The Effect of Cataract Surgery on the Risk of Dementia: A Nationwide Cohort Study
Abstract
:1. Introduction
2. Method and Material
2.1. Study Setting and Source of Data
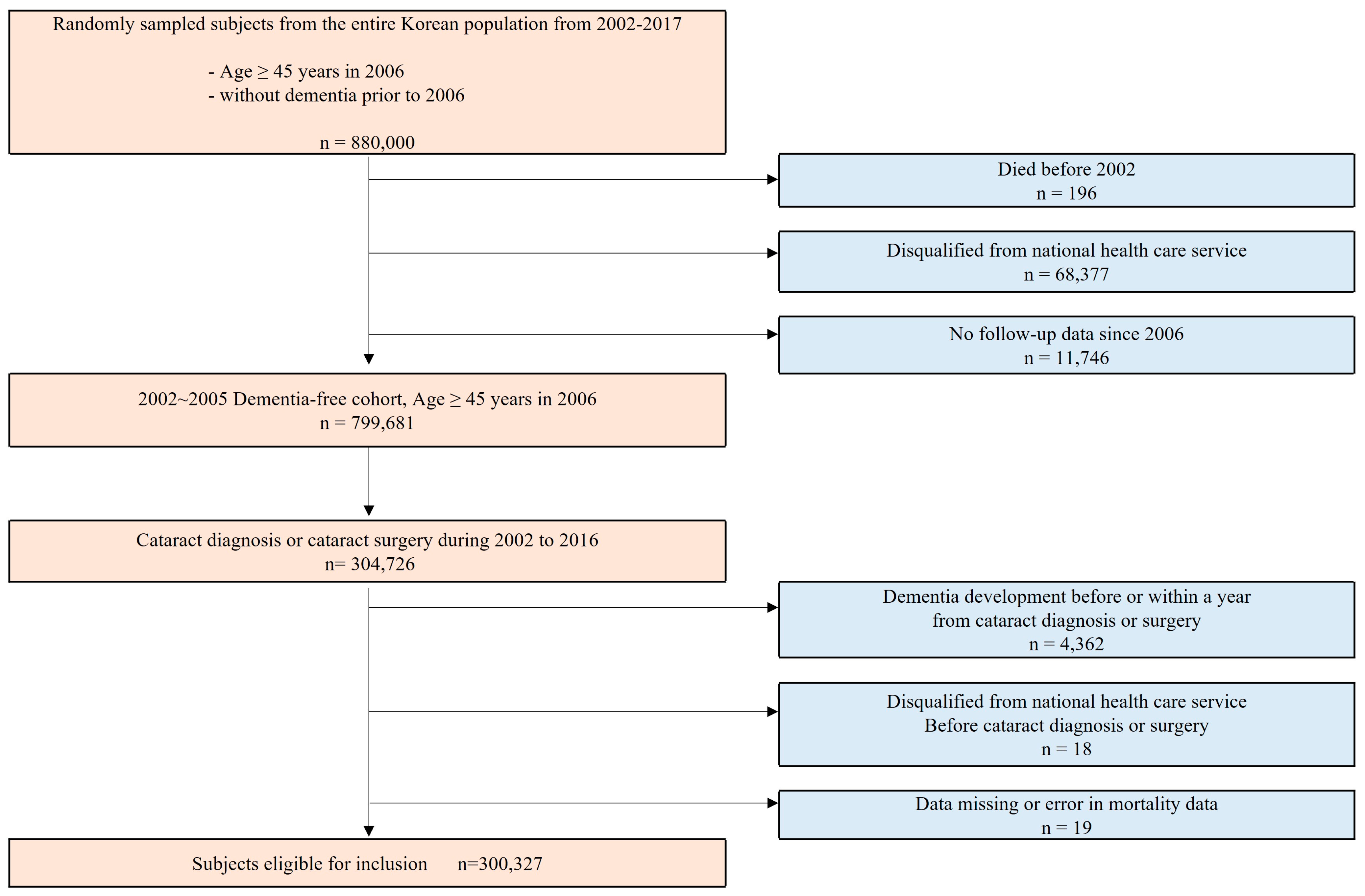
2.2. Study Participants
2.3. Variables for Exposure and Outcome
2.4. Other Variables and Follow-Up
2.5. Statistical Procedures
3. Results
3.1. Initial Characteristics of the Study Cohort
3.2. Univariate Analysis
3.3. Multivariate Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Li, C.Y.; Sun, Y.; Hu, S.C. Gender and Age Differences and the Trend in the Incidence and Prevalence of Dementia and Alzheimer’s Disease in Taiwan: A 7-Year National Population-Based Study. Biomed. Res. Int. 2019, 2019, 5378540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Jefferis, J.M.; Mosimann, U.P.; Clarke, M.P. Cataract and cognitive impairment: A review of the literature. Br. J. Ophthalmol. 2011, 95, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, E.; Littlejohns, T.J.; Khawaja, A.P.; Llewellyn, D.J.; Ukoumunne, O.C.; Thiem, U. Visual Impairment, Eye Diseases, and Dementia Risk: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2021, 83, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Gibbons, L.E.; Lee, A.Y.; Yanagihara, R.T.; Blazes, M.S.; Lee, M.L.; McCurry, S.M.; Bowen, J.D.; McCormick, W.C.; Crane, P.K.; et al. Association Between Cataract Extraction and Development of Dementia. JAMA Intern. Med. 2022, 182, 134–141. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Hayat, S.; Luben, R.; Brayne, C.; Conroy, M.; Foster, P.J.; Khawaja, A.P.; Kuźma, E. Visual Impairment and Risk of Dementia in 2 Population-Based Prospective Cohorts: UK Biobank and EPIC-Norfolk. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 697–704. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, D.; Liao, H.; Ha, J.; Shang, X.; Huang, Y.; Zhang, X.; Jiang, Y.; Li, L.; Yu, H.; et al. Visual Impairment and Risk of Dementia: The UK Biobank Study. Am. J. Ophthalmol. 2022, 235, 7–14. [Google Scholar] [CrossRef]
- Pellegrini, M.; Bernabei, F.; Schiavi, C.; Giannaccare, G. Impact of cataract surgery on depression and cognitive function: Systematic review and meta-analysis. Clin. Exp. Ophthalmol. 2020, 48, 593–601. [Google Scholar] [CrossRef]
- Yu, W.K.; Chen, Y.T.; Wang, S.J.; Kuo, S.C.; Shia, B.C.; Liu, C.J. Cataract surgery is associated with a reduced risk of dementia: A nationwide population-based cohort study. Eur. J. Neurol. 2015, 22, 1370–1377, e1379–1380. [Google Scholar] [CrossRef]
- Lee, Y.H.; Han, K.; Ko, S.H.; Ko, K.S.; Lee, K.U. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data Analytic Process of a Nationwide Population-Based Study Using National Health Information Database Established by National Health Insurance Service. Diabetes Metab. J. 2016, 40, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Anuurad, E.; Shiwaku, K.; Nogi, A.; Kitajima, K.; Enkhmaa, B.; Shimono, K.; Yamane, Y. The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J. Occup. Health 2003, 45, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Yoshikawa, T.; Morikawa, M.; Mine, M.; Okamoto, N.; Kurumatani, N.; Ogata, N. Effect of cataract surgery on cognitive function in elderly: Results of Fujiwara-kyo Eye Study. PLoS ONE 2018, 13, e0192677. [Google Scholar] [CrossRef]
- Hall, T.A.; McGwin, G.; Jr Owsley, C. Effect of cataract surgery on cognitive function in older adults. J. Am. Geriatr. Soc. 2005, 53, 2140–2144. [Google Scholar] [CrossRef]
- Su, C.W.; Lin, C.C.; Kao, C.H.; Chen, H.Y. Association Between Glaucoma and the Risk of Dementia. Medicine 2016, 95, e2833. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, X.; Yang, S.; Wang, G.; Ning, G. Association between Diabetic Retinopathy and Cognitive Impairment: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 692911. [Google Scholar] [CrossRef]
- Seden, D.; Alime, G.; Kadir, D.; Serpil, D.; Levent, T.; Özlem, T. Is Alzheimer disease related to age-related macular degeneration? Turk. J. Med. Sci. 2015, 45, 1115–1121. [Google Scholar]
- Rong, S.S.; Lee, B.Y.; Kuk, A.K.; Yu, X.T.; Li, S.S.; Li, J.; Guo, Y.; Yin, Y.; Osterbur, D.L.; Yam, J.C.S.; et al. Comorbidity of dementia and age-related macular degeneration calls for clinical awareness: A meta-analysis. Br. J. Ophthalmol. 2019, 103, 1777–1783. [Google Scholar] [CrossRef]
- Cox, J.T.; Subburaman, G.B.; Munoz, B.; Friedman, D.S.; Ravindran, R.D. Visual Acuity Outcomes after Cataract Surgery: High-Volume versus Low-Volume Surgeons. Ophthalmology 2019, 126, 1480–1489. [Google Scholar] [CrossRef]
- Dervenis, N.; Praidou, A.; Dervenis, P.; Chiras, D.; Little, B. Visual Acuity Outcomes after Phacoemulsification in Eyes with Good Visual Acuity before Cataract Surgery. Med. Princ. Pract. 2021, 30, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hecht, I.; Kanclerz, P.; Tuuminen, R. Secondary outcomes of lens and cataract surgery: More than just “best-corrected visual acuity”. Prog. Retin. Eye Res. 2023, 95, 101150. [Google Scholar] [CrossRef] [PubMed]
- Gębska-Tołoczko, M.; Kałużny, J.J.; Żaroń, A.; Danek, B.; Pulkowska-Ulfig, J.; Beck, O.; Wojciechowska, M.; Kałużny, B.J. Cognitive functions in patieCognitive functions in patients after cataract phacoemulsification and implantation of multifocal and monofocal intraocular lnts after cataract phacoemulsification and implantation of multifocal and monofocal intraocular lenses. Med. Res. J. 2018, 3, 70–75. [Google Scholar] [CrossRef]
| Variables | Total (n = 300,327) | Cataract Surgery | p-Value | |
|---|---|---|---|---|
| Yes (n = 111,208) | No (n = 189,119) | |||
| 1. Demographic Factors | ||||
| Age (mean ± SD) | 66.79 ± 8.90 | 67.79 ± 8.74 | 66.21 ± 8.94 | <0.0001 |
| Sex, female (%) | 181,253 | 68,489 (61.6%) | 112,764 (59.6%) | <0.0001 |
| Income (grade) | ||||
| Q1 (low) | 72,395 | 26,909 (24.2%) | 45,486 (24.1%) | 0.5233 |
| Q2 (lower-middle) | 47,061 | 17,514 (15.7%) | 29,547 (15.6%) | |
| Q3 (higher-middle) | 68,595 | 25,301 (22.8%) | 43,294 (22.9%) | |
| Q4 (high) | 112,276 | 41,484 (37.3%) | 70,792 (37.4%) | |
| Follow-up period (years) | 6.83 ± 3.68 | 6.08 ± 3.06 | 6.29 ± 3.75 | <0.0001 |
| 2. Systemic Comorbidities | ||||
| Dementia | 32,895 | 14,016 (12.6%) | 18,879 (10.0%) | <0.0001 |
| DM | 72,867 | 28,471 (25.6%) | 44,396 (23.5%) | <0.0001 |
| HTN | 193,044 | 73,803 (66.4%) | 119,241 (63.1%) | <0.0001 |
| Dyslipidemia | 111,242 | 39,325 (35.4%) | 71,917 (38.0%) | <0.0001 |
| Stroke | 35,416 | 13,220 (11.9%) | 22,196 (11.7%) | 0.2150 |
| Depression | 56,792 | 19,636 (17.7%) | 37,156 (19.6%) | <0.0001 |
| CHD | 80,856 | 29,776 (26.8%) | 51,080 (27.0%) | 0.1620 |
| Glaucoma | 6382 | 2840 (2.6%) | 3542 (1.9%) | <0.0001 |
| Diabetic retinopathy | 34,361 | 16,868 (15.2%) | 17,493 (9.2%) | <0.0001 |
| AMD | 34,234 | 19,528 (17.6%) | 14,706 (7.8%) | <0.0001 |
| 3. Behavioral Factors | ||||
| BMI (kg/m2) a | ||||
| <18.5 | 5773 | 2436 (2.2%) | 3337 (1.8%) | 0.6989 |
| 18.5 to <23 | 72,275 | 27,214 (24.5%) | 45,061 (23.8%) | |
| 23 to <25 | 58,184 | 21,676 (19.5%) | 36,508 (19.3%) | |
| ≥25 | 72,482 | 27,665 (24.9%) | 44,817 (23.7%) | |
| Smoking b | ||||
| Never smoked | 158,460 | 60,525 (54.4%) | 97,935 (51.8%) | <0.0001 |
| Ex-smoker | 28,190 | 10,417 (9.4%) | 17,773 (9.4%) | |
| Current smoker | 27,622 | 10,331 (9.3%) | 17,291 (9.1%) | |
| Drinking (heavy) | ||||
| Heavy | 30,235 | 10,576 (9.5%) | 19,659 (10.4%) | <0.0001 |
| No | 41,620 | 73,891 (9.9) | 3483 (8.8) | |
| 4. Ophthalmic Factors (VA) | ||||
| Good (≥20/66) | 207,562 | 73,316 (65.9%) | 134,246 (71.0%) | 0.0186 |
| Bad (<20/66) | 6642 | 2253 (2.0%) | 4389 (2.3%) | |
| Cataract Surgery | Subject No. | Case No. | Event (%) | Crude | Model 1 * | Model 2 ** | Model 3 *** | Model 4 **** |
|---|---|---|---|---|---|---|---|---|
| HR [95% CI] | HR [95% CI] | HR [95% CI] | HR [95% CI] | HR [95% CI] | ||||
| No | 189,119 | 18,879 | 10.0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 111,208 | 14,016 | 12.6 | 1.352 (1.322, 1.382) | 1.155 (1.1, 1.181) | 1.217 (1.181, 1.254) | 1.243 (1.167, 1.323) | 1.1 (1.021, 1.185) |
| Subgroup and Variables | aHR (95% CI) † | p-Value | |||
|---|---|---|---|---|---|
| Subgroups | Cataract Surgery | Visual Acuity | Subject Number | ||
| Whole population | No | bad | 4499 | 1 (ref.) | |
| No | good | 184,620 | 0.881 (0.714, 1.087) | 0.236 | |
| Yes | bad | 2074 | 1.331 (0.967, 1.83) | 0.0791 | |
| Yes | good | 109,134 | 0.879 (0.711, 1.085) | 0.2292 | |
| Age ≥ 65 years | No | bad | 3434 | 1 (ref.) | |
| No | good | 102,090 | 0.888 (0.71, 1.111) | 0.3002 | |
| Yes | bad | 1588 | 1.372 (0.981, 1.917) | 0.0642 | |
| Yes | good | 71,679 | 0.886 (0.708, 1.109) | 0.2889 | |
| Age < 65 years | No | bad | 1,065 | 1 (ref.) | |
| No | good | 82,530 | 0.903 (0.804, 1.013) | 0.0805 | |
| Yes | bad | 486 | 1.057 (0.925, 1.209) | 0.417 | |
| Yes | good | 37,455 | 0.88 (0.785, 0.987) | 0.0288 | |
| Male sex | No | bad | 1024 | 1 (ref.) | |
| No | good | 75,331 | 0.485 (0.264, 0.888) | 0.0191 | |
| Yes | bad | 515 | 0.855 (0.295, 2.481) | 0.7734 | |
| Yes | good | 42,204 | 0.486 (0.263, 0.899) | 0.0215 | |
| Female sex | No | bad | 3475 | 1 (ref.) | |
| No | good | 109,289 | 1.144 (0.827, 1.583) | 0.4166 | |
| Yes | bad | 1559 | 1.456 (0.905, 2.343) | 0.1218 | |
| Yes | good | 66,930 | 1.028 (0.742, 1.424) | 0.8684 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Shin, E.; Kim, M.; Bae, Y.; Chung, T.-Y.; Seo, S.W.; Jang, H.; Lim, D.H. The Effect of Cataract Surgery on the Risk of Dementia: A Nationwide Cohort Study. J. Clin. Med. 2023, 12, 6441. https://doi.org/10.3390/jcm12206441
Lee C, Shin E, Kim M, Bae Y, Chung T-Y, Seo SW, Jang H, Lim DH. The Effect of Cataract Surgery on the Risk of Dementia: A Nationwide Cohort Study. Journal of Clinical Medicine. 2023; 12(20):6441. https://doi.org/10.3390/jcm12206441
Chicago/Turabian StyleLee, Chaeyeon, Eunhae Shin, Mina Kim, Yoonjong Bae, Tae-Young Chung, Sang Won Seo, Hyemin Jang, and Dong Hui Lim. 2023. "The Effect of Cataract Surgery on the Risk of Dementia: A Nationwide Cohort Study" Journal of Clinical Medicine 12, no. 20: 6441. https://doi.org/10.3390/jcm12206441
APA StyleLee, C., Shin, E., Kim, M., Bae, Y., Chung, T.-Y., Seo, S. W., Jang, H., & Lim, D. H. (2023). The Effect of Cataract Surgery on the Risk of Dementia: A Nationwide Cohort Study. Journal of Clinical Medicine, 12(20), 6441. https://doi.org/10.3390/jcm12206441









