The Renal Composite Benefit of Sodium Glucose Co-Transporter 2 Inhibitors Should Ideally Be Assessed Based on a Standardised Definition: A Meta-Analysis of Randomised Controlled Trials
Abstract
:1. Introduction
- P (patient population) = patients diagnosed with T2D.
- I (intervention) = received drugs belonging to the SGLT-2i group.
- C (control group) = compared to placebo.
- O (outcome) = beneficial effects of SGLT-2is on renal composite outcomes compared with placebo irrespective of the definitions used, followed by the reanalysis of the data using the different definitions used to describe the eGFR decline aspect of the renal composite outcomes.
2. Materials and Methods
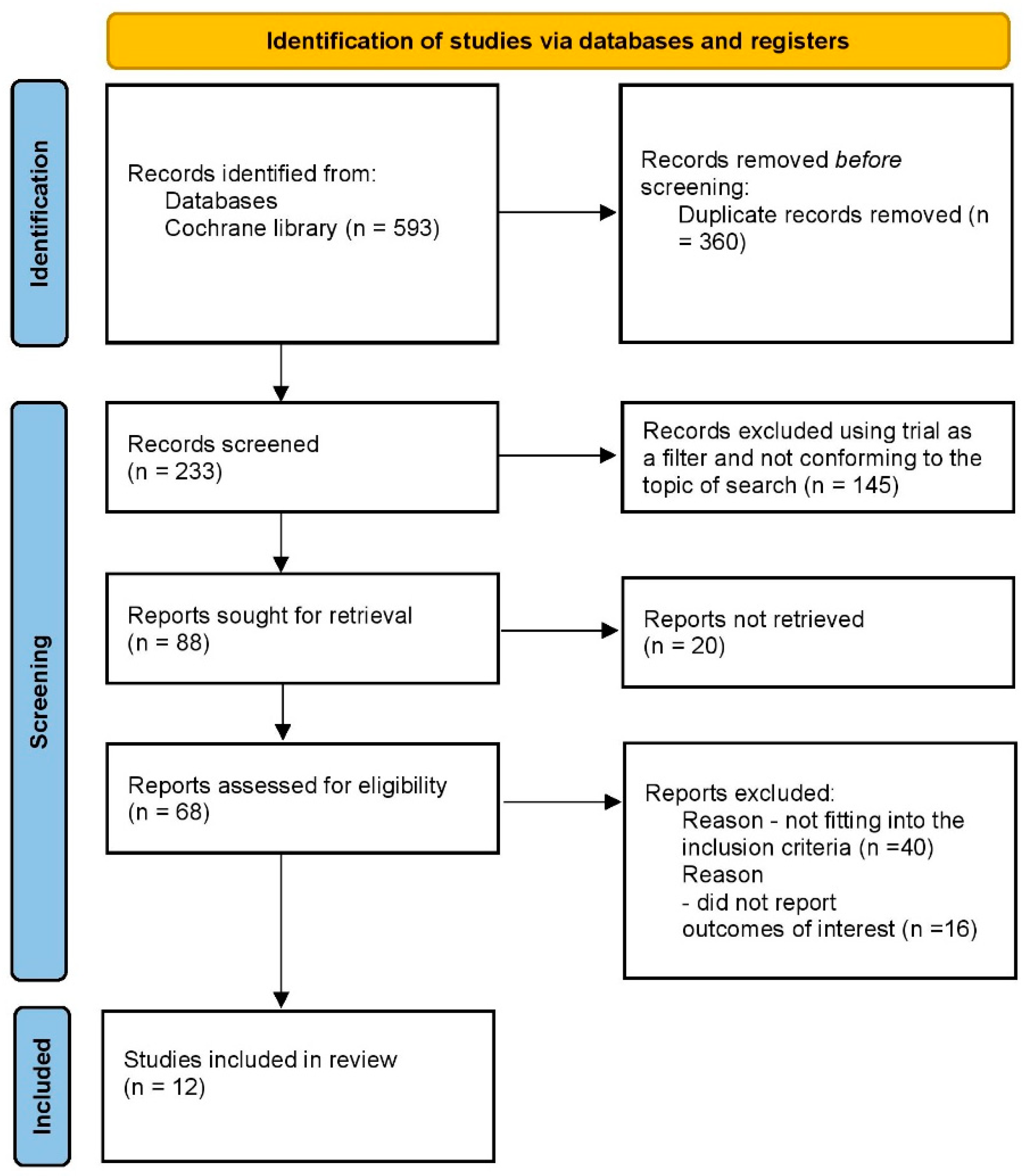
2.1. Search Strategy and Eligibility Criteria
2.2. Study Selection and Eligibility Criteria
- T2D.
- Age: more than or equal to 18 years.
- Comparator arm: placebo.
- Clear mention of the renal composite outcomes, including the individual components in the selected citations.
- Non-T2D patients (type 1 diabetes, gestational diabetes, type 3c diabetes, etc.).
- Acute or decompensated disorder pertaining to any organ.
- Non-RCTs.
- Less than eighteen years of age.
- The comparator arm including agents capable of altering the primary outcome.
2.3. Data Extraction
2.4. Risk of Bias
2.5. Statistical Analysis
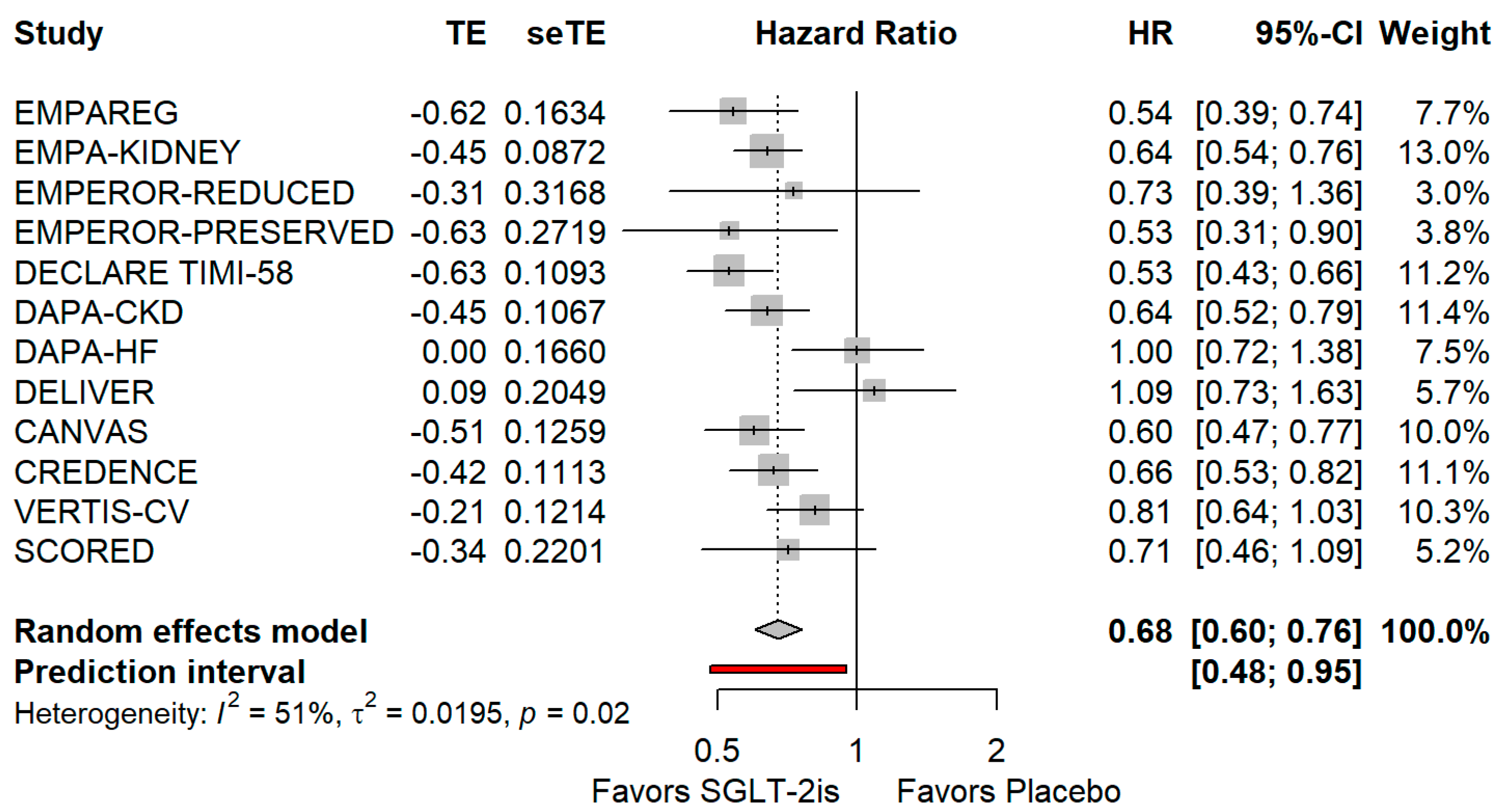
3. Results
3.1. Baseline Characteristics and Risk of Bias
3.2. Renal Composite Outcomes Irrespective of Baseline Definition
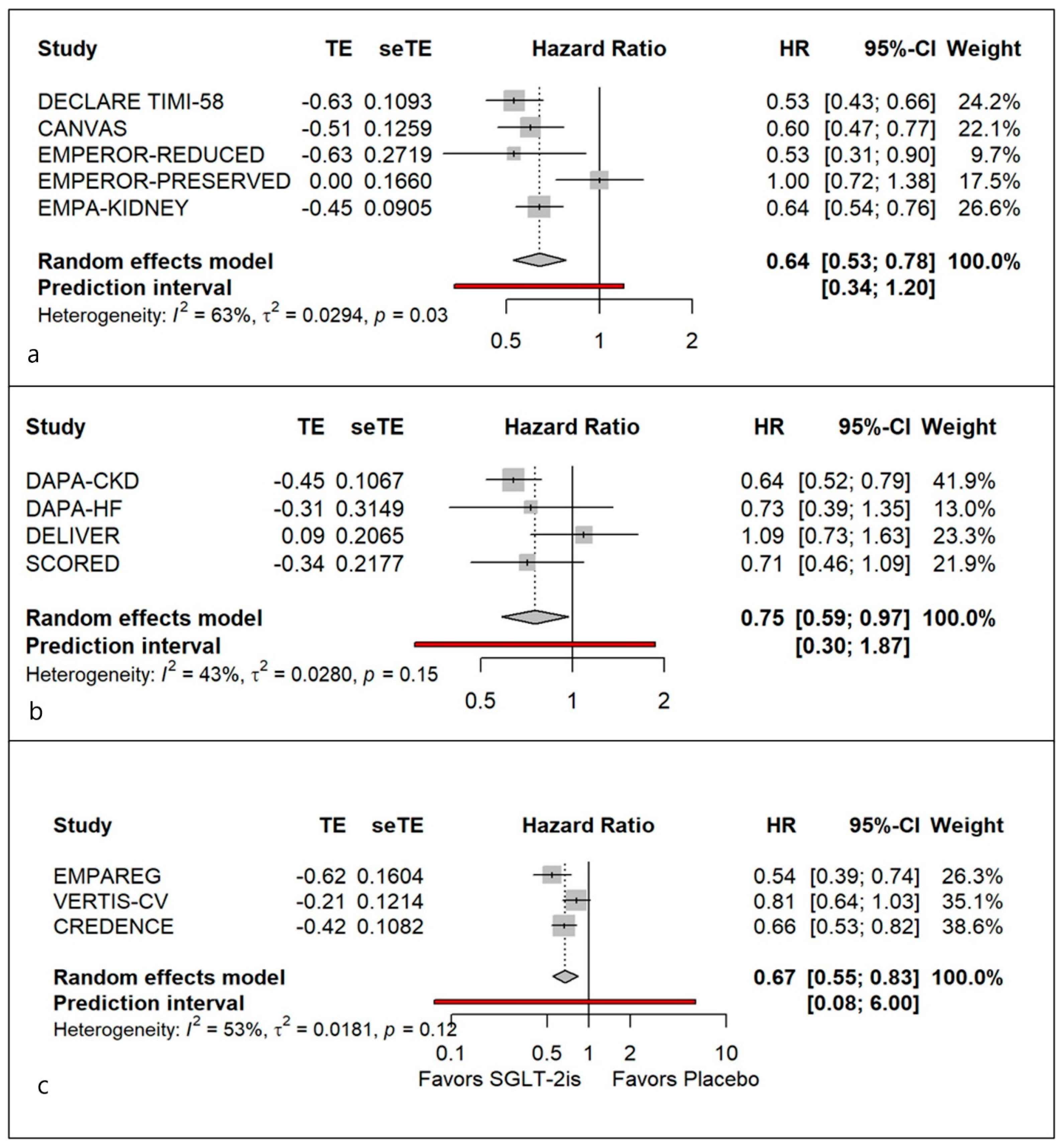
3.3. Uniform Definition 1: Preventing ≥ 40% GFR Decline in eGFR
3.4. Uniform Definition 2: Preventing a ≥ 50% GFR Decline in eGFR
3.5. Uniform Definition 3: Preventing Doubling of Serum Creatinine
4. Discussion
4.1. Literature Review
4.2. Findings from This Meta-Analysis
4.3. Limitations and Strengths
4.4. Research Recommendations
- It is necessary to standardize the definition of renal composite end points.
- To develop a standardized definition of renal composite benefits of SGLT-2is in T2D patients, more data need to be accumulated with a uniform definition.
- In view of the heterogeneity detected (leading to a lack of generalizability) with all three definitions of eGFR decline, additional studies are required to identify clinical and biochemical attributes that can be used to identify responders and non-responders to SGLT-2is.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yau, K.; Dharia, A.; Alrowiyti, I.; Cherney, D.Z.I. Prescribing SGLT2 Inhibitors in Patients with CKD: Expanding Indications and Practical Considerations. Kidney Int. Rep. 2022, 7, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Wissing, K.M.; Scheen, A.J. Sodium-glucose cotransporter 2 inhibitors: Renal outcomes according to baseline albuminuria. Clin. Kidney J. 2021, 14, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.M.; Persson, F.; McGill, J.B.; Rossing, P. Clinical implications and guidelines for CKD in type 2 diabetes. Nephrol. Dial. Transplant. 2023, 38, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, D.; Inzucchi, S.E.; Lachin, J.M.; Wanner, C.; van de Borne, P.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; George, J.T.; et al. Cardiovascular Mortality Reduction with Empagliflozin in Patients with Type 2 Diabetes and Cardiovascular Disease. J. Am. Coll. Cardiol. 2018, 71, 364–366. [Google Scholar] [CrossRef]
- Kaul, S. Is the Mortality Benefit with Empagliflozin in Type 2 Diabetes Mellitus Too Good to Be True? Circulation 2016, 134, 94–96. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ghosal, S.; Ghosal, A.; Ghosal, S. The Renal Composite Benefits of Sodium Glucose Co-Transporter 2 Inhibitor Must Be Assessed Based on a Standardised Defi-nition: A Meta-Analysis of Randomised Controlled Trial. Prospero 2023 CRD42023435521. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023435521 (accessed on 2 September 2023).
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Biomath, D.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S140–S157. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Longo, M.; Scappaticcio, L.; Bellastella, G.; Maiorino, M.I.; Esposito, K. SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: A meta-analysis of 11 CVOTs. Cardiovasc. Diabetol. 2021, 20, 236. [Google Scholar] [CrossRef]
- The Nuffield Department of Population Health Renal Studies Group; the SGLT2 Inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Higgins, J.P.; Hedges, L.V.; Rothstein, H.R. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res. Synth. Methods 2017, 8, 5–18. [Google Scholar] [CrossRef] [PubMed]
| Study | Year | Intervention | Intervention/Placebo (n) | Mean Age | Renal Composite | Baseline eGFR (mL/min) |
|---|---|---|---|---|---|---|
| EMPA KIDNEY [8] | 2022 | Empagliflozin | 1525/1515 | 63.9 ± 13.9 | End-stage kidney disease, a sustained estimated glomerular filtration rate (eGFR) < 10 mL/min/1.73 m2, renal death, or a sustained decline of ≥40% in eGFR from randomisation. | 37.4 ± 14.5 (mean) |
| EMPAREG [9] | 2015 | Empagliflozin | 4645/2323 | 63.0 ± 8.6 | Doubling of serum creatinine, initiation of renal replacement therapy, or death due to renal disease | ≥60 (74.1%), 45 to <60 (17.8%), <45 (8.1%) |
| EMPEROR PRESERVED [10] | 2021 | Empagliflozin | 1466/1472 | 70.9 ± 9.0 | Chronic dialysis, renal transplantation, sustained reduction of ≥40% in estimated GFR | 59.7 ± 20.7 (Mean) |
| EMPEROR REDUCED [11] | 2022 | Empagliflozin | 927/929 | 67.6 ± 11.6 | Chronic dialysis, kidney transplant, sustained reduction of ≥40% eGFR, or sustained eGFR < 15 mL/min/1.73 m2 if eGFR was >30 mL/min/1.73 m2 or <10 mL/min/1.73 m2 for patients with baseline eGFR ≤ 30 mL/min/1.73 m2 | 62.7 ± 21.1 (Mean) |
| DECLARE TIMI-58 [12] | 2019 | Dapagliflozin | 8582/8578 | 63.9 + 6.8 | A 40% decrease in eGFR, ESRD, or renal death | 85.4 ± 15.8 |
| DAPA-HF [13] | 2019 | Dapagliflozin | 2139/2139 | 66.2 + 11.0 | A reduction of 50% or more in the estimated GFR sustained for at least 28 days, end-stage renal disease, or death from renal causes. | 66.0 ± 19.6 (Mean) |
| DELIVER [14] | 2022 | Dapagliflozin | 3131/3131 | 71.8 ± 9.6 | Composite of decline in estimated GFR of ≥50%, end-stage kidney disease, or death from renal causes | 61 ± 19 (Mean) |
| DAPA-CKD [15] | 2020 | Dapagliflozin | 1455/1451 | 61.8 ± 12.1 | Composite of decline in estimated GFR of ≥50%, end-stage kidney disease, or death from renal causes | 43.2 ± 12.3 (Mean) |
| CANVAS [16] | 2017 | Canagliflozin | 5795/4347 | 63.2 + 8 3 | A 40% decrease in eGFR, renal death or renal replacement therapy requirement | 76.7 ± 20.3 (Mean) |
| CREDENCE [17] | 2019 | Canagliflozin | 2202/2199 | 62.9 ± 9.2 | End stage kidney disease, doubling of serum creatinine, renal death | 56.3 ± 18.2 (Mean) |
| VERTIS-CV [18] | 2021 | Ertugliflozin | 5499/2747 | 64.4 ± 8.1 | Doubling creatinine, ESKD, renal death | 76.1 ± 20.9 (Mean) |
| SCORED [19] | 2021 | Sotagliflozin | 5292/5292 | 69 (63–74) [Median–IQR] | A ≥50% sustained decline eGFR or end-stage renal disease or renal death | 44.4 (37.0–51.3) [Median–IQR] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosal, S.; Ghosal, S.; Ghosal, A. The Renal Composite Benefit of Sodium Glucose Co-Transporter 2 Inhibitors Should Ideally Be Assessed Based on a Standardised Definition: A Meta-Analysis of Randomised Controlled Trials. J. Clin. Med. 2023, 12, 6462. https://doi.org/10.3390/jcm12206462
Ghosal S, Ghosal S, Ghosal A. The Renal Composite Benefit of Sodium Glucose Co-Transporter 2 Inhibitors Should Ideally Be Assessed Based on a Standardised Definition: A Meta-Analysis of Randomised Controlled Trials. Journal of Clinical Medicine. 2023; 12(20):6462. https://doi.org/10.3390/jcm12206462
Chicago/Turabian StyleGhosal, Samit, Shamita Ghosal, and Anuradha Ghosal. 2023. "The Renal Composite Benefit of Sodium Glucose Co-Transporter 2 Inhibitors Should Ideally Be Assessed Based on a Standardised Definition: A Meta-Analysis of Randomised Controlled Trials" Journal of Clinical Medicine 12, no. 20: 6462. https://doi.org/10.3390/jcm12206462
APA StyleGhosal, S., Ghosal, S., & Ghosal, A. (2023). The Renal Composite Benefit of Sodium Glucose Co-Transporter 2 Inhibitors Should Ideally Be Assessed Based on a Standardised Definition: A Meta-Analysis of Randomised Controlled Trials. Journal of Clinical Medicine, 12(20), 6462. https://doi.org/10.3390/jcm12206462






