Chronic Fatigue, Depression and Anxiety Symptoms in Long COVID Are Strongly Predicted by Neuroimmune and Neuro-Oxidative Pathways Which Are Caused by the Inflammation during Acute Infection
Abstract
1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Clinical Measurements
2.3. Assays
2.4. Statistical Analysis
3. Results
3.1. Socio-Demographic Data
3.2. Differences in Psychiatric Rating Scales between Study Groups
3.3. Differences in Biomarkers between Study Groups
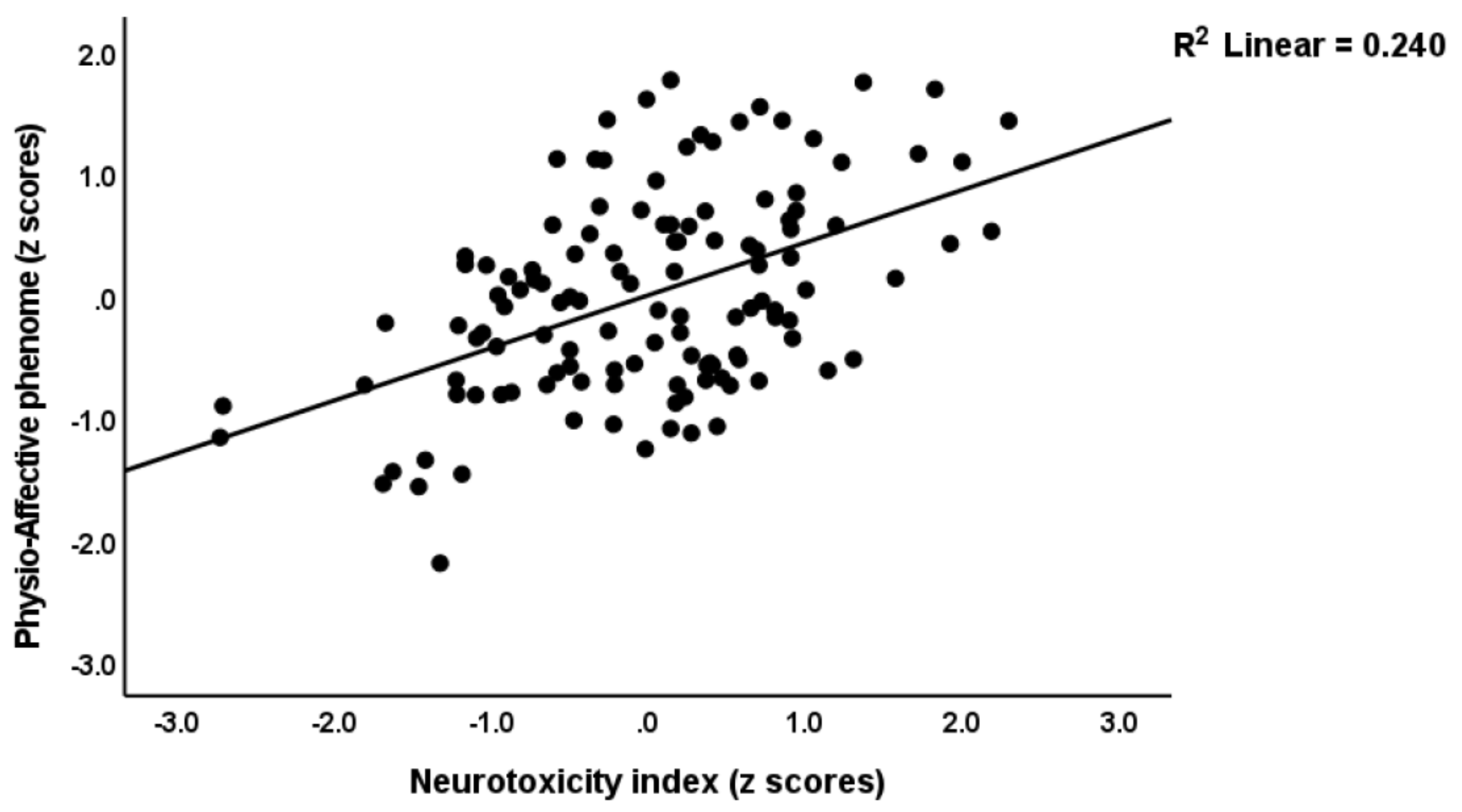
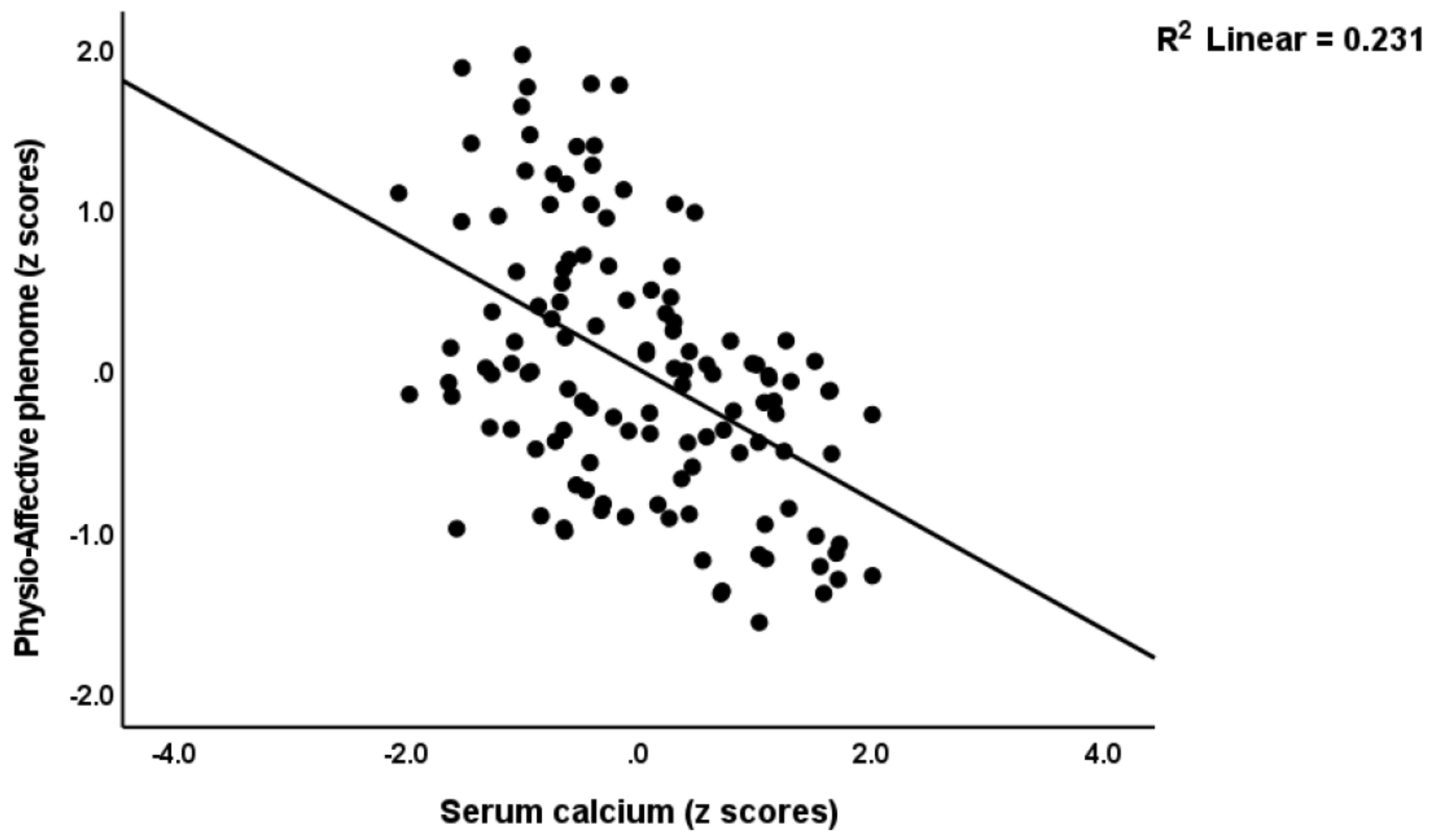
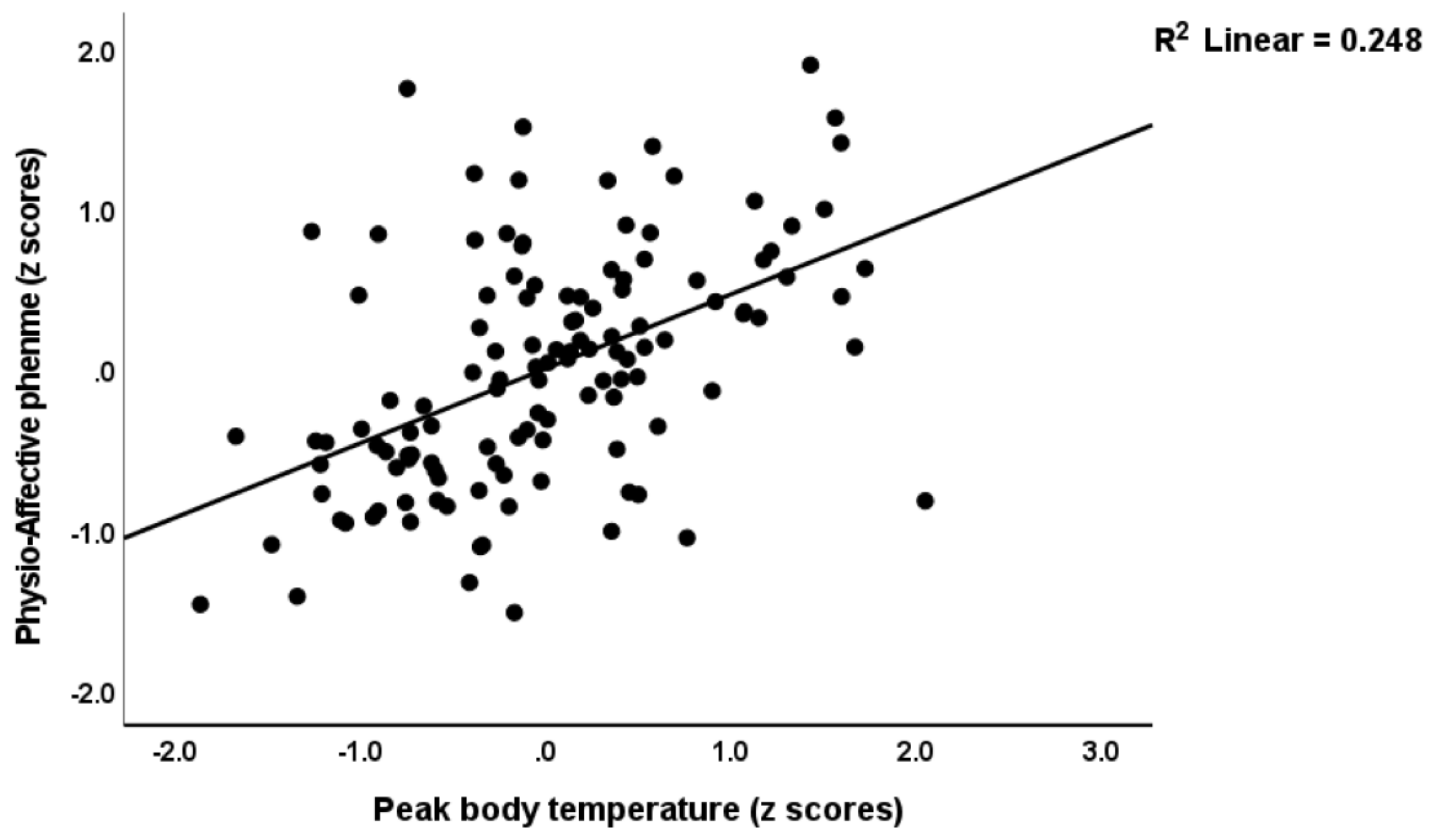
3.4. Prediction of the Physio-Affective Phenome Using TO2, NT, and Total Ca
3.5. Prediction of the Physio-Somatic and Affective Domains Using Biomarkers
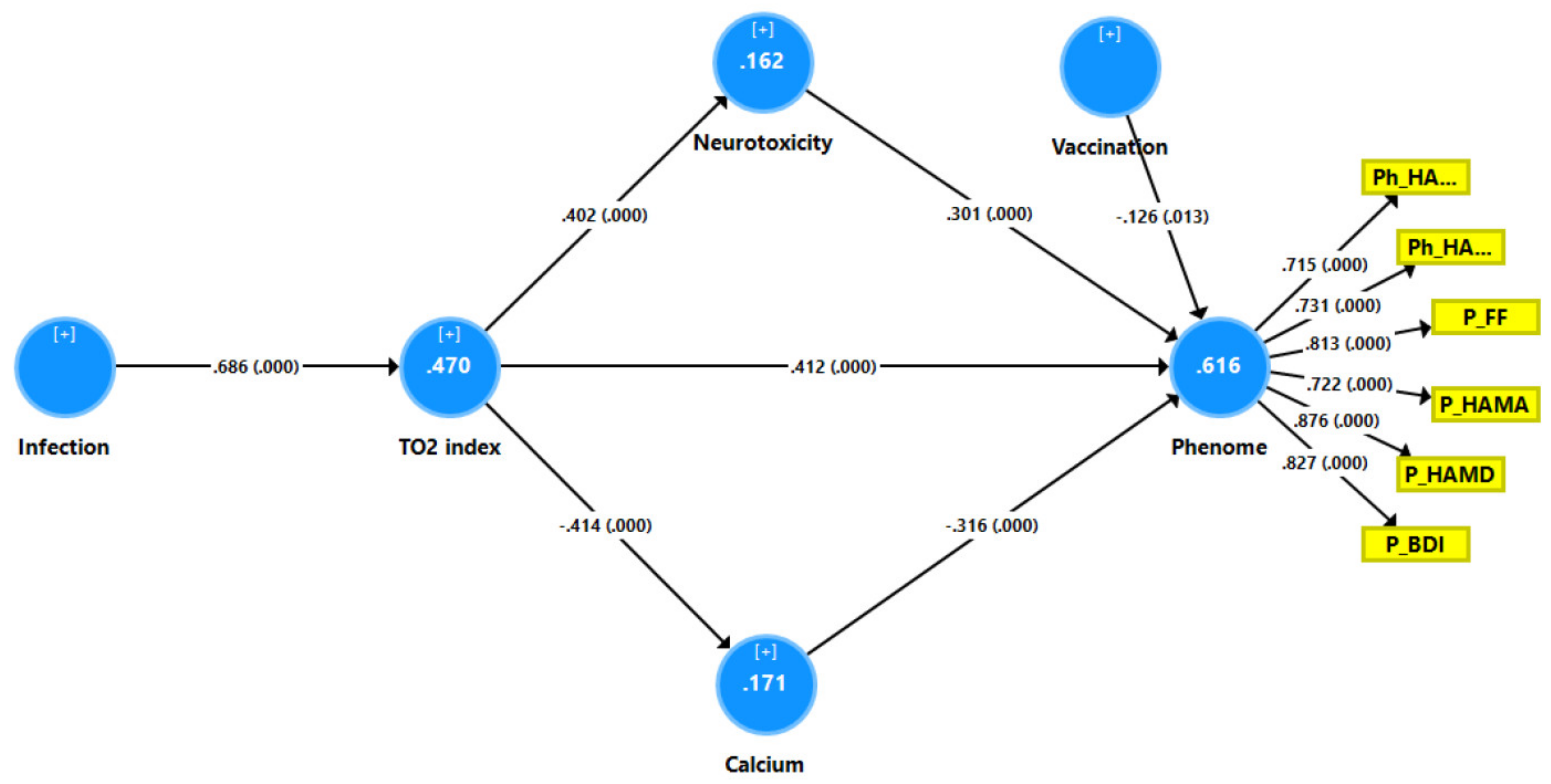
3.6. Results of PLS Analysis
4. Discussion
4.1. The Physio-Affective Phenome of Long COVID
4.2. Increased NT due to NLRP3 Activation Predicts the Physio-Affective Phenome
4.3. Increased NT due to Increased CRP Predicts the Physio-Affective Core
4.4. Increased NT due to Oxidative Stress Predicts the Physio-Affective Phenome
4.5. Lowered Total Ca Levels Predict the Physio-Affective Phenome
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.M.; Robinson, D.A.; Yang, L.; Williams, C.F.; Newman, L.M.; Breen, J.J.; Eisinger, R.W.; Schneider, J.S.; Adimora, A.A.; Erbelding, E.J. Toward Understanding COVID-19 Recovery: National Institutes of Health Workshop on Postacute COVID-19. Ann. Intern. Med. 2021, 174, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Renaud-Charest, O.; Lui, L.M.W.; Eskander, S.; Ceban, F.; Ho, R.; Di Vincenzo, J.D.; Rosenblat, J.D.; Lee, Y.; Subramaniapillai, M.; McIntyre, R.S. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J. Psychiatr. Res. 2021, 144, 129–137. [Google Scholar] [CrossRef]
- Sandler, C.X.; Wyller, V.B.B.; Moss-Morris, R.; Buchwald, D.; Crawley, E.; Hautvast, J.; Katz, B.Z.; Knoop, H.; Little, P.; Taylor, R.; et al. Long COVID and Post-infective Fatigue Syndrome: A Review. Open Forum Infect. Dis. 2021, 8, ofab440. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of COVID-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef]
- Cares-Marambio, K.; Montenegro-Jiménez, Y.; Torres-Castro, R.; Vera-Uribe, R.; Torralba, Y.; Alsina-Restoy, X.; Vasconcello-Castillo, L.; Vilaró, J. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chron. Respir. Dis. 2021, 18, 14799731211002240. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Simani, L.; Ramezani, M.; Darazam, I.A.; Sagharichi, M.; Aalipour, M.A.; Ghorbani, F.; Pakdaman, H. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J. Neurovirol. 2021, 27, 154–159. [Google Scholar] [CrossRef]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and Psychophysical Sequelae Among Patients With COVID-19 Four Months After Hospital Discharge. JAMA Netw. Open 2021, 4, e2036142. [Google Scholar] [CrossRef]
- Cirulli, E.T.; Schiabor Barrett, K.M.; Riffle, S.; Bolze, A.; Neveux, I.; Dabe, S.; Grzymski, J.J.; Lu, J.T.; Washington, N.L. Long-term COVID-19 symptoms in a large unselected population. medRxiv 2010. [Google Scholar] [CrossRef]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020, 81, e4–e6. [Google Scholar] [CrossRef]
- Van den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; van Hees, H.W.H.; van Helvoort, H.; van den Boogaard, M.; van der Hoeven, H.; et al. Comprehensive Health Assessment 3 Months after Recovery from Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, e1089–e1098. [Google Scholar] [CrossRef]
- Maes, M.; Tedesco Junior, W.L.D.; Lozovoy, M.A.B.; Mori, M.T.E.; Danelli, T.; Almeida, E.R.D.d.; Tejo, A.M.; Tano, Z.N.; Reiche, E.M.V.; Simão, A.N.C. In COVID-19, NLRP3 inflammasome genetic variants are associated with critical disease and these effects are partly mediated by the sickness symptom complex: A nomothetic network approach. Mol. Psychiatry 2022, 27, 1945–1955. [Google Scholar] [CrossRef]
- Yalcinkaya, M.; Liu, W.; Islam, M.N.; Kotini, A.G.; Gusarova, G.A.; Fidler, T.P.; Papapetrou, E.P.; Bhattacharya, J.; Wang, N.; Tall, A.R. Modulation of the NLRP3 inflammasome by SARS-CoV-2 Envelope protein. Sci. Rep. 2021, 11, 24432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Di, B.; Xu, L.-L. The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Sagulkoo, P.; Plaimas, K.; Suratanee, A.; Colado Simão, A.N.; Vissoci Reiche, E.M.; Maes, M. Immunopathogenesis and Immunogenetic Variants in COVID-19. Curr. Pharm. Des. 2022, 28, 1780–1797. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, C.; Crespo, Ã.n.; Ranjbar, S.; Lewandrowski, M.; Ingber, J.; de Lacerda, L.B.; Parry, B.; Ravid, S.; Clark, S.; Ho, F.; et al. SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. Res. Sq. 2021, 1–42. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Tharakan, S.; Nomoto, K.; Miyashita, S.; Ishikawa, K. Body temperature correlates with mortality in COVID-19 patients. Crit. Care 2020, 24, 298. [Google Scholar] [CrossRef]
- Al-Hadrawi, D.S.; Al-Rubaye, H.T.; Almulla, A.F.; Al-Hakeim, H.K.; Maes, M. Lowered oxygen saturation and increased body temperature in acute COVID-19 largely predict chronic fatigue syndrome and affective symptoms due to Long COVID: A precision nomothetic approach. Acta Neuropsychiatr. 2022, 1–12. [Google Scholar] [CrossRef]
- Maes, M. Precision Nomothetic Medicine in Depression Research: A New Depression Model, and New Endophenotype Classes and Pathway Phenotypes, and A Digital Self. J. Pers. Med. 2022, 12, 403. [Google Scholar] [CrossRef]
- Al-Jassas, H.K.; Al-Hakeim, H.K.; Maes, M. Intersections between pneumonia, lowered oxygen saturation percentage and immune activation mediate depression, anxiety, and chronic fatigue syndrome-like symptoms due to COVID-19: A nomothetic network approach. J. Affect. Disord. 2022, 297, 233–245. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2022. [CrossRef]
- Maes, M.; Carvalho, A.F. The Compensatory Immune-Regulatory Reflex System (CIRS) in Depression and Bipolar Disorder. Mol. Neurobiol. 2018, 55, 8885–8903. [Google Scholar] [CrossRef]
- Maes, M.; Bonifacio, K.L.; Morelli, N.R.; Vargas, H.O.; Moreira, E.G.; St Stoyanov, D.; Barbosa, D.S.; Carvalho, A.F.; Nunes, S.O.V. Generalized Anxiety Disorder (GAD) and Comorbid Major Depression with GAD Are Characterized by Enhanced Nitro-oxidative Stress, Increased Lipid Peroxidation, and Lowered Lipid-Associated Antioxidant Defenses. Neurotox. Res. 2018, 34, 489–510. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Kotańska, M. Aberrations in the Cross-Talks Among Redox, Nuclear Factor-κB, and Wnt/β-Catenin Pathway Signaling Underpin Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. Front. Psychiatry 2022, 13, 822382. [Google Scholar] [CrossRef]
- Almulla, F.A.; Maes, M. The Tryptophan Catabolite or Kynurenine Pathway’s Role in Major Depression. Curr. Top. Med. Chem. 2022, 22, 1731–1735. [Google Scholar] [CrossRef]
- Zhou, X.; Fernando, S.M.; Pan, A.Y.; Laposa, R.; Cullen, K.R.; Klimes-Dougan, B.; Andreazza, A.C. Characterizing the NLRP3 Inflammasome in Mood Disorders: Overview, Technical Development, and Measures of Peripheral Activation in Adolescent Patients. Int. J. Mol. Sci. 2021, 22, 12513. [Google Scholar] [CrossRef]
- Kaufmann, F.N.; Costa, A.P.; Ghisleni, G.; Diaz, A.P.; Rodrigues, A.L.S.; Peluffo, H.; Kaster, M.P. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav. Immun. 2017, 64, 367–383. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Du, X.M.; Ma, X.J.; Zong, Y.; Chen, J.K.; Yu, C.L.; Liu, Y.G.; Chen, Y.C.; Zhao, L.J.; Lu, G.C. Activation of the NLRP3 inflammasome in lipopolysaccharide-induced mouse fatigue and its relevance to chronic fatigue syndrome. J. Neuroinflamm. 2016, 13, 71. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Xia, Z.; Chen, J.; Liu, Y.; Chen, Y.; Zhu, J.; Li, J.; Yu, H.; Zong, Y.; et al. NLRP3 inflammasome activation mediates fatigue-like behaviors in mice via neuroinflammation. Neuroscience 2017, 358, 115–123. [Google Scholar] [CrossRef]
- Kuwar, R.; Rolfe, A.; Di, L.; Blevins, H.; Xu, Y.; Sun, X.; Bloom, G.S.; Zhang, S.; Sun, D. A Novel Inhibitor Targeting NLRP3 Inflammasome Reduces Neuropathology and Improves Cognitive Function in Alzheimer’s Disease Transgenic Mice. J. Alzheimers Dis. 2021, 82, 1769–1783. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Trageser, K.J.; Wu, H.; Herman, F.J.; Iqbal, U.H.; Sebastian-Valverde, M.; Frolinger, T.; Zeng, E.; Pasinetti, G.M. Anxiolytic effects of NLRP3 inflammasome inhibition in a model of chronic sleep deprivation. Transl. Psychiatry 2021, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Aghaie, F.; Moradifar, F.; Hosseini, A. Rapamycin attenuates depression and anxiety-like behaviors through modulation of the NLRP3 pathway in pentylenetetrazole-kindled male Wistar rats. Fundam. Clin. Pharmacol. 2021, 35, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.H.; Stoyanov, D. False dogmas in mood disorders research: Towards a nomothetic network approach. World J. Psychiatry 2022, 12, 651–667. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Morris, G.; Maes, M. Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. 2013, 11, 205. [Google Scholar] [CrossRef]
- Zachrisson, O.; Regland, B.; Jahreskog, M.; Kron, M.; Gottfries, C.G. A rating scale for fibromyalgia and chronic fatigue syndrome (the FibroFatigue scale). J. Psychosom. Res. 2002, 52, 501–509. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II (BDI-II) [Database Record]. In APA PsycTests; American Psychological Association: Washington, DC, USA; p. 1996. [CrossRef]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Lin, L.; Xu, L.; Lv, W.; Han, L.; Xiang, Y.; Fu, L.; Jin, M.; Zhou, R.; Chen, H.; Zhang, A. An NLRP3 inflammasome-triggered cytokine storm contributes to Streptococcal toxic shock-like syndrome (STSLS). PLoS Pathog. 2019, 15, e1007795. [Google Scholar] [CrossRef]
- Tisoncik Jennifer, R.; Korth Marcus, J.; Simmons Cameron, P.; Farrar, J.; Martin Thomas, R.; Katze Michael, G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Li, M.X.; Zheng, H.L.; Luo, Y.; He, J.G.; Wang, W.; Han, J.; Zhang, L.; Wang, X.; Ni, L.; Zhou, H.Y.; et al. Gene deficiency and pharmacological inhibition of caspase-1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Mol. Psychiatry 2018, 23, 556–568. [Google Scholar] [CrossRef]
- Towers, A.E.; Oelschlager, M.L.; Patel, J.; Gainey, S.J.; McCusker, R.H.; Freund, G.G. Acute fasting inhibits central caspase-1 activity reducing anxiety-like behavior and increasing novel object and object location recognition. Metabolism 2017, 71, 70–82. [Google Scholar] [CrossRef]
- Swartz, J.R.; Prather, A.A.; Di Iorio, C.R.; Bogdan, R.; Hariri, A.R. A Functional Interleukin-18 Haplotype Predicts Depression and Anxiety through Increased Threat-Related Amygdala Reactivity in Women but Not Men. Neuropsychopharmacology 2017, 42, 419–426. [Google Scholar] [CrossRef]
- Hardcastle, S.L.; Brenu, E.W.; Johnston, S.; Nguyen, T.; Huth, T.; Ramos, S.; Staines, D.; Marshall-Gradisnik, S. Serum Immune Proteins in Moderate and Severe Chronic Fatigue Syndrome/Myalgic Encephalomyelitis Patients. Int. J. Med. Sci. 2015, 12, 764–772. [Google Scholar] [CrossRef]
- VanElzakker, M.B.; Brumfield, S.A.; Lara Mejia, P.S. Neuroinflammation and Cytokines in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Critical Review of Research Methods. Front. Neurol. 2019, 9, 1033. [Google Scholar] [CrossRef]
- Song, L.; Pei, L.; Yao, S.; Wu, Y.; Shang, Y. NLRP3 Inflammasome in Neurological Diseases, from Functions to Therapies. Front. Cell. Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef]
- Cannon, J.G. Inflammatory Cytokines in Nonpathological States. News Physiol Sci. 2000, 15, 298–303. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Depino, A.M.; Prada, F.; Muraro, N.; Campbell, S.; Podhajcer, O.; Perry, V.H.; Anthony, D.C.; Pitossi, F.J. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am. J. Pathol. 2004, 165, 1827–1837. [Google Scholar] [CrossRef]
- Nakahira, M.; Ahn, H.J.; Park, W.R.; Gao, P.; Tomura, M.; Park, C.S.; Hamaoka, T.; Ohta, T.; Kurimoto, M.; Fujiwara, H. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J. Immunol. 2002, 168, 1146–1153. [Google Scholar] [CrossRef]
- Bossù, P.; Ciaramella, A.; Salani, F.; Vanni, D.; Palladino, I.; Caltagirone, C.; Scapigliati, G. Interleukin-18, from neuroinflammation to Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 4213–4224. [Google Scholar] [CrossRef] [PubMed]
- Felderhoff-Mueser, U.; Schmidt, O.I.; Oberholzer, A.; Bührer, C.; Stahel, P.F. IL-18: A key player in neuroinflammation and neurodegeneration? Trends Neurosci. 2005, 28, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Licastro, F.; Pedrini, S.; Caputo, L.; Annoni, G.; Davis, L.J.; Ferri, C.; Casadei, V.; Grimaldi, L.M. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: Peripheral inflammation or signals from the brain? J. Neuroimmunol. 2000, 103, 97–102. [Google Scholar] [CrossRef] [PubMed]
- De Jong, B.A.; Huizinga, T.W.; Bollen, E.L.; Uitdehaag, B.M.; Bosma, G.P.; van Buchem, M.A.; Remarque, E.J.; Burgmans, A.C.; Kalkers, N.F.; Polman, C.H.; et al. Production of IL-1beta and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis. J. Neuroimmunol. 2002, 126, 172–179. [Google Scholar] [CrossRef]
- Huang, W.X.; Huang, P.; Hillert, J. Increased expression of caspase-1 and interleukin-18 in peripheral blood mononuclear cells in patients with multiple sclerosis. Mult. Scler. J. 2004, 10, 482–487. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396. [Google Scholar] [CrossRef]
- Gameil, M.A.; Marzouk, R.E.; Elsebaie, A.H.; Rozaik, S.E. Long-term clinical and biochemical residue after COVID-19 recovery. Egypt. Liver J. 2021, 11, 74. [Google Scholar] [CrossRef]
- Kuta, A.E.; Baum, L.L. C-reactive protein is produced by a small number of normal human peripheral blood lymphocytes. J. Exp. Med. 1986, 164, 321–326. [Google Scholar] [CrossRef]
- Song, I.U.; Kim, J.S.; Chung, S.W.; Lee, K.S. Is there an association between the level of high-sensitivity C-reactive protein and idiopathic Parkinson’s disease? A comparison of Parkinson’s disease patients, disease controls and healthy individuals. Eur. Neurol. 2009, 62, 99–104. [Google Scholar] [CrossRef]
- Windgassen, E.B.; Funtowicz, L.; Lunsford, T.N.; Harris, L.A.; Mulvagh, S.L. C-reactive protein and high-sensitivity C-reactive protein: An update for clinicians. Postgrad. Med. 2011, 123, 114–119. [Google Scholar] [CrossRef]
- Hsuchou, H.; Kastin, A.J.; Mishra, P.K.; Pan, W. C-reactive protein increases BBB permeability: Implications for obesity and neuroinflammation. Cell. Physiol. Biochem. 2012, 30, 1109–1119. [Google Scholar] [CrossRef]
- Belin, C.; Devic, P.; Ayrignac, X.; Dos Santos, A.; Paix, A.; Sirven-Villaros, L.; Simard, C.; Lamure, S.; Gastinne, T.; Ursu, R.; et al. Description of neurotoxicity in a series of patients treated with CAR T-cell therapy. Sci. Rep. 2020, 10, 18997. [Google Scholar] [CrossRef]
- Ge, X.; Xu, X.-y.; Feng, C.-h.; Wang, Y.; Li, Y.-l.; Feng, B. Relationships among serum C-reactive protein, receptor for advanced glycation products, metabolic dysfunction, and cognitive impairments. BMC Neurol. 2013, 13, 110. [Google Scholar] [CrossRef]
- Elkind, M.S.; Tai, W.; Coates, K.; Paik, M.C.; Sacco, R.L. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern. Med. 2006, 166, 2073–2080. [Google Scholar] [CrossRef]
- Di Napoli, M.; Papa, F.; Bocola, V. C-reactive protein in ischemic stroke: An independent prognostic factor. Stroke 2001, 32, 917–924. [Google Scholar] [CrossRef]
- Masotti, L.; Ceccarelli, E.; Forconi, S.; Cappelli, R. Prognostic role of C-reactive protein in very old patients with acute ischaemic stroke. J. Intern. Med. 2005, 258, 145–152. [Google Scholar] [CrossRef]
- Montaner, J.; Fernandez-Cadenas, I.; Molina, C.A.; Ribó, M.; Huertas, R.; Rosell, A.; Penalba, A.; Ortega, L.; Chacón, P.; Alvarez-Sabín, J. Poststroke C-reactive protein is a powerful prognostic tool among candidates for thrombolysis. Stroke 2006, 37, 1205–1210. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Kanchanatawan, B.; Sirivichayakul, S.; Mahieu, B.; Nowak, G.; Maes, M. Lower Serum Zinc and Higher CRP Strongly Predict Prenatal Depression and Physio-somatic Symptoms, Which All Together Predict Postnatal Depressive Symptoms. Mol. Neurobiol. 2017, 54, 1500–1512. [Google Scholar] [CrossRef]
- Vasupanrajit, A.; Jirakran, K.; Tunvirachaisakul, C.; Solmi, M.; Maes, M. Inflammation and nitro-oxidative stress in current suicidal attempts and current suicidal ideation: A systematic review and meta-analysis. Mol. Psychiatry 2022, 27, 1350–1361. [Google Scholar] [CrossRef]
- Costello, H.; Gould, R.L.; Abrol, E.; Howard, R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open 2019, 9, e027925. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, R.; Sartor, M.L.; Scott, F.; Cleare, A.J. Inflammatory proteins are altered in chronic fatigue syndrome-A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2019, 107, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Arce Rentería, M.; Gillett, S.R.; McClure, L.A.; Wadley, V.G.; Glasser, S.P.; Howard, V.J.; Kissela, B.M.; Unverzagt, F.W.; Jenny, N.S.; Manly, J.J.; et al. C-reactive protein and risk of cognitive decline: The REGARDS study. PLoS ONE 2020, 15, e0244612. [Google Scholar] [CrossRef] [PubMed]
- Mathy-Hartert, M.; Bourgeois, E.; Grülke, S.; Deby-Dupont, G.; Caudron, I.; Deby, C.; Lamy, M.; Serteyn, D. Purification of myeloperoxidase from equine polymorphonuclear leucocytes. Can. J. Vet. Res. 1998, 62, 127–132. [Google Scholar] [PubMed]
- Da Cruz Nizer, W.S.; Inkovskiy, V.; Overhage, J. Surviving Reactive Chlorine Stress: Responses of Gram-Negative Bacteria to Hypochlorous Acid. Microorganisms 2020, 8, 1220. [Google Scholar] [CrossRef]
- Maes, M.; Landucci Bonifacio, K.; Morelli, N.R.; Vargas, H.O.; Barbosa, D.S.; Carvalho, A.F.; Nunes, S.O.V. Major Differences in Neurooxidative and Neuronitrosative Stress Pathways Between Major Depressive Disorder and Types I and II Bipolar Disorder. Mol. Neurobiol. 2019, 56, 141–156. [Google Scholar] [CrossRef]
- Gray, E.; Thomas, T.L.; Betmouni, S.; Scolding, N.; Love, S. Elevated Activity and Microglial Expression of Myeloperoxidase in Demyelinated Cerebral Cortex in Multiple Sclerosis. Brain Pathol. 2008, 18, 86–95. [Google Scholar] [CrossRef]
- Green, P.S.; Mendez, A.J.; Jacob, J.S.; Crowley, J.R.; Growdon, W.; Hyman, B.T.; Heinecke, J.W. Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. J. Neurochem. 2004, 90, 724–733. [Google Scholar] [CrossRef]
- Vaccarino, V.; Brennan, M.-L.; Miller, A.H.; Bremner, J.D.; Ritchie, J.C.; Lindau, F.; Veledar, E.; Su, S.; Murrah, N.V.; Jones, L.; et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: A twin study. Biol. Psychiatry 2008, 64, 476–483. [Google Scholar] [CrossRef]
- Liang, S.; Li, X.; Huang, W.; Gong, H. Change of serum myeloperoxidase and lipoxin A4 level in coronary heart disease patients with anxiety and/or depression. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2013, 38, 370–375. [Google Scholar] [CrossRef]
- Talarowska, M.; Szemraj, J.; Gałecki, P. Myeloperoxidase gene expression and cognitive functions in depression. Adv. Med. Sci. 2015, 60, 1–5. [Google Scholar] [CrossRef]
- Shrivastava, S.; Chelluboina, S.; Jedge, P.; Doke, P.; Palkar, S.; Mishra, A.C.; Arankalle, V.A. Elevated Levels of Neutrophil Activated Proteins, Alpha-Defensins (DEFA1), Calprotectin (S100A8/A9) and Myeloperoxidase (MPO) Are Associated with Disease Severity in COVID-19 Patients. Front. Cell. Infect. Microbiol. 2021, 11, 1056. [Google Scholar] [CrossRef]
- Islam, M.R.; Islam, M.R.; Shalahuddin Qusar, M.M.A.; Islam, M.S.; Kabir, M.H.; Mustafizur Rahman, G.K.M.; Islam, M.S.; Hasnat, A. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: A case-control study. BMC Psychiatry 2018, 18, 94. [Google Scholar] [CrossRef]
- Bowden, C.L.; Huang, L.G.; Javors, M.A.; Johnson, J.M.; Seleshi, E.; McIntyre, K.; Contreras, S.; Maas, J.W. Calcium function in affective disorders and healthy controls. Biol. Psychiatry 1988, 23, 367–376. [Google Scholar] [CrossRef]
- Al-Dujaili, A.H.; Al-Hakeim, H.K.; Twayej, A.J.; Maes, M. Total and ionized calcium and magnesium are significantly lowered in drug-naïve depressed patients: Effects of antidepressants and associations with immune activation. Metab. Brain Dis. 2019, 34, 1493–1503. [Google Scholar] [CrossRef]
- Jung, K.I.; Ock, S.M.; Chung, J.H.; Song, C.H. Associations of serum Ca and Mg levels with mental health in adult women without psychiatric disorders. Biol. Trace Elem. Res. 2010, 133, 153–161. [Google Scholar] [CrossRef]
- Paul, I.A. Antidepressant activity and calcium signaling cascades. Hum. Psychopharmacol. 2001, 16, 71–80. [Google Scholar] [CrossRef]
- Toescu, E.C.; Verkhratsky, A. The importance of being subtle: Small changes in calcium homeostasis control cognitive decline in normal aging. Aging Cell 2007, 6, 267–273. [Google Scholar] [CrossRef]
- Hurst, K. Primary hyperparathyroidism as a secondary cause of depression. J. Am. Board Fam. Med. 2010, 23, 677–680. [Google Scholar] [CrossRef]
- Grützner, T.M.; Listunova, L.; Fabian, G.A.; Kramer, B.A.; Flach, D.; Weisbrod, M.; Roesch-Ely, D.; Sharma, A. Serum calcium levels and neuropsychological performance in depression and matched healthy controls: Reversal of correlation a marker of the aging cognitive clock? Psychoneuroendocrinology 2018, 91, 198–205. [Google Scholar] [CrossRef]
- Almulla, A.F.; Thipakorn, Y.; Vasupanrajit, A.; Abo Algon, A.A.; Tunvirachaisakul, C.; Hashim Aljanabi, A.A.; Ox-enkrug, G.; Al-Hakeim, H.K.; Maes, M. The tryptophan catabolite or kynurenine pathway in major depressive and bi-polar disorder: A systematic review and meta-analysis. Brain Behav. Immun. Health 2022, 26, 100537. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Leonard, B.E.; Myint, A.M.; Kubera, M.; Verkerk, R. The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 702–721. [Google Scholar] [CrossRef]
- Maes, M.; Song, C.; Lin, A.H.; Bonaccorso, S.; Kenis, G.; De Jongh, R.; Bosmans, E.; Scharpé, S. Negative immunoregulatory effects of antidepressants: Inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology 1999, 20, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef]
- Couzin-Frankel, J.; Vogel, G. Vaccines may cause rare, Long COVID-like symptoms. Science 2022, 375, 364–366. [Google Scholar] [CrossRef]
- Spinicci, M. Different SARS-CoV-2 variants may give rise to different long COVID symptoms, study suggests. In Proceedings of the European Congress of Clinical Microbiology & Infectious Diseases (ECCMID 2022), Lisbon, Portugal, 23–26 April 2022. [Google Scholar]
- Al-Rashedi, N.A.M.; Alburkat, H.; Hadi, A.O.; Munahi, M.G.; Jasim, A.; Hameed, A.; Oda, B.S.; Lilo, K.M.; AlObaidi, L.A.H.; Vapalahti, O.; et al. High prevalence of an alpha variant lineage with a premature stop codon in ORF7a in Iraq, winter 2020–2021. PLoS ONE 2022, 17, e0267295. [Google Scholar] [CrossRef]
- Huaxia. Iraq Reports First Cases of COVID-19 Omicron Variant. Available online: https://english.news.cn/20220106/e6eedae158c44eea8433caf983a10738/c.html (accessed on 13 October 2022).

| Variables | HC (n = 39) A | Low TO-NT (n = 56) B | High TO-NT (n = 30) C | F/X2 | df | p |
|---|---|---|---|---|---|---|
| Age (years) | 28.3 (7.6) | 27.6 (5.4) | 29.8 (7.3) | 1.15 | 2/122 | 0.320 |
| Sex (M/F) | 42/15 | 40/16 | 22/8 | 1.42 | 2 | 0.512 |
| Marital state (Ma/S) | 22/17 | 31/25 | 18/12 | 0.18 | 2 | 0.946 |
| Smoking (Y/N) | 13/26 | 16/40 | 11/19 | 0.64 | 2 | 0.732 |
| Residency (U/R) | 31/8 | 46/10 | 25/5 | 0.19 | 2 | 0.914 |
| Vaccination (A/Pf/S) | 9/21/9 | 14/31/11 | 6/17/7 | 0.42 | 4 | 0.979 |
| BMI (Kg/m2) | 25.60 (3.97) | 25.71 (3.74) | 26.96 (5.75) | 0.99 | 2/122 | 0.372 |
| Education (years) | 15.0 (1.3) | 15.7 (1.8) | 15.5 (1.7) | 2.73 | 2/122 | 0.069 |
| Peak BT (℃) | 36.86 (0.25) B,C | 38.20 (0.64) A,C | 39.31 (0.83) A,B | 138.38 | 2/122 | <0.001 |
| Lowest SpO2 (%) | 95.08 (1.52) B,C | 92.41 (2.74) A,C | 88.27 (4.62) A,B | 42.81 | 2/122 | <0.001 |
| TO2 index (z scores) | −1.01 (0.240) B,C | 0.054 (0.52) A,C | 1.21 (0.86) A,B | 131.43 | 2/122 | <0.001 |
| Composite NT (z score) | −0.595 (0.85) B,C | −0.143 (0.80) A,C | 1.04 (0.66) A,B | 37.91 | 2/122 | <0.001 |
| Variables | HC (n = 39) A | Low TO-NT (n = 56) B | High TO-NT (n = 30) C | F/X2 df = 2/119 | p |
|---|---|---|---|---|---|
| Total HAMD | 5.5 (0.67) B,C | 15.7 (0.6) A,C | 19.1 (0.8) A,B | 105.21 | <0.001 |
| Total BDI | 8.6 (1.0) B,C | 23.5 (0.9) A,C | 27.0 (1.2) A,B | 85.35 | <0.001 |
| Total HAMA | 7.8 (1.1) B,C | 15.4 (0.9) A,C | 19.5 (1.3) A,B | 26.23 | <0.001 |
| Total FF | 10.9 (1.8) B,C | 24.7 (1.5) A,C | 35.4 (2.0) A,B | 43.05 | <0.001 |
| Pure FF (z score) | −0.877 (0.122) B,C | 0.146 (0.101) A,C | 0.867 (0.138) A,B | 46.06 | <0.001 |
| Pure HAMD (z score) | −0.964 (0.118) B,C | 0.296 (0.098) A,C | 0.702 (0.133) A,B | 51.04 | <0.001 |
| Pure BDI (z score) | −1.030 (0.113) B,C | 0.348 (0.094) A,C | 0.700 (0.128) A,B | 62.79 | <0.001 |
| Pure HAMA (z score) | −0.606 (0.139) B,C | 0.190 (0.115) A | 0.432 (0.157) A | 14.37 | <0.001 |
| Physiosom HAMA (z score) | −0.495 (0.145) B,C | 0.029 (0.120) A,C | 0.588 (0.164) A,B | 12.21 | <0.001 |
| Physiosom HAMD (z score) | −1.031 (0.118) B,C | 0.347 (0.098) A,C | 0.702 (0.133) A,B | 57.93 | <0.001 |
| Biomarkers | HC (n = 39) A | Low TO-NT (n = 56) B | High TO-NT (n = 30) C | F/X2 df = 2/117 | p |
|---|---|---|---|---|---|
| Caspase 1 (pg/mL) | 73.90 (3.27) C | 71.83(2.75) C | 85.57(3.75) A,B | 4.54 | 0.013 |
| IL-1β (pg/mL) | 4.58 (0.33) C | 5.21(0.282) | 5.81(0.385) A | 2.95 | 0.056 |
| IL-18 (pg/mL) | 233.9 (11.91) | 231.62(10.02) | 261.32(13.67) | 1.67 | 0.192 |
| IL-10 (pg/mL) | 9.09 (1.08) B,C | 14.10(0.911) A | 13.06(1.24) A | 6.50 | 0.002 |
| CRP (mg/L) | 5.02 (0.53) B,C | 6.32 (0.443) A,C | 10.11 (0.604) A,B | 27.08 | <0.001 |
| MPO (ng/mL) | 43.1 (3.3) | 49.9 (2.8) | 51.3 (3.8) | 1.72 | 0.184 |
| TAC (U/mL) | 6.74 (0.51) | 6.78 (0.43) | 7.02 (0.58) | 0.08 | 0.925 |
| AOPP (µmol/g) | 0.92 (0.14) B,C | 1.29 (0.12) A,C | 1.76 (0.16) B,C | 7.80 | 0.001 |
| Total calcium (mM) | 2.56 (0.03) B,C | 2.26 (0.02) A | 2.33 (0.03) A | 40.45 | <0.001 |
| Dependent Variables | Explanatory Variables | B | t | p | Model R2 | F | df | p |
|---|---|---|---|---|---|---|---|---|
| #1 Physio-somatic phenome | Model Total calcium NT BMI | −0.414 0.420 0.164 | −6.00 6.16 2.42 | <0.001 <0.001 0.017 | 0.460 | 34.06 | 3/120 | <0.001 |
| #2 Physio-somatic phenome | Model Peak BT Calcium NT | 0.472 −0.253 0.229 | 6.29 −3.76 3.38 | <0.001 <0.001 <0.001 | 0.574 | 53.88 | 3/120 | <0.001 |
| #3 Pure FF | Model Total calcium CRP Education AOPP AstraZeneca vaccination BMI | −0.346 0.242 0.192 0.214 0.166 0.149 | −4.56 3.09 2.59 2.74 2.23 1.99 | <0.001 0.002 0.011 0.007 0.027 0.048 | 0.371 | 11.61 | 6/118 | <0.001 |
| #4 Pure HAMD | Model Total calcium CRP Education AstraZeneca vaccination AOPP MPO | −0.381 0.224 0.244 0.186 0.201 0.155 | −5.25 2.97 3.37 2.58 2.67 2.11 | <0.001 0.004 0.001 0.011 0.009 0.037 | 0.413 | 13.86 | 6/118 | <0.001 |
| #5 Pure BDI | Model Total calcium AOPP Education CRP Interleukin-1β | −0.368 0.247 0.197 0.218 0.174 | −4.87 3.17 2.61 2.80 2.22 | <0.001 0.002 0.010 0.006 0.028 | 0.372 | 14.12 | 5/119 | <0.001 |
| #6 Pure HAMA | Model Total calcium CRP MPO | −0.296 0.204 0.170 | −3.52 2.42 2.06 | 0.001 0.017 0.041 | 0.183 | 9.06 | 3/121 | <0.001 |
| #7 Physiosom HAMD | Model Total calcium CRP Interleukin 18 Vaccination Sinopharm | −0.432 0.301 0.181 −0.159 | −5.84 4.07 2.49 −2.19 | <0.001 <0.001 0.014 0.030 | 0.374 | 17.88 | 4/120 | <0.001 |
| #8 Physiosom HAMA | Model Total calcium MPO BMI | −0.301 0.231 0.207 | −3.70 2.85 2.53 | <0.001 0.005 0.013 | 0.217 | 11.17 | 3/121 | <0.001 |
| Sets | Variables | Canonical Loadings |
|---|---|---|
| Set 1 Dependent | Pure FF | 0.845 |
| Pure HAMD | 0.815 | |
| Physiosom HAMD | 0.784 | |
| Pure HAMA | 0.585 | |
| Physiosom HAMA | 0.629 | |
| Pure BDI | 0.825 | |
| Set 2 Explanatory | NT composite | 0.670 |
| TO2 index | 0.875 | |
| Total calcium | 0.674 | |
| Statistics | F (df) | 8.675 (18/325) |
| P | <0.001 | |
| Correlation | 0.799 | |
| Set 1/set 2 | 0.355 | |
| Set 2 by itself | 0.556 | |
| Set 1 by itself | 0.569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hakeim, H.K.; Al-Rubaye, H.T.; Almulla, A.F.; Al-Hadrawi, D.S.; Maes, M. Chronic Fatigue, Depression and Anxiety Symptoms in Long COVID Are Strongly Predicted by Neuroimmune and Neuro-Oxidative Pathways Which Are Caused by the Inflammation during Acute Infection. J. Clin. Med. 2023, 12, 511. https://doi.org/10.3390/jcm12020511
Al-Hakeim HK, Al-Rubaye HT, Almulla AF, Al-Hadrawi DS, Maes M. Chronic Fatigue, Depression and Anxiety Symptoms in Long COVID Are Strongly Predicted by Neuroimmune and Neuro-Oxidative Pathways Which Are Caused by the Inflammation during Acute Infection. Journal of Clinical Medicine. 2023; 12(2):511. https://doi.org/10.3390/jcm12020511
Chicago/Turabian StyleAl-Hakeim, Hussein Kadhem, Haneen Tahseen Al-Rubaye, Abbas F. Almulla, Dhurgham Shihab Al-Hadrawi, and Michael Maes. 2023. "Chronic Fatigue, Depression and Anxiety Symptoms in Long COVID Are Strongly Predicted by Neuroimmune and Neuro-Oxidative Pathways Which Are Caused by the Inflammation during Acute Infection" Journal of Clinical Medicine 12, no. 2: 511. https://doi.org/10.3390/jcm12020511
APA StyleAl-Hakeim, H. K., Al-Rubaye, H. T., Almulla, A. F., Al-Hadrawi, D. S., & Maes, M. (2023). Chronic Fatigue, Depression and Anxiety Symptoms in Long COVID Are Strongly Predicted by Neuroimmune and Neuro-Oxidative Pathways Which Are Caused by the Inflammation during Acute Infection. Journal of Clinical Medicine, 12(2), 511. https://doi.org/10.3390/jcm12020511









