FAPI PET/CT in Diagnostic and Treatment Management of Colorectal Cancer: Review of Current Research Status
Abstract
1. Introduction
2. Fibroblast Activation Protein (FAP) and FAPI
3. Colorectal Cancer and CAFs
4. FAPI PET/CT Imaging
4.1. FAPI PET/CT Imaging in Normal Biological Organs
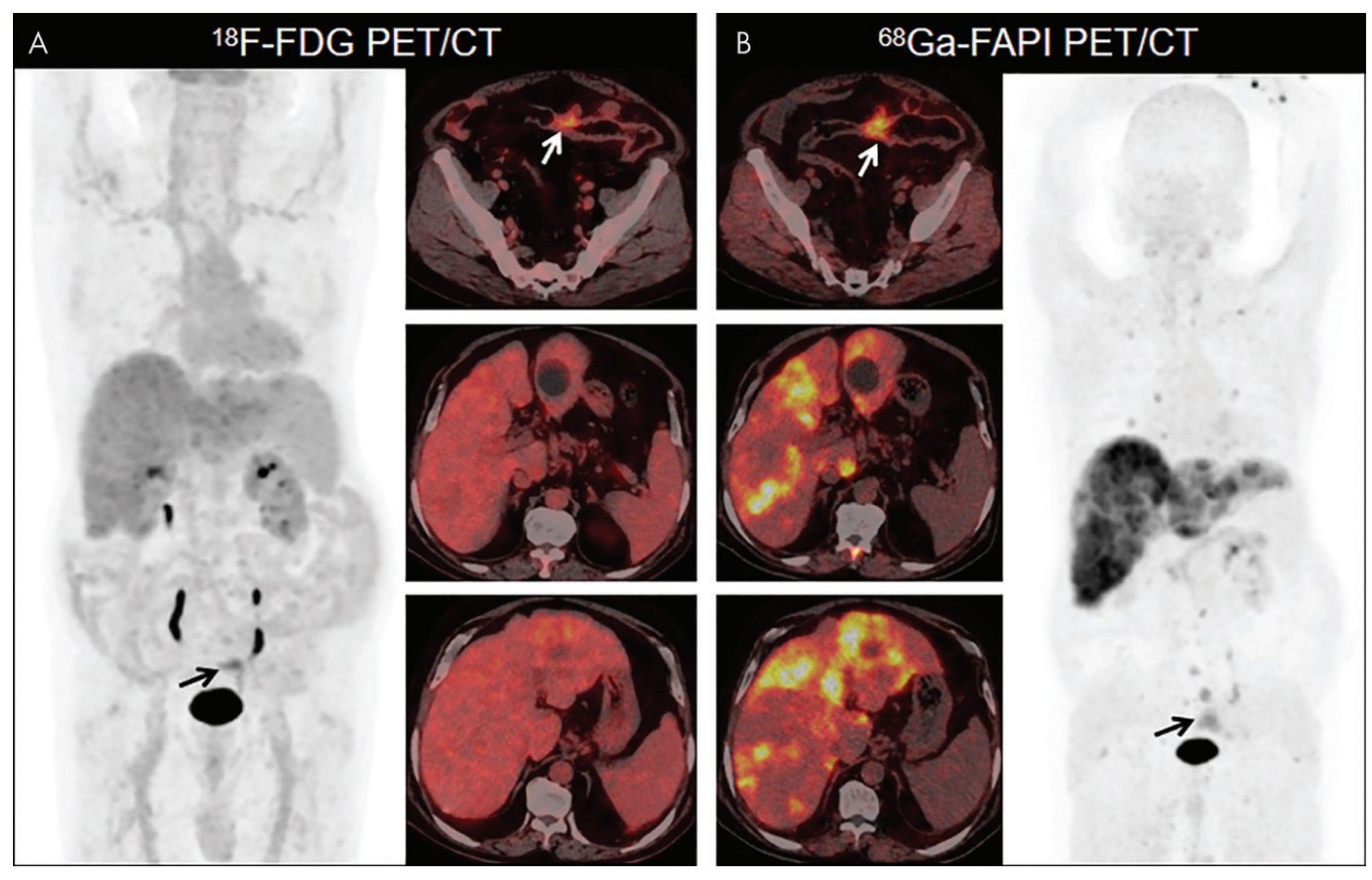
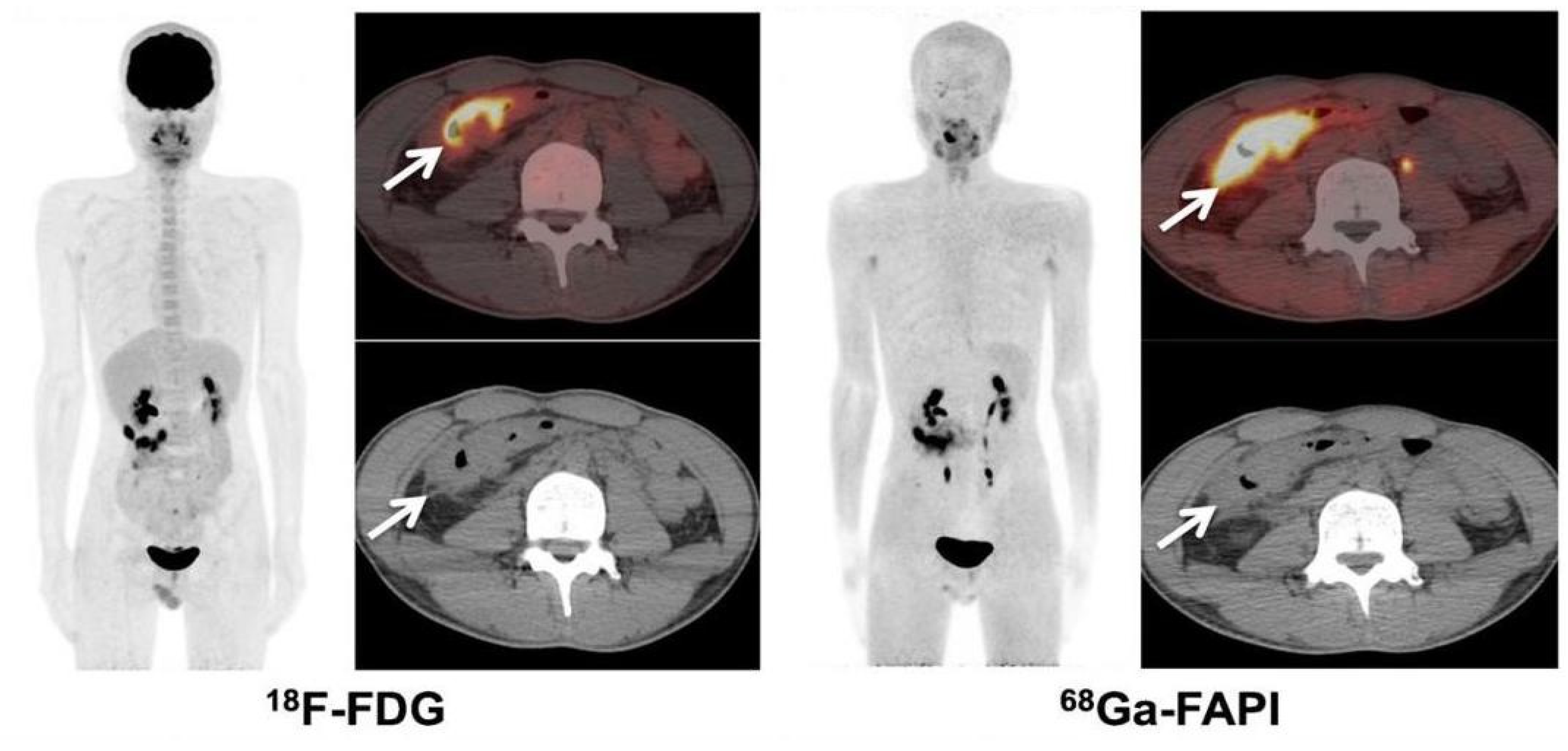
4.2. FAPI PET/CT Features of Primary and Metastatic Colorectal Cancer
4.3. FAPI vs. FDG Imaging in Colorectal Cancer
5. Limitations of FAPI PET/CT Imaging
6. Discussion
7. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Provenzale, D.; Ness, R.M.; Llor, X.; Weiss, J.M.; Abbadessa, B.; Cooper, G.; Early, D.S.; Friedman, M.; Giardiello, F.M.; Glaser, K.; et al. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 2.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 1312–1320. [Google Scholar] [CrossRef]
- Bae, S.U.; Won, K.S.; Song, B.I.; Jeong, W.K.; Baek, S.K.; Kim, H.W. Accuracy of F-18 FDG PET/CT with optimal cut-offs of maximum standardized uptake value according to size for diagnosis of regional lymph node metastasis in patients with rectal cancer. Cancer Imaging 2018, 18, 32. [Google Scholar] [CrossRef]
- Furtado, F.S.; Suarez-Weiss, K.E.; Vangelm, M.; Clark, J.W.; Cusack, J.C.; Hong, T.; Blaszkowsky, L.; Wo, J.; Striar, R.; Umutlu, L.; et al. Clinical impact of PET/MRI in oligometastatic colorectal cancer. Br. J. Cancer 2021, 125, 975–982. [Google Scholar] [CrossRef]
- Dagallier, C.; Avry, F.; Touchefeu, Y.; Buron, F.; Routier, S.; Chérel, M.; Arlicot, N. Development of PET Radioligands Targeting COX-2 for Colorectal Cancer Staging, a Review of in vitro and Preclinical Imaging Studies. Front. Med. 2021, 8, 675209. [Google Scholar] [CrossRef]
- Yang, T.; Ma, L.; Hou, H.; Gao, F.; Tao, W. FAPI PET/CT in the Diagnosis of Abdominal and Pelvic Tumors. Front. Oncol. 2021, 11, 797960. [Google Scholar] [CrossRef]
- Puré, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357. [Google Scholar] [CrossRef]
- Lindner, T.; Giesel, F.L.; Kratochwil, C.; Serfling, S.E. Radioligands Targeting Fibroblast Activation Protein (FAP). Cancers 2021, 13, 5744. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Kratochwil, C.; Cardinale, J.; Finck, R.; Dabir, M.; Novruzov, E.; Watabe, T.; Kramer, V.; Choyke, P.L.; et al. FAP and FAPI-PET/CT in Malignant and Non-Malignant Diseases: A Perfect Symbiosis? Cancers 2021, 13, 4946. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef]
- Langbein, T.; Weber, W.A.; Eiber, M. Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine. J. Nucl. Med. 2019, 60, 13S–19S. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Kirienko, M.; Gelardi, F.; Fiz, F.; Gozzi, N.; Chiti, A. State-of-the-art of FAPI-PET imaging: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4396–4414. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Schuchardt, C.; Singh, A.; Chantadisai, M.; Robiller, F.C.; Zhang, J.; Mueller, D.; Eismant, A.; Almaguel, F.; Zboralski, D.; et al. Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using 177Lu-FAP-2286: First-in-Humans Results. J. Nucl. Med. 2022, 63, 415–423. [Google Scholar] [CrossRef]
- Calais, J. FAP: The Next Billion Dollar Nuclear Theranostics Target? J. Nucl. Med. 2020, 61, 163–165. [Google Scholar] [CrossRef]
- Huang, R.; Pu, Y.; Huang, S.; Yang, C.; Yang, F.; Pu, Y.; Li, J.; Chen, L.; Huang, Y. FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century. Front. Oncol. 2022, 12, 854658. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef]
- Frank, M.H.; Wilson, B.J.; Gold, J.S.; Frank, N.Y. Clinical Implications of Colorectal Cancer Stem Cells in the Age of Single-Cell Omics and Targeted Therapies. Gastroenterology 2021, 160, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A.; Baiocchi, M. Colorectal Cancer Stem Cells: An Overview of Evolving Methods and Concepts. Cancers 2021, 13, 5910. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Mhaidly, R.; Mechta-Grigoriou, F. Fibroblast heterogeneity in tumor micro-environment: Role in immunosuppression and new therapies. Semin. Immunol. 2020, 48, 101417. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Ganguly, D.; Chandra, R.; Karalis, J.; Teke, M.; Aguilera, T.; Maddipati, R.; Wachsmann, M.B.; Ghersi, D.; Siravegna, G.; Zeh, H.J., 3rd; et al. Cancer-Associated Fibroblasts: Versatile Players in the Tumor Microenvironment. Cancers 2020, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ng, A.S.; Cai, S.; Li, Q.; Yang, L.; Kerr, D. Novel therapeutic strategies: Targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol. 2021, 22, e358–e368. [Google Scholar] [CrossRef]
- Siveke, J.T. Fibroblast-Activating Protein: Targeting the Roots of the Tumor Microenvironment. J. Nucl. Med. 2018, 59, 1412–1414. [Google Scholar] [CrossRef]
- Glabman, R.A.; Choyke, P.L.; Sato, N. Cancer-Associated Fibroblasts: Tumorigenicity and Targeting for Cancer Therapy. Cancers 2022, 14, 3906. [Google Scholar] [CrossRef]
- Barsoumian, H.B.; Ramapriyan, R.; Younes, A.I.; Caetano, M.S.; Menon, H.; Comeaux, N.I.; Cushman, T.R.; Schoenhals, J.E.; Cadena, A.P.; Reilly, T.P.; et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J. Immunother. Cancer 2020, 8, e000537. [Google Scholar] [CrossRef]
- Miyashita, N.; Saito, A. Organ Specificity and Heterogeneity of Cancer-Associated Fibroblasts in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 10973. [Google Scholar] [CrossRef] [PubMed]
- Fotsitzoudis, C.; Koulouridi, A.; Messaritakis, I.; Konstantinidis, T.; Gouvas, N.; Tsiaoussis, J.; Souglakos, J. Cancer-Associated Fibroblasts: The Origin, Biological Characteristics and Role in Cancer-A Glance on Colorectal Cancer. Cancers 2022, 14, 4394. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, T.P.; Stuart, M.P.M.E.; Oosting, J.; Tollenaar, R.A.E.M.; Sier, C.F.M.; Mesker, W.E. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer 2019, 19, 284. [Google Scholar] [CrossRef]
- Rathke, H.; Fuxius, S.; Giesel, F.L.; Lindner, T.; Debus, J.; Haberkorn, U.; Kratochwil, C. Two Tumors, One Target: Preliminary Experience With 90Y-FAPI Therapy in a Patient With Metastasized Breast and Colorectal Cancer. Clin. Nucl. Med. 2021, 46, 842–844. [Google Scholar] [CrossRef]
- Meyer, C.; Dahlbom, M.; Lindner, T.; Vauclin, S.; Mona, C.; Slavik, R.; Czernin, J.; Haberkorn, U.; Calais, J. Radiation Dosimetry and Biodistribution of 68Ga-FAPI-46 PET Imaging in Cancer Patients. J. Nucl. Med. 2020, 61, 1171–1177. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Tripathi, M.; ArunRaj, S.T.; Sarswat, S.; Bal, C. Biodistribution, pharmacokinetics, dosimetry of [68Ga]Ga-DOTA.SA.FAPi, and the head-to-head comparison with [18F]F-FDG PET/CT in patients with various cancers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1915–1931. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of 68Ga-FAPI and 18F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodist.tribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Mona, C.E.; Benz, M.R.; Hikmat, F.; Grogan, T.R.; Lueckerath, K.; Razmaria, A.; Riahi, R.; Slavik, R.; Girgis, M.D.; Carlucci, G.; et al. Correlation of 68Ga-FAPi-46 PET Biodistribution with FAP Expression by Immunohistochemistry in Patients with Solid Cancers: Interim Analysis of a Prospective Translational Exploratory Study. J. Nucl. Med. 2022, 63, 1021–1026. [Google Scholar] [CrossRef]
- Strating, E.; Wassenaar, E.; Verhagen, M.; Rauwerdink, P.; van Schelven, S.; de Hingh, I.; Rinkes, I.B.; Boerma, D.; Witkamp, A.; Lacle, M.; et al. Fibroblast activation protein identifies Consensus Molecular Subtype 4 in colorectal cancer and allows its detection by 68Ga-FAPI-PET imaging. Br. J. Cancer 2022, 127, 145–155. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, S.; Ma, J.; Chen, Z.; Song, G.; Rao, D.; Cheng, Y.; Huang, S.; Liu, Y.; Jiang, S.; et al. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov. 2022, 12, 134–153. [Google Scholar] [CrossRef]
- Zeng, X.; Ward, S.E.; Zhou, J.; Cheng, A.S.L. Liver Immune Microenvironment and Metastasis from Colorectal Cancer-Pathogenesis and Therapeutic Perspectives. Cancers 2021, 13, 2418. [Google Scholar] [CrossRef]
- Welt, S.; Divgi, C.R.; Scott, A.M.; Garin-Chesa, P.; Finn, R.D.; Graham, M.; Carswell, E.A.; Cohen, A.; Larson, S.M.; Old, L.J.; et al. Antibody targeting in metastatic colon cancer: A phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J. Clin. Oncol. 1994, 12, 1193–1203. [Google Scholar] [CrossRef]
- Şahin, E.; Elboğa, U.; Çelen, Y.Z.; Sever, Ö.N.; Çayırlı, Y.B.; Çimen, U. Comparison of 68Ga-DOTA-FAPI and 18FDG PET/CT imaging modalities in the detection of liver metastases in patients with gastrointestinal system cancer. Eur. J. Radiol. 2021, 142, 109867. [Google Scholar] [CrossRef]
- Koerber, S.A.; Staudinger, F.; Kratochwil, C.; Adeberg, S.; Haefner, M.F.; Ungerechts, G.; Rathke, H.; Winter, E.; Lindner, T.; Syed, M.; et al. The Role of 68Ga-FAPI PET/CT for Patients with Malignancies of the Lower Gastrointestinal Tract: First Clinical Experience. J. Nucl. Med. 2020, 61, 1331–1336. [Google Scholar] [CrossRef]
- Polack, M.; Hagenaars, S.C.; Couwenberg, A.; Kool, W.; Tollenaar, R.A.E.M.; Vogel, W.V.; Snaebjornsson, P.; Mesker, W.E. Characteristics of tumor stroma in regional lymph node metastases in colorectal cancer patients: A theoretical framework for future diagnostic imaging with FAPI PET/CT. Clin. Transl. Oncol. 2022, 24, 1776–1784. [Google Scholar] [CrossRef]
- Chen, S.H.; Miles, K.; Taylor, S.A.; Ganeshan, B.; Rodriquez, M.; Fraioli, F.; Wan, S.; Afaq, A.; Shortman, R.; Walls, D.; et al. FDG-PET/CT in colorectal cancer: Potential for vascular-metabolic imaging to provide markers of prognosis. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 371–384. [Google Scholar] [CrossRef]
- Liu, H.; Ye, Z.; Yang, T.; Xie, H.; Duan, T.; Li, M.; Wu, M.; Song, B. Predictive Value of Metabolic Parameters Derived From 18F-FDG PET/CT for Microsatellite Instability in Patients with Colorectal Carcinoma. Front. Immunol. 2021, 12, 724464. [Google Scholar] [CrossRef]
- van Helden, E.J.; Vacher, Y.J.L.; van Wieringen, W.N.; van Velden, F.H.P.; Verheul, H.M.W.; Hoekstra, O.S.; Boellaard, R.; Menke-van der Houven van Oordt, C.W. Radiomics analysis of pre-treatment [18F]FDG PET/CT for patients with metastatic colorectal cancer undergoing palliative systemic treatment. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2307–2317. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Tamburini, K.; Mori, Y.; Cardinale, J.; Haberkorn, U.; Giesel, F.L. Advancement and Future Perspective of FAPI PET/CT In Gynecological Malignancies. Semin. Nucl. Med. 2022, 52, 628–634. [Google Scholar] [CrossRef]
- Zygulska, A.L.; Pierzchalski, P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 852. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.F.; Shi, Q.; Yang, Y.; Yao, B.H.; Wang, S.C.; Geng, G.Y. Prediction value of 18F-FDG PET/CT intratumor metabolic heterogeneity parameters for recurrence after radical surgery of stage II/III colorectal cancer. Front. Oncol. 2022, 12, 945939. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Finck, R.; Mokoala, K.M.G.; Staudinger, F.; Schillings, L.; Heger, U.; Röhrich, M.; Kratochwil, C.; Sathekge, M.; et al. 68Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4089–4100. [Google Scholar] [CrossRef] [PubMed]
- Kömek, H.; Can, C.; Güzel, Y.; Oruç, Z.; Gündoğan, C.; Yildirim, Ö.A.; Kaplan, İ.; Erdur, E.; Yıldırım, M.S.; Çakabay, B. 68Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: A comparative pilot study with the 18F-FDG PET/CT. Ann. Nucl. Med. 2021, 35, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Liu, H.; Wang, Y.; Deng, J.; Peng, D.; Feng, Y.; Wang, L.; Chen, Y.; Qiu, L. The potential utility of [68Ga]Ga-DOTA-FAPI-04 as a novel broad-spectrum oncological and non-oncological imaging agent-comparison with [18F]FDG. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Roselt, P.J.; Kallur, K.G.; Tothill, R.W.; Mileshkin, L. FAPI PET/CT: Will It End the Hegemony of 18F-FDG in Oncology? J. Nucl. Med. 2021, 62, 296–302. [Google Scholar] [CrossRef]
- Qin, C.; Song, Y.; Gai, Y.; Ruan, W.; Liu, Q.; Liu, F.; Zheng, D.; Zhang, P.; Liu, H.; Zhang, T.; et al. Gallium-68-labeled fibroblast activation protein inhibitor PET in gastrointestinal cancer: Insights into diagnosis and management. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4228–4240. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, L.; Luo, Z.; Hao, B.; Wu, H.; Lin, Q.; Sun, L.; Chen, H. Comparison of 68Ga-FAPI and 18F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology 2021, 298, 393–402. [Google Scholar] [CrossRef]
- Elboga, U.; Sahin, E.; Kus, T.; Cayirli, Y.B.; Aktas, G.; Okuyan, M.; Cinkir, H.Y.; Teker, F.; Sever, O.N.; Aytekin, A.; et al. Comparison of 68Ga-FAPI PET/CT and 18FDG PET/CT Modalities in Gastrointestinal System Malignancies with Peritoneal Involvement. Mol. Imaging Biol. 2022, 24, 789–797. [Google Scholar] [CrossRef]
- Fu, L.; Hu, K.; Tang, G.; Wu, H.; Zhou, W. [68Ga]Ga-FAPI-04 PET/CT imaging in signet-ring cell carcinoma of sigmoid colon. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1690–1691. [Google Scholar] [CrossRef]
- Güzel, Y.; Kaplan, İ.; Kepenek, F.; Söğütçü, N.; Kömek, H. Perirenal Infiltration of Signet Ring Cell Colon Carcinoma Shown by 68Ga-FAPI PET/CT. Clin. Nucl. Med. 2022, 47, e509–e512. [Google Scholar] [CrossRef] [PubMed]
- Kömek, H.; Can, C.; Kaplan, İ.; Gündoğan, C.; Kepenek, F.; Karaoglan, H.; Demirkıran, A.; Ebinç, S.; Güzel, Y.; Gündeş, E. Comparison of [68Ga]Ga-DOTA-FAPI-04 PET/CT and [18F]FDG PET/CT in colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3898–3909. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; Ruan, D.; Pang, Y.; Hao, B.; Dai, Y.; Wu, X.; Guo, W.; Fan, C.; Wu, J.; et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Lin, L.; Sun, L.; Wu, H.; Lin, Q.; Chen, H. [68Ga]Ga-DOTA-FAPI-04 improves tumor staging and monitors early response to chemoradiotherapy in a patient with esophageal cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3188–3189. [Google Scholar] [CrossRef]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular Imaging of Fibroblast Activity After Myocardial Infarction Using a 68Ga-Labeled Fibroblast Activation Protein Inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Rauber, S.; Atzinger, A.; Agarwal, R.; Götz, T.I.; Soare, A.; Cordes, M.; Prante, O.; Bergmann, C.; Kleyer, A.; et al. Ramming, A. Disentangling inflammatory from fibrotic disease activity by fibroblast activation protein imaging. Ann. Rheum. Dis. 2020, 79, 1485–1491. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Q.; Yang, H.; Peng, L.; Zhang, W.; Li, F. Fibroblast Activation Protein-Targeted PET/CT with 68Ga-FAPI for Imaging IgG4-Related Disease: Comparison to 18F-FDG PET/CT. J. Nucl. Med. 2021, 62, 266–271. [Google Scholar] [CrossRef]
- Zidar, N.; Langner, C.; Jerala, M.; Boštjančič, E.; Drobne, D.; Tomažič, A. Pathology of Fibrosis in Crohn’s Disease-Contribution to Understanding Its Pathogenesis. Front. Med. 2020, 7, 167. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Q.; Xu, H.; Zhang, R.; Li, J.; Li, F. Active uptake of 68Ga-FAPI in Crohn’s disease but not in ulcerative colitis. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 1682–1683. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lin, K.; Zheng, S.; Yao, S.; Miao, W. 68Ga-FAPI and 18F-PET/CT Images in Intestinal Tuberculosis. Clin. Nucl. Med. 2022, 47, 239. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Type | Key Message |
|---|---|---|---|
| Loktev et al. | 2018 | Article | Cancer-associated fibroblasts are abundant in colon cancer and can be imaged using molecular probes targeting FAP. |
| Kratochwil et al. | 2019 | Article | FAPI PET/CT has intermediate strength uptake in colorectal cancer. |
| Koerber et al. | 2020 | Article | 68Ga-FAPI PET/CT can detect primary and metastatic tumors of the lower digestive tract, identify clinical stages and provide evidence for tumor management. |
| Rathke et al. | 2021 | Case | FAPI uptake was high in colorectal cancer-related metastases, and peritoneal metastases disappeared after treatment with 90Y-FAPI46. |
| Giesel et al. | 2021 | Article | In the background tissues of colon, the SUVmax of 68Ga-FAPI was significantly lower than that of 18F-FDG. |
| Şahin et al. | 2021 | Article | 68Ga-FAPI is superior to 18F-FDG in detecting liver metastasis of colorectal cancer. |
| Pang et al. | 2021 | Article | 68Ga-FAPI was superior to 18F-FDG in the detection of colorectal cancer primary and metastatic sites, showing higher uptake in most primary and metastatic sites. |
| Fu et al. | 2021 | Case | 68Ga-FAPI has strong uptake in sigmoid signet ring cell carcinoma and is more sensitive to primary lesions and peritoneal carcinomas than 18F-FDG. |
| Mona et al. | 2022 | Article | FAPI PET/CT has strong uptake in colorectal cancer patients, and the intensity of FAPI uptake is related to the intensity of immunohistochemical staining of FAP. |
| Strating et al. | 2022 | Article | FAPI PET/CT may have important potential value in evaluating CMS4 as an adverse prognostic factor for colorectal cancer. |
| Qin et al. | 2022 | Article | 68Ga-FAPI is of great significance in the accurate staging and clinical management of colorectal cancer. |
| Elboga et al. | 2022 | Article | The uptake level of FAPI in primary and metastatic lesions of colorectal cancer (especially liver metastasis) was significantly higher than that of FDG and had a better tumor-to-background ratio. |
| Güzel et al. | 2022 | Case | 68Ga-FAPI was superior to 18F-FDG in evaluating recurrent signet ring cell colon cancer. |
| Kömek et al. | 2022 | Article | 68Ga-FAPI has higher sensitivity and specificity in detecting primary lesions of colorectal cancer and lymph node and peritoneal metastasis. |
| Author | Year | Lesion Location | FAPI # SUVmax | FDG SUVmax |
|---|---|---|---|---|
| Koerber et al. | 2020 | Primary lesions | 15.7 | - |
| Local relapse | 6.56 | - | ||
| Lymph node metastases | 8.33 | - | ||
| Liver metastases | 9.54 | - | ||
| Şahin et al. | 2021 | Primary lesions | 5.5 | 5.0 |
| Pang et al. | 2021 | Primary lesions | 15.9 | 2.2 |
| Lymph node metastases * | 6.7 | 2.4 | ||
| Liver metastases * | 9.7 | 1.7 | ||
| Elboga et al. | 2022 | Primary lesions | 14.6 | 8.4 |
| Lymph node metastases | 9.9 | 4.9 | ||
| Peritoneal metastases | 10.7 | 3.1 | ||
| Liver metastases | 12.2 | 5.0 | ||
| Kömek et al. | 2022 | Primary lesions | 11.54 | 18.93 |
| Lymph node metastases | 3.6 | 2.25 | ||
| Peritoneal metastases | 5.14 | 3.59 | ||
| Liver metastases | 6.15 | 9.66 |
| Author | Year | Lesion Location | FAPI # Sensitivity | FAPI # Specificity | FDG Sensitivity | FDG Specificity |
|---|---|---|---|---|---|---|
| Şahin et al. | 2021 | Liver metastases | 96.6% | - | 70.8% | - |
| Pang et al. | 2021 | Lymph node metastases * | 79% | 82% | 54% | 89% |
| Kömek et al. | 2022 | Primary lesions | 100% | 100% | 100% | 85.3% |
| Lymph node metastases | 90% | 100% | 80% | 81.8% | ||
| Peritoneal metastases | 100% | 100% | 55% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Wang, S.; Xu, S.; Du, B.; Li, X.; Li, Y. FAPI PET/CT in Diagnostic and Treatment Management of Colorectal Cancer: Review of Current Research Status. J. Clin. Med. 2023, 12, 577. https://doi.org/10.3390/jcm12020577
Cheng Z, Wang S, Xu S, Du B, Li X, Li Y. FAPI PET/CT in Diagnostic and Treatment Management of Colorectal Cancer: Review of Current Research Status. Journal of Clinical Medicine. 2023; 12(2):577. https://doi.org/10.3390/jcm12020577
Chicago/Turabian StyleCheng, Zhiming, Shu Wang, Shuoyan Xu, Bulin Du, Xuena Li, and Yaming Li. 2023. "FAPI PET/CT in Diagnostic and Treatment Management of Colorectal Cancer: Review of Current Research Status" Journal of Clinical Medicine 12, no. 2: 577. https://doi.org/10.3390/jcm12020577
APA StyleCheng, Z., Wang, S., Xu, S., Du, B., Li, X., & Li, Y. (2023). FAPI PET/CT in Diagnostic and Treatment Management of Colorectal Cancer: Review of Current Research Status. Journal of Clinical Medicine, 12(2), 577. https://doi.org/10.3390/jcm12020577






