Abstract
Introduction: Surgical site infection (SSI) is a frequently reported complication after ankle fracture surgery. To our knowledge, no study has been conducted on its incidence in Sweden. The present study aimed to determine the incidence of, risk factors for, and most common causative pathogen of SSI. Methods: Patients who underwent primary surgery for an ankle fracture between 1 September 2017 and 31 August 2019 at the Sahlgrenska University Hospital were identified. Data on potential SSI risk factors and clinical outcome (infected/non-infected) were retrieved from medical records. Cox regression analysis and descriptive statistics were used. Results: Of the 480 reviewed patients, 49 developed SSI (10.2%), of which 35 (7.3%) were superficial and 14 (2.9%) were deep. Open fractures (p < 0.001) and age (p = 0.016) were statistically significant risk factors for SSI in the univariate analysis. In the multivariable analysis, only open fracture was statistically significant (HR = 3.0; 95% C.I. = 1.3–6.9, p = 0.013). Cases of Staphylococcus aureus (S. aureus) were most common (n = 12, 24.5%). Methicillin resistance was uncommon (n = 2, 4.1%). Conclusions: An incidence of 10.2% was established, which is comparable to international findings. Infection monitoring is an important part of tackling the global challenge of antibiotic resistance. Future prospective studies to further establish risk factors are warranted to decrease the incidence of SSI.
1. Introduction
Ankle fractures are the third most common type of fracture in Sweden, with an incidence of 127 per 100,000 person-years [1]. The Sahlgrenska University Hospital (SU) alone treats approximately 700 ankle fractures annually [2]. One of the most common complications after ankle fracture surgery is surgical site infection (SSI) [3], with an incidence of between 1 and 28% [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. The increase of antibiotic resistance and its recognition as one of the greatest threats to global health [19] validates the importance of SSI research. Within ankle fracture surgery alone, methicillin-resistant Staphylococcus aureus (MRSA) causes 5.8–44% of SSIs [8,14,15,16,18], warranting the need for both infection monitoring and new strategies for infection prevention.
SSI is associated with increased morbidity and mortality, and high healthcare-related costs. The costs for a patient with a SSI can be up to double that of a non-infected patient [20]. Furthermore, SSIs have negative psychological, social and economic effects on patients due to long-lasting pain, isolation and insecurity [21], leading to a significant reduction in their quality of life [22]. A cornerstone of infection prevention is patient selection, i.e., assessing the risk of whether a patient may develop an infection. However, depending on the severity of the fracture, conservative treatment is sometimes not an option, or not sufficient. There are a number of suggested risk factors for SSI to be considered. For ankle fracture surgery, advanced age [4,5,9,16,23]; diabetes mellitus [4,6,11,12,23]; smoking tobacco [9,10,12,14,17]; ASA (American Society of Anaesthesiologists Physical Status) class 3–4 [4,11,23]; the presence of an open fracture [9,11,16]; and obesity [4,12,15,16] have been highlighted as patient-related risk factors. Moreover, as SSIs are multifactorial, other non-patient-specific factors need consideration, such as surgical details in the form of prolonged duration of surgery [9,10,15,24]; delay of surgery [13,25,26]; and the surgeons’ level of experience [15]. Patient selection and optimisation of surgery can be difficult, yet it is of great importance to increase the knowledge of which factors should be considered to improve infection prevention.
To the authors’ knowledge, no study on the incidence and risk factors for SSI after ankle fracture surgery has been conducted in Sweden. The aim of this study was to investigate the incidence of SSI after ankle fracture surgery. A secondary aim was to identify risk factors for SSI following ankle fracture surgery and to determine the most common pathogen causing these infections. The hypothesis was that the incidence of SSI after ankle facture surgery in Sweden is 1–28%.
2. Materials and Methods
This is a retrospective observational study conducted at SU in Gothenburg, Sweden. All patients ≥18 years of age who were diagnosed with an ankle fracture between 1 September 2017 and 31 August 2019 and underwent primary surgery were identified using the Swedish Fracture Register (SFR). Only patients with a Swedish personal identity number who experienced a fracture in Sweden are registered in the SFR.
The SFR is a Swedish national quality register started in 2011 [27]. Data, such as fracture classification, injury date and treatment details, are manually registered in SFR by treating physicians. When controlled against the National Patient Register in Sweden, the SFR has a completeness of approximately 70–90% for most of the orthopaedic departments [28].
Data on patient-, injury- and surgery-related variables were collected by reviewing medical records and the local surgery database at the hospital. Data on microbiology were retrieved from microbiological records. Exclusion criteria were <18 years of age, bilateral ankle fractures and residence in a city other than Gothenburg, Sweden. Records were reviewed on 9 September 2021.
All patients were given prophylactic intravenous antibiotics—Cloxacillin 2 g or, if allergic, Clindamycin 600 mg—30 min before surgery according to hospital routine. If an open fracture was present, antibiotic treatment was given at the emergency department. Patients were followed up three weeks post operation for wound status and suture removal, and additionally at six weeks post operation according to local routine. Data on signs of infection, positive microbiological cultures, use of antibiotics and re-operations were retrieved from medical records.
2.1. Definitions
The definition of SSI was assessed though a modified version of Metsemakers et al.’s definition of suggestive criteria for fracture-related infection (FRI) [29]. Infection status (infected/non-infected) was recorded within one year of the index surgery. SSI was defined based on wound status, antibiotic use, microbiological findings and need for reoperation, and further classified as superficial or deep (Table 1). Wound status at follow-up was classified according to the physician’s assessment: normal healing, delayed healing without suspicion of SSI, and SSI. Delayed healing was defined as a wound not healed at the follow-up six weeks post operation. Patients were excluded from the SSI group if the suspicion of infection was discarded at a later follow-up and treatment with antibiotics was ceased. For reoperations, SSI was ruled out if no intraoperative signs of infection were present and antibiotics were discontinued postoperatively.

Table 1.
The definition of surgical site infection used in the current study.
Microbiological culture samples were defined as negative if no bacterial growth was present. Positive samples were categorised as monomicrobial, or polymicrobial if more than one species or strain was found. Cultures showing mixed Gram-positive flora were considered as a separate group due to their low clinical significance.
Surgeon experience was defined as resident, specialist, or consultant level. For analysis, only the main surgeon’s level of experience was included in cases where there was more than one surgeon present during the operation.
2.2. Statistical Analysis
Means with standard deviation (SD) and medians with interquartile range (IQR) were used for descriptive statistics. To compare the non-infected and infected groups, the chi-square or Fisher’s exact test were used for statistical evaluation of categorical variables, and the Mann–Whitney U test was used for continuous variables. A multivariable logistic regression analysis (Cox regression) was performed to further analyse risk factors for SSI. The significance level was set at p-value < 0.05. Statistical analysis was conducted in IBM SPSS version 26.
3. Results
3.1. Study Cohort
During the study period, 494 patients underwent primary surgery for an ankle fracture; of these, 14 patients were excluded from the study (9 patients resided in another city and were lost to follow-up, 4 patients were <18 years old and 1 patient had bilateral ankle fractures). In total, 480 patients were included (Figure 1).
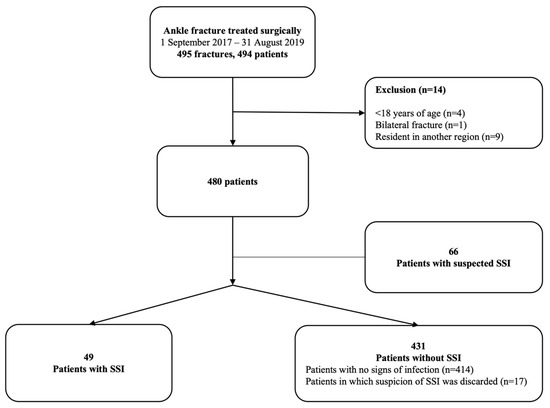
Figure 1.
Flow chart of patient inclusion. Abbreviation: SSI, surgical site infection.
Of the 480 included patients, the median age was 55 years (IQR: 29), BMI was 27.0 (IQR: 6.5) and the majority of patients were female (59.6%). Further characteristics of the study population are presented in Table 2. Within one year of surgery, seven patients died, whereof three experienced a SSI. However, no death was caused by a SSI.

Table 2.
Characteristics of the study population (n = 480).
3.2. Patients with a SSI
Of the 480 patients, 49 (10.2%) developed a SSI. The rate of superficial SSI was 7.3%, and for deep SSI, 2.9%. Of the 449 patients who had closed fractures, 39 (8.7%) developed a SSI; and of the 31 patients with an open fracture, 10 (32.3%) developed a SSI (Table 3).

Table 3.
Infection incidence according to fracture type.
Infected patients were older than non-infected patients (p = 0.016) and had a higher rate of open fractures (p < 0.001) (Table 4). Surgical characteristics did not statistically significantly differ between the two groups, although the infected group had a higher proportion of patients who waited more than 72 h for surgery and the non-infected group were, to a greater extent, operated on in ORs with laminar airflow (LAF).

Table 4.
Characteristics of the infected and non-infected patients with univariate analyses.
The infected group had a longer hospital stay at the initial admission (p = 0.037) and a greater number of follow-ups (p < 0.001) compared to the non-infected group. The median length of antibiotic treatment of the infected patients was 10 days (IQR; 20, range 7–164). Patients who underwent reoperation due to infection had one to four reoperations before infection resolution.
3.3. Microbiological Findings in the Infected Patients
Of the 49 infected cases, 33 (67.3%) were sampled with microbiological counts and 16 (32.7%) had no samples available. Of the 33 sampled cases, 26 (78.8%) were positive for bacterial growth, making the incidence of SSI verified with cultures 5.4%. A total of 23 patients with SSI had either growth of mixed Gram-negative bacteria, no growth, or no samples available. There were 18 monomicrobial infections and 8 polymicrobial infections. S. aureus was the most common monomicrobial pathogen and was identified in 12 patients (24.5%). Two infections (4.1%) were caused by MRSA, whereof one was monomicrobial and one was polymicrobial. Further microbiological characteristics of the infected patients are presented in Table 5.

Table 5.
Microbiological characteristics of infected patients.
3.4. Risk factors for SSI
In the univariate analysis (Table 4), infected patients were older than non-infected patients (p = 0.016) and had a higher rate of open fractures (p = <0.001). However, in the multivariable analysis, only the presence of an open fracture was a statistically significant risk factor, with a threefold greater risk of SSI compared to patients with a closed fracture (HR = 3.0, 95% C.I. = 1.3–6.9, p = 0.013) (Table 6).

Table 6.
Cox regression analysis of risk factors for SSI.
4. Discussion
This retrospective observational study of 480 patients with a surgically treated ankle fracture reveals a 10.2% incidence of SSI. Presence of an open fracture implied a threefold greater risk of SSI. The most common causative pathogen was S. aureus, and there was a low incidence of MRSA (4.1% of the infected patients).
The overall rate of infection (10.2%), with rates of superficial SSI at 7.3% and deep SSI at 2.9%, was comparable with previous findings of overall rates between 1 and 28% [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18], rates of superficial SSI at 2.1–14.0% [8,17] and rates of deep SSI at 0.8–6.8% [8,10]. Differences in incidence rate may depend on the definition of SSI used, study design and inclusion criteria, as well as the country and level of hospital. The infection rate presented in the current study could be an underestimate, as some patients may seek care in the primary health care sector for wound problems that hence remain unknown to us. However, an overestimation may be more likely, due to the broad inclusion criteria of superficial infection, where some of the included cases may be a result of antibiotic overuse. Nonetheless, the incidence of SSI in the current study mirrors the clinical reality of patients treated for suspected SSI at our department.
S. aureus is a common pathogen in orthopaedic infections, which is confirmed in the current study with a rate of 24.5%. Similar incidence rates have been found in studies from both Finland (32.8%) [10] and China (17.8%) [8]. In the current study, the rate of MRSA (n = 2, 4.1%) is low in comparison to existing literature where rates of 5.8–44% have been reported [8,14,15,16,18]. This finding was not surprising though as Sweden, in general, has a low rate of MRSA compared to other countries [30]. Similar studies should be conducted in the future to continue the surveillance of MRSA and other drug-resistant bacteria.
In the current study, open fractures implied a threefold increased risk for SSI. The presence of an open fracture has previously been established as an independent risk factor for SSI [9,11,16,31]. The most obvious explanation may be that the fracture punctures the skin, at which point bacteria can contaminate the tissues. Several other patient-related risk factors have been reported to contribute to SSI risk, such as advanced age; smoking tobacco; diabetes mellitus; male sex; high ASA class (3–4); and obesity [4,5,6,9,10,11,12,14,15,16,17,23]. However, the current study could not confirm this, which may be due to the size of the cohort, but also due to selection bias. Patients with well-known risk factors, such as advanced age, diabetes or smoking, may receive non-surgical treatment instead of surgery to a greater extent, due to a higher risk of infection. This selection might contribute to the idea that that these well-known risk factors do not stand out as risk factors in the analysis of this retrospective study. Interestingly, Schade et al. reported that only insulin-dependent diabetes was a risk factor for SSI, as opposed to non-insulin-dependent diabetes [23]. Similarly, Neuman et al. stated that there was an increased risk of complication postoperatively in posterior malleolus fractures when insulin-dependent diabetes mellitus was present ref. [32]. The current data was not controlled for this possible confounding. No association was found between SSI and surgical characteristics, such as delay of surgery from admission, use of temporary external fixation, duration of surgery, or use of LAF in the OR. Again, this might be due to the size of our study population, as both delay of surgery and duration of surgery have previously been reported as risk factors for SSI [9,10,13,15,24,25,26].
This study has limitations. Firstly, the retrospective design and the reliance on data assessed through medical records is a limitation. Medical records as a data source can compromise data accuracy, due to the lack of a standardised way to document signs of infection. Details of the extent of soft tissue damage and the classifications of open fractures were not stated in the medical records; therefore, we were not able to obtain this type of information. The retrospective design did not allow us to classify the surgical wound according to current definitions of SSI, such as the CDC [33] or FRI classifications [29]. Moreover, it is of great importance to use the available diagnostic criteria of SSI when possible, to reduce the risk of overuse of antibiotics. In 23 cases, the diagnosis of SSI can be questioned as the infection was not verified with cultures, making the incidence of SSI verified with cultures 5.4% (26/480). Notably, in three cases, antibiotics had been started prior to sampling which could have led to false negative results. However, in all included SSI cases, a clinical suspicion of infection was confirmed by a physician in an orthopaedic department, and despite the difficulties of SSI diagnostics, the staff are experienced in this field. The incidence found in the present study reflects the clinical reality of treated SSI. Current SSI definitions, such as the CDC’s [33], have their limitations as the depth of the SSI needs to be evaluated, which requires a reopening of the incision. In fracture surgery, this exposes the fracture and hardware, which risks bacterial contamination. However, to optimise the possibility of SSI classification, a prospective study is warranted. Further, all cases defined as SSI resulted in the use of antibiotics and additional follow-ups, which increase costs, making the chosen definition of SSI clinically meaningful. Patients treated with prolonged antibiotics, who need multiple hospital visits and experience local symptoms, can be assumed to suffer to some extent from this [21], making these cases important to acknowledge.
Compared to larger studies on more than a thousand patients [8,10], the cohort of the current study is rather small; this might be the reason why this study did not reach statistical significance for the tested variables. A rule of thumb for Cox regression is the “one in ten rule” which implies that there is a need for a minimum of ten outcomes per variable [34]. According to this rule, there is a risk of overfit in the present study. However, the “one in ten rule” has been questioned and may be too conservative; instead, five to nine outcomes per variable has been suggested as a valid option [35].
Despite its limitations, the current study was able to gather data from one of Scandinavia’s largest orthopaedic departments. The standardised times for follow-up at the hospital allowed for consistency for all patients regarding wound examination. We also had access to several variables which, combined, could be used to determine the presence of SSI. Furthermore, to the authors’ knowledge, the current study was the first in Sweden to assess the incidence of SSI after surgically treated ankle fractures. Additionally, the causative pathogens and the rate of MRSA, as well as risk factors of SSI, were presented. This knowledge is important for further development of safer surgery and infection prevention.
5. Conclusions
In the present study, surgically treated ankle fractures had a 10.2% risk of SSI. S. aureus was the most common pathogen and the rate of MRSA was, as expected, low compared to international results. Patients with an open fracture had a three times higher risk of SSI, which should be considered when managing these injuries. The current study contributes to infection monitoring, which is a crucial part of the global fight against antibiotic resistance. However, there is still a need for large, prospective multicentre studies to further establish risk factors and thereby identify possible infection preventative measures.
Author Contributions
Conceptualization, K.S.M.; methodology, K.S.M. and J.B.; validation, K.S.M.; formal analysis, J.B. and K.S.M.; investigation, J.B. and K.S.M.; data curation, D.W., E.M.R. and J.B.; writing—original draft preparation, J.B. and K.S.M.; writing—review and editing, J.B., K.S.M., E.M.R. and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Regional Ethical Committee, Gothenburg (Dnr 1011-15).
Informed Consent Statement
Patient consent was waived due to the Swedish legislation that states that no signed consent is needed for each registered patient in National Quality Registers [36]. Upon registration in the SFR, all patients were informed that they have the right to withdraw.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
All doctors and employees who register data in the SFR, thank you for making this study possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bergh, C.; Wennergren, D.; Möller, M.; Brisby, H. Fracture incidence in adults in relation to age and gender: A study of 27,169 fractures in the Swedish Fracture Register in a well-defined catchment area. PLoS ONE 2020, 15, e0244291. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, E.M.; Zorko, T.; Sundfeldt, M.; Möller, M.; Wennergren, D. Classification and treatment of lateral malleolar fractures—A single-center analysis of 439 ankle fractures using the Swedish Fracture Register. BMC Musculoskelet. Disord. 2020, 21, 521. [Google Scholar] [CrossRef] [PubMed]
- SooHoo, N.F.; Krenek, L.; Eagan, M.J.; Gurbani, B.; Ko, C.Y.; Zingmond, D.S. Complication rates following open reduction and internal fixation of ankle fractures. J. Bone Joint Surg. Am. 2009, 91, 1042–1049. [Google Scholar] [CrossRef]
- Olsen, L.L.; Møller, A.M.; Brorson, S.; Hasselager, R.B.; Sort, R. The impact of lifestyle risk factors on the rate of infection after surgery for a fracture of the ankle. Bone Joint J. 2017, 99, 225–230. [Google Scholar] [CrossRef]
- Kelly, E.G.; Cashman, J.P.; Groarke, P.J.; Morris, S.F. Risk factors for surgical site infection following operative ankle fracture fixation. Ir. J. Med. Sci. 2013, 182, 453–456. [Google Scholar] [CrossRef]
- Korim, M.T.; Payne, R.; Bhatia, M. A case-control study of surgical site infection following operative fixation of fractures of the ankle in a large, U.K. trauma unit. Bone Joint J. 2014, 96-b, 636–640. [Google Scholar] [CrossRef]
- Lachman, J.R.; Elkrief, J.I.; Pipitone, P.S.; Haydel, C.L. Comparison of Surgical Site Infections in Ankle Fracture Surgery With or Without the Use of Postoperative Antibiotics. Foot Ankle Int. 2018, 39, 1278–1282. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, Y.; Chen, W.; Li, M.; Liu, S.; Zhang, Y. Multiple preoperative biomarkers are associated with incidence of surgical site infection following surgeries of ankle fractures. Int. Wound J. 2020, 17, 842–850. [Google Scholar] [CrossRef]
- Meng, J.; Sun, T.; Zhang, F.; Qin, S.; Li, Y.; Zhao, H. Deep surgical site infection after ankle fractures treated by open reduction and internal fixation in adults: A retrospective case-control study. Int. Wound J. 2018, 15, 971–977. [Google Scholar] [CrossRef]
- Ovaska, M.T.; Mäkinen, T.J.; Madanat, R.; Huotari, K.; Vahlberg, T.; Hirvensalo, E.; Lindahl, J. Risk factors for deep surgical site infection following operative treatment of ankle fractures. J. Bone Joint Surg. Am. 2013, 95, 348–353. [Google Scholar]
- Rascoe, A.S.; Kavanagh, M.D.; Audet, M.A.; Hu, E.; Vallier, H.A. Factors associating with surgical site infection following operative management of malleolar fractures at an urban level 1 trauma center. OTA Int. 2020, 3, e077. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.G.; Swiggett, S.J.; Pasternack, J.B.; Vakharia, R.M.; Kang, K.K.; Abdelgawad, A. Comparison study of patient demographics and risk factors for surgical site infections following open reduction and internal fixation for lateral malleolar ankle fractures within the medicare population. Foot Ankle Surg. 2021, 27, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Saithna, A.; Moody, W.; Jenkinson, E.; Almazedi, B.; Sargeant, I. The influence of timing of surgery on soft tissue complications in closed ankle fractures. Eur. J. Orthop. Surg. Traumatol. 2009, 19, 481–484. [Google Scholar] [CrossRef]
- Sato, T.; Takegami, Y.; Sugino, T.; Bando, K.; Fujita, T.; Imagama, S. Smoking and trimalleolar fractures are risk factors for infection after open reduction and internal fixation of closed ankle fractures: A multicenter retrospective study of 1201 fractures. Injury 2021, 52, 1959–1963. [Google Scholar] [CrossRef]
- Sun, R.; Li, M.; Wang, X.; Li, X.; Wu, L.; Chen, Z.; Chen, K. Surgical site infection following open reduction and internal fixation of a closed ankle fractures: A retrospective multicenter cohort study. Int. J. Surg. 2017, 48, 86–91. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Tang, Y.; Zhao, H.; Qin, S.; Xu, L.; Xia, Z.; Zhang, F. Incidence and risk factors for surgical site infection after open reduction and internal fixation of ankle fracture: A retrospective multicenter study. Medicine 2018, 97, e9901. [Google Scholar] [CrossRef]
- Thangarajah, T.; Prasad, P.S.; Narayan, B. Surgical site infections following open reduction and internal fixation of ankle fractures. Open Orthop. J. 2009, 3, 56–60. [Google Scholar] [CrossRef]
- Zalavras, C.G.; Christensen, T.; Rigopoulos, N.; Holtom, P.; Patzakis, M.J. Infection following operative treatment of ankle fractures. Clin. Orthop. Relat. Res. 2009, 467, 1715–1720. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Broex, E.C.; van Asselt, A.D.; Bruggeman, C.A.; van Tiel, F.H. Surgical site infections: How high are the costs? J. Hosp. Infect. 2009, 72, 193–201. [Google Scholar] [CrossRef]
- Andersson, A.E.; Bergh, I.; Karlsson, J.; Nilsson, K. Patients’ experiences of acquiring a deep surgical site infection: An interview study. Am. J. Infect. Control. 2010, 38, 711–717. [Google Scholar] [CrossRef]
- Cahill, J.L.; Shadbolt, B.; Scarvell, J.M.; Smith, P.N. Quality of life after infection in total joint replacement. J. Orthop. Surg. 2008, 16, 58–65. [Google Scholar] [CrossRef]
- Schade, M.A.; Hollenbeak, C.S. Early Postoperative Infection Following Open Reduction Internal Fixation Repair of Closed Malleolar Fractures. Foot Ankle Spec. 2018, 11, 335–341. [Google Scholar] [CrossRef]
- Gowd, A.K.; Bohl, D.D.; Hamid, K.S.; Lee, S.; Holmes, G.B.; Lin, J. Longer Operative Time Is Independently Associated With Surgical Site Infection and Wound Dehiscence Following Open Reduction and Internal Fixation of the Ankle. Foot Ankle Spec. 2020, 13, 104–111. [Google Scholar] [CrossRef]
- Carragee, E.J.; Csongradi, J.J.; Bleck, E.E. Early complications in the operative treatment of ankle fractures : Influence of delay before operation. J. Bone Jt. Surg. Br. Vol. 1991, 73, 79–82. [Google Scholar] [CrossRef]
- Schepers, T.; De Vries, M.R.; Van Lieshout, E.M.; Van der Elst, M. The timing of ankle fracture surgery and the effect on infectious complications; a case series and systematic review of the literature. Int. Orthop. 2013, 37, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Wennergren, D.; Ekholm, C.; Sandelin, A.; Möller, M. The Swedish fracture register: 103,000 fractures registered. BMC Musculoskelet. Disord. 2015, 16, 338. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Wolf, O.; Bergdahl, C.; Mukka, S.; Rydberg, E.M.; Hailer, N.P.; Ekelund, J.; Wennergren, D. The Swedish Fracture Register—Ten years of experience and 600,000 fractures collected in a National Quality Register. BMC Musculoskelet. Disord. 2022, 23, 141. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.J.; Morgenstern, M.; McNally, M.A.; Moriarty, T.F.; McFadyen, I.; Scarborough, M.; Athanasou, N.A.; Ochsner, P.E.; Kuehl, R.; Raschke, M.; et al. Fracture-related infection: A consensus on definition from an international expert group. Injury 2018, 49, 505–510. [Google Scholar] [CrossRef] [PubMed]
- ECDC. European Centre for Disease Prevention and Control (ECDC)/European Medicines Agency (EMEA) Joint Technical report: The Bacterial Challenge: Time to React; ECDC: Solna, Stockholm, 2009. [Google Scholar]
- Shao, J.; Zhang, H.; Yin, B.; Li, J.; Zhu, Y.; Zhang, Y. Risk factors for surgical site infection following operative treatment of ankle fractures: A systematic review and meta-analysis. Int. J. Surg. 2018, 56, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.P.; Kroker, L.; Beyer, F.; Rammelt, S. Complications following surgical treatment of posterior malleolar fractures: An analysis of 300 cases. Arch. Orthop. Trauma. Surg. 2023, 143, 3129–3136. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 1999, 27, 97–132, quiz 3–4, discussion 96. [Google Scholar] [CrossRef] [PubMed]
- Concato, J.; Peduzzi, P.; Holford, T.R.; Feinstein, A.R. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J. Clin. Epidemiol. 1995, 48, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef] [PubMed]
- SFS 2008:355 Patient Data Act (Swe). Sect. Chapter 7. Available online: https://www.riksdagen.se/sv/dokument-och-lagar/dokument/svensk-forfattningssamling/patientdatalag-2008355_sfs-2008-355/ (accessed on 8 October 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).