gonzalez Garcia, J.; Puig Rullan, A.M.; Lopez Perez, R.; Carbajo Vicente, M. Coronary artery dissection in alpha-1-antitrypsin deficiency. Histopathology 1999, 34, 376–378. [Google Scholar] [CrossRef]
- McElvaney, O.F.; Murphy, M.P.; Reeves, E.P.; McElvaney, N.G. Anti-cytokines as a Strategy in Alpha-1 Antitrypsin Deficiency. Chronic Obstr. Pulm. Dis. 2020, 7, 203–213. [Google Scholar] [CrossRef]
- Kenn, K.; Gloeckl, R.; Soennichsen, A.; Sczepanski, B.; Winterkamp, S.; Boensch, M.; Welte, T. Predictors of success for pulmonary rehabilitation in patients awaiting lung transplantation. Transplantation 2015, 99, 1072–1077. [Google Scholar] [CrossRef]
- Spruit, M.A.; Pitta, F.; McAuley, E.; ZuWallack, R.L.; Nici, L. Pulmonary Rehabilitation and Physical Activity in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Dowson, L.J.; Newall, C.; Guest, P.J.; Hill, S.L.; Stockley, R.A. Exercise capacity predicts health status in alpha(1)-antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 2001, 163, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Jarosch, I.; Hitzl, W.; Koczulla, A.R.; Wencker, M.; Welte, T.; Gloeckl, R.; Janciauskiene, S.; Kenn, K. Comparison of exercise training responses in COPD patients with and without Alpha-1 antitrypsin deficiency. Respir. Med. 2017, 130, 98–101. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Study | Subjects (n) | Males n (%) | Age (Years) | BMI (kg/m2) | Diabetes n (%) | Hypertension n (%) | Dyslipidemia n (%) | Smokers n (%) | Ex-smokers n (%) | COPD n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dahl 2003 [17] | 546 AATD | - | - | - | - | - | - | - | - | - |
| 9064 Controls | - | - | - | - | - | - | - | - | - | |
| Elzouki 1999 [29] | 6 AATD | 6 (100) | 74.0 | - | 0 | 4 (66.7) | - | - | - | - |
| 74 Controls | 74 (100) | 72.0 | - | 4 (5.4) | 29 (39.2) | - | - | - | - | |
| Fähndrich 2017 * [27] | 139 AATD | 77 (55.4) | 59.8 | 24.6 | 5 (3.6) | 57 (41.0) | - | - | - | 139 (100) |
| 2506 Controls | 1495 (59.7) | 65.3 | 27.1 | 356 (14.2) | 1428 (57.0) | - | - | - | 2506 (100) | |
| Greulich 2017 [30] | 590 AATD | 298 (50.5) | 61.0 | - | 161 (27.3) | 374 (63.4) | 44 (31.6) | - | - | 189 (32.0) |
| 5900 Controls | 2980 (50.5) | 61.1 | - | 1558 (26.4) | 3863 (65.5) | 987 (39.4) | - | - | 5900 (100) | |
| Nakanishi 2020 [31] | 19,003 AATD | 8650 (45.5) | 57.9 | 27.3 | - | - | - | 1801 (9.5) | 6607 (34.8) | 789 (4.1) |
| 398,424 Controls | 182,344 (45.8) | 58.0 | 27.4 | - | - | - | 41,735 (10.5) | 21,525 (53.3) | 15,502 (3.9) | |
| Tanash 2020 [26] | 1545 AATD | 768 (50.0) | 47.0 | - | 30 (1.9) | 63 (4.1) | 24 (1.5) | 130 (8.4) | 713 (46.1) | 711 (46.0) |
| 5883 Controls | 2977 (50.0) | 45.0 | - | 95 (1.6) | 184 (3.1) | 52 (0.9) | 1528 (26.0) | 3115 (52.9) | 219 (3.7) | |
| Winther 2022 ** [18] | 392 AATD | 173 (44.1) | 55.8 | 26.2 | - | 86 (21.9) § | - | 43 (11.0) | 157 (40.0) | 125 (31.9) |
| 91,148 Controls | 40,942 (44.9) | 57.8 | 26.2 | - | 17,898 (19.6) § | - | 15,861 (18.1) | 35,533 (39.0) | 5137 (5.6) | |
| Winther 2023 [28] | 2209 AATD | 1154 (52.2) | 44.8 | - | - | 408 (18.5) | 1167 (52.8) | |||
| 21,869 Controls | 11426 (52.2) | 44.7 | - | - | 3900 (17.8) | 1500 (6.8) |
| Study | Subjects (n) | PiZZ n (%) | PiSZ n (%) | PiMZ n (%) | PiSS n (%) | AT n (%) |
|---|---|---|---|---|---|---|
| Dahl 2003 [17] | 546 AATD | 8 (1.5) | 12 (2.2) | 514 (94.1) | 12 (2.2) | 0 |
| Elzouki 1999 [29] | 6 AATD | 0 | 0 | 6 (100) | 0 | 0 |
| Fähndrich 2017 * [27] | 139 AATD | 106 (76.3) | 2 (1.4) | - | - | 110 (79.1) |
| Greulich 2017 [30] | 590 AATD | - | - | - | - | - |
| Nakanishi 2020 [31] | 19,003 AATD | 140 (0.7) | 867 (4.6) | 16,983 (89.4) | 1013 (5.3) | 0 |
| Tanash 2020 [26] | 1545 AATD | 1545 (100) | 0 | 0 | 0 | 0 |
| Winther 2022 ** [18] | 392 AATD | 185 (47.2) | 207 (52.8) | 0 | 0 | 0 |
| Winther 2023 *** [28] | 2209 AATD | 876 (88.1) | 118 (11.9) | 0 | 0 | 0 |
| N. of Studies | N. of Datasets | N. of Patients | Effect Size | |
|---|---|---|---|---|
| SENSITIVITY ANALYSES | A. “Better quality” studies | |||
| 6 | 6 | 23,283 AATD 532,068 controls | OR: 0.826 (95%CI: 0.756–0.903); p < 0.001 I2 = 40.6%; p = 0.135 | |
| B. Exclusion of studies without AATD genotyping | ||||
| 6 | 6 | 22,080 AATD 510,279 controls | OR: 0.807 (95%CI: 0.723–0.901); p < 0.001 I2 = 46.2%; p = 0.098 | |
| C. Exclusion of potentially duplicate populations | ||||
| 7 | 7 | 24,289 AATD 532,148 controls | OR: 0.825 (95%CI: 0.749–0.908); p < 0.001 I2 = 43.6%; p = 0.100 | |
| SUBGROUP ANALYSES | D. Prospective | |||
| 4 | 4 | 4483 AATD 36,158 controls | OR: 0.641 (95%CI: 0.421, 0.977); p = 0.039 I2 = 88.4%; p < 0.0001 | |
| E. Retrospective | ||||
| 4 | 4 | 20,145 AATD 413,462 controls | OR: 0.831 (95%CI: 0.799, 0.865); p < 0.001 I2 = 0%; p = 0.430 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosino, P.; Marcuccio, G.; Lombardi, C.; D’Anna, S.E.; Sanduzzi Zamparelli, S.; Mancusi, C.; Spedicato, G.A.; Motta, A.; Maniscalco, M. Cardiovascular Risk Associated with Alpha-1 Antitrypsin Deficiency (AATD) Genotypes: A Meta-Analysis with Meta-Regressions. J. Clin. Med. 2023, 12, 6490. https://doi.org/10.3390/jcm12206490
Ambrosino P, Marcuccio G, Lombardi C, D’Anna SE, Sanduzzi Zamparelli S, Mancusi C, Spedicato GA, Motta A, Maniscalco M. Cardiovascular Risk Associated with Alpha-1 Antitrypsin Deficiency (AATD) Genotypes: A Meta-Analysis with Meta-Regressions. Journal of Clinical Medicine. 2023; 12(20):6490. https://doi.org/10.3390/jcm12206490
Chicago/Turabian StyleAmbrosino, Pasquale, Giuseppina Marcuccio, Carmen Lombardi, Silvestro Ennio D’Anna, Stefano Sanduzzi Zamparelli, Costantino Mancusi, Giorgio Alfredo Spedicato, Andrea Motta, and Mauro Maniscalco. 2023. "Cardiovascular Risk Associated with Alpha-1 Antitrypsin Deficiency (AATD) Genotypes: A Meta-Analysis with Meta-Regressions" Journal of Clinical Medicine 12, no. 20: 6490. https://doi.org/10.3390/jcm12206490
APA StyleAmbrosino, P., Marcuccio, G., Lombardi, C., D’Anna, S. E., Sanduzzi Zamparelli, S., Mancusi, C., Spedicato, G. A., Motta, A., & Maniscalco, M. (2023). Cardiovascular Risk Associated with Alpha-1 Antitrypsin Deficiency (AATD) Genotypes: A Meta-Analysis with Meta-Regressions. Journal of Clinical Medicine, 12(20), 6490. https://doi.org/10.3390/jcm12206490









