Association of the Cumulative Live Birth Rate with the Factors in Assisted Reproductive Technology: A Retrospective Study of 16,583 Women
Abstract
1. Introduction
2. Materials and Methods
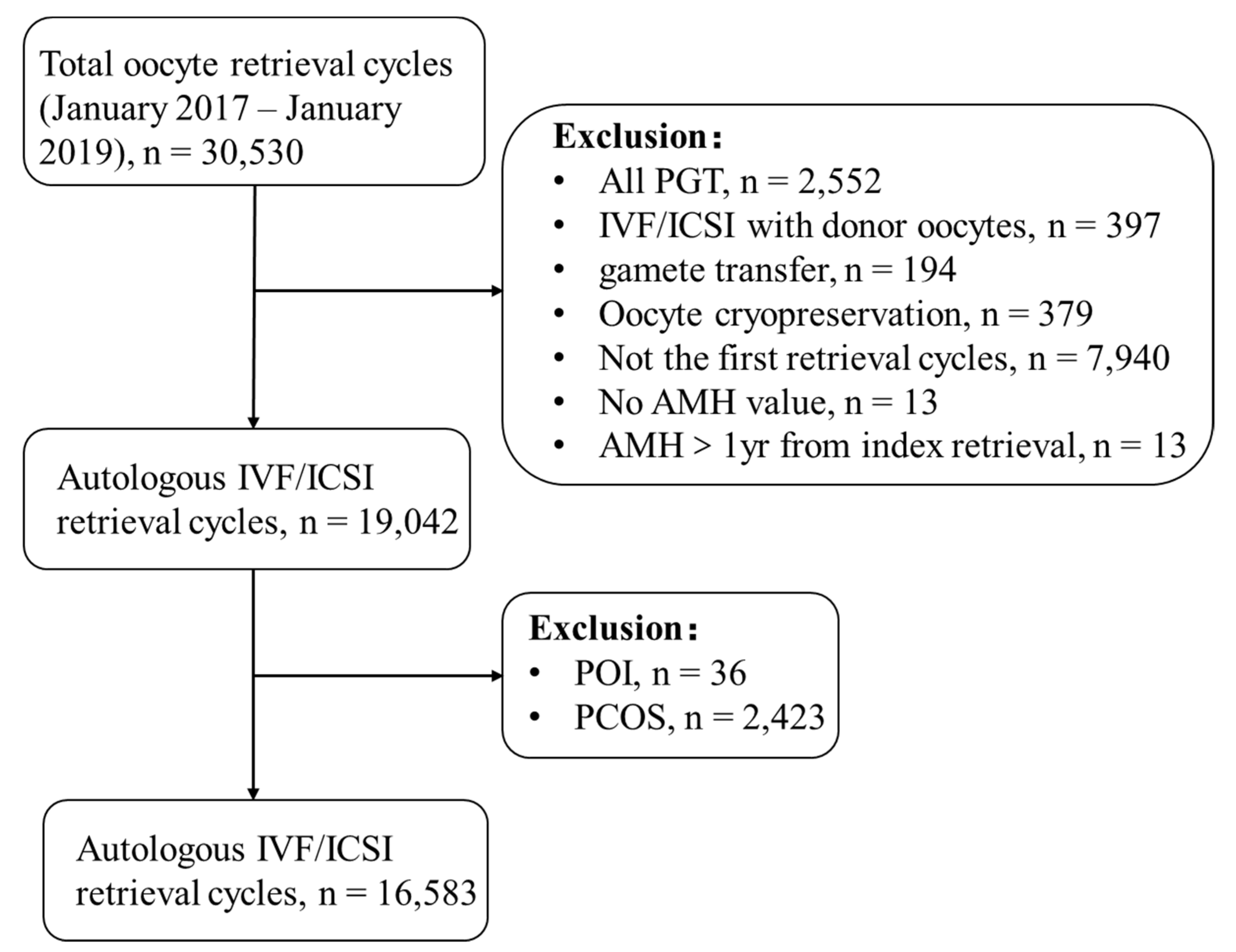
2.1. Study Design and Population
2.2. IVF/ICSI Protocols
2.3. Outcome
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
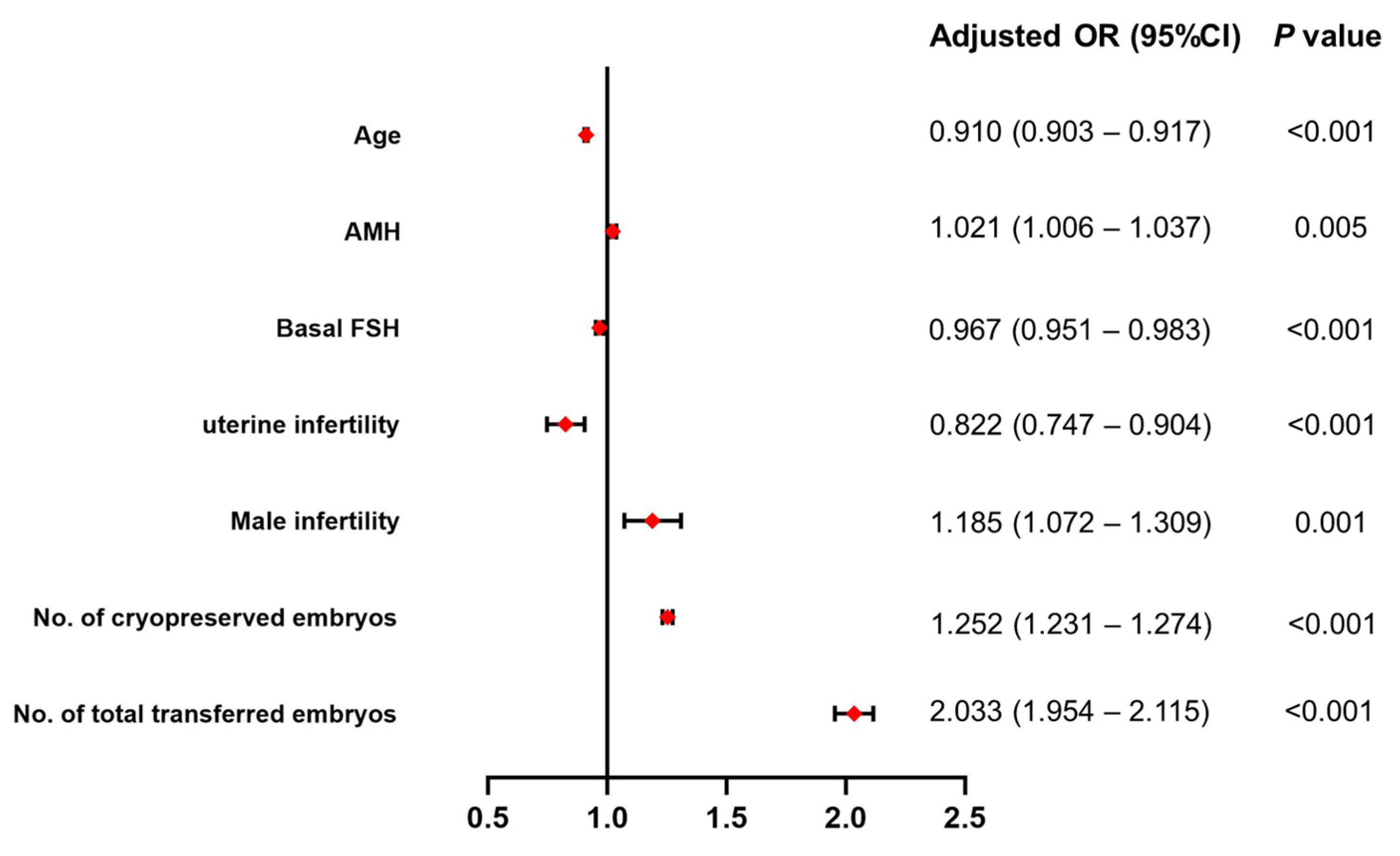
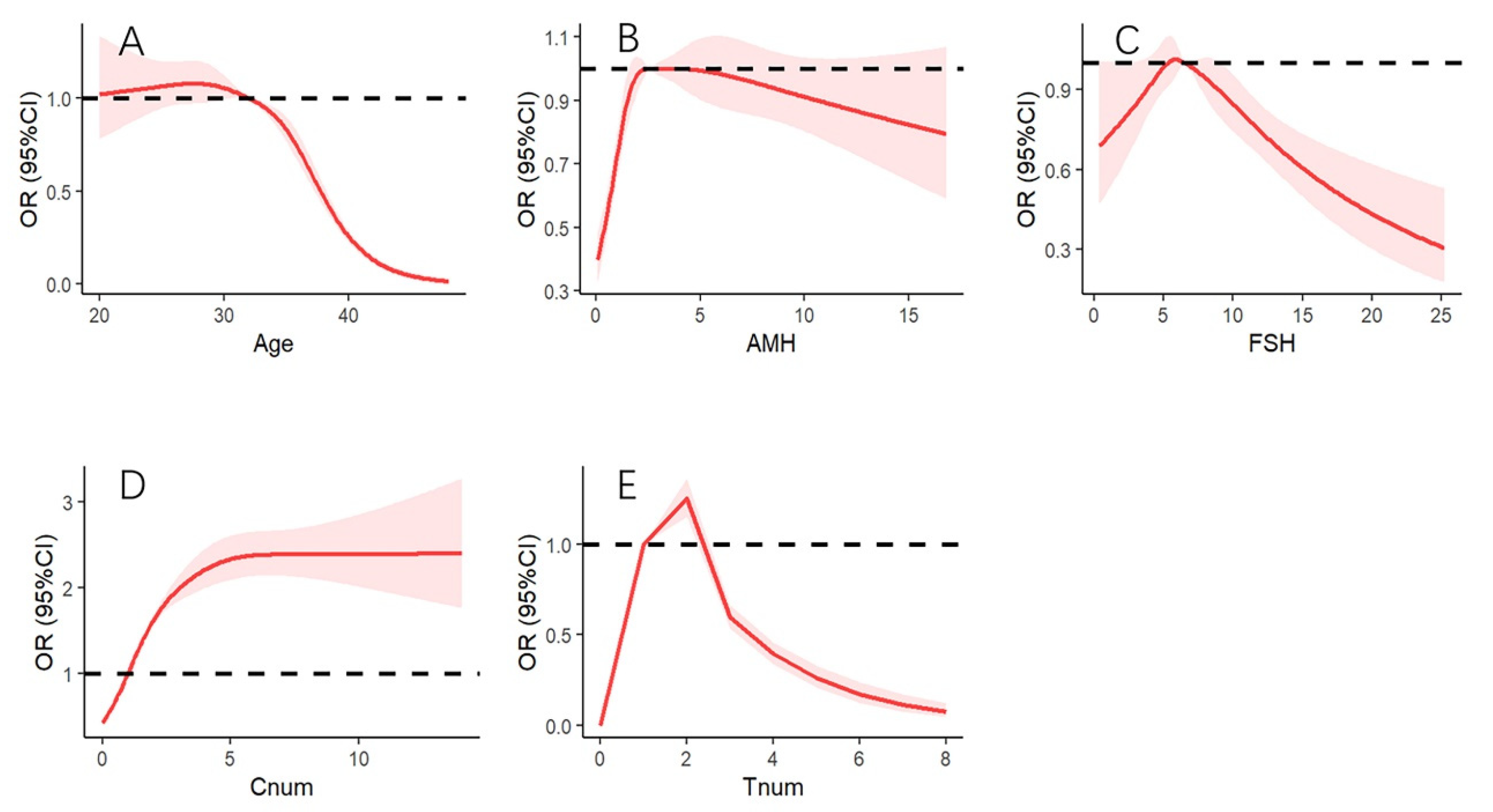
3.2. Association of AMH and Other Factors with CLBR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Gong, T.T.; Jiang, Y.T.; Zhang, S.; Zhao, Y.H.; Wu, Q.J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging 2019, 11, 10952–10991. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zheng, D.; Wu, H.; Li, R.; Xu, S.; Kang, Y.; Cao, Y.; Chen, X.; Zhu, Y.; Xu, S.; et al. Epidemiology of infertility in China: A population-based study. BJOG 2018, 125, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Sarac, M.; Koc, I. Prevalence and Risk Factors of Infertility in Turkey: Evidence from Demographic and Health Surveys, 1993–2013. J. Biosoc. Sci. 2018, 50, 472–490. [Google Scholar] [CrossRef] [PubMed]
- Roque, M.; Haahr, T.; Geber, S.; Esteves, S.C.; Humaidan, P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: A systematic review and meta-analysis of reproductive outcomes. Hum. Reprod. Update 2019, 25, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, Y.; Hao, C.; Zhang, H.; Wei, D.; Zhang, Y.; Zhu, Y.; Deng, X.; Qi, X.; Li, H.; et al. Transfer of Fresh versus Frozen Embryos in Ovulatory Women. N. Engl. J. Med. 2018, 378, 126–136. [Google Scholar] [CrossRef]
- Maheshwari, A.; Pandey, S.; Amalraj Raja, E.; Shetty, A.; Hamilton, M.; Bhattacharya, S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum. Reprod. Update 2018, 24, 35–58. [Google Scholar] [CrossRef]
- Maheshwari, A.; McLernon, D.; Bhattacharya, S. Cumulative live birth rate: Time for a consensus? Hum. Reprod. 2015, 30, 2703–2707. [Google Scholar] [CrossRef]
- Zhou, Q.W.; Jing, S.; Xu, L.; Guo, H.; Lu, C.F.; Gong, F.; Lu, G.X.; Lin, G.; Gu, Y.F. Clinical and neonatal outcomes of patients of different ages following transfer of thawed cleavage embryos and blastocysts cultured from thawed cleavage-stage embryos. PLoS ONE 2018, 13, e0207340. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, F.L.; Liu, X.C.; Hu, L.L.; Dai, S.J.; Li, G.; Kong, H.J.; Guo, Y.H. Impact of Female Obesity on Cumulative Live Birth Rates in the First Complete Ovarian Stimulation Cycle. Front. Endocrinol. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.E.; Brown, M.B.; Wantman, E.; Kalra, S.K.; Luke, B. Live birth rates and birth outcomes by diagnosis using linked cycles from the SART CORS database. J. Assist. Reprod. Genet. 2013, 30, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Lew, R. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Seifer, D.B.; Tal, R.; Granger, E.; Wantman, E.; Tal, O. AMH Highly Correlates with Cumulative Live Birth Rate in Women with Diminished Ovarian Reserve Independent of Age. J. Clin. Endocrinol. Metab. 2021, 106, 2754–2766. [Google Scholar] [CrossRef] [PubMed]
- Ata, B.; Seyhan, A.; Seli, E. Diminished ovarian reserve versus ovarian aging: Overlaps and differences. Curr. Opin. Obstet. Gynecol. 2019, 31, 139–147. [Google Scholar] [CrossRef] [PubMed]
- McLernon, D.J.; Raja, E.A.; Toner, J.P.; Baker, V.L.; Doody, K.J.; Seifer, D.B.; Sparks, A.E.; Wantman, E.; Lin, P.C.; Bhattacharya, S.; et al. Predicting personalized cumulative live birth following in vitro fertilization. Fertil. Steril. 2022, 117, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hao, G.; Wang, Q.; Liu, H.; Wang, Z.; Jiang, Q.; Shi, Y.; Chen, Z.-J. Major Factors Affecting the Live Birth Rate after Frozen Embryo Transfer Among Young Women. Front. Med. 2020, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xu, X.; Zhang, L.; Zhang, L.; Yan, L.; Ma, J. Endometrial thickness is associated with incidence of small-for-gestational-age infants in fresh in vitro fertilization-intracytoplasmic sperm injection and embryo transfer cycles. Fertil. Steril. 2020, 113, 745–752. [Google Scholar] [CrossRef]
- Zong, L.; Liu, P.; Zhou, L.; Wei, D.; Ding, L.; Qin, Y. Increased risk of maternal and neonatal complications in hormone replacement therapy cycles in frozen embryo transfer. Reprod. Biol. Endocrinol. 2020, 18, 36. [Google Scholar] [CrossRef]
- Chen, Z.J.; Shi, Y.; Sun, Y.; Zhang, B.; Liang, X.; Cao, Y.; Yang, J.; Liu, J.; Wei, D.; Weng, N.; et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 523–533. [Google Scholar] [CrossRef]
- Xin, A.; Qu, R.; Chen, G.; Zhang, L.; Chen, J.; Tao, C.; Fu, J.; Tang, J.; Ru, Y.; Chen, Y.; et al. Disruption in ACTL7A causes acrosomal ultrastructural defects in human and mouse sperm as a novel male factor inducing early embryonic arrest. Sci. Adv. 2020, 6, eaaz4796. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ma, Y.; Li, L.; Hu, L.; Wang, F.; Zhang, Y.; Dai, S.; Sun, Y. Factors Associated with Ovarian Hyperstimulation Syndrome (OHSS) Severity in Women with Polycystic Ovary Syndrome Undergoing IVF/ICSI. Front. Endocrinol. 2020, 11, 615957. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Carvalho, J.F.; Martinhago, C.D.; Melo, A.A.; Bento, F.C.; Humaidan, P.; Alviggi, C. Estimation of age-dependent decrease in blastocyst euploidy by next generation sequencing: Development of a novel prediction model. Panminerva Med. 2019, 61, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.H.; Ho, P.C. Ageing and ART: A waste of time and money? Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Hogan, R.G.; Wang, A.Y.; Li, Z.; Hammarberg, K.; Johnson, L.; Mol, B.W.; Sullivan, E.A. Oocyte donor age has a significant impact on oocyte recipients’ cumulative live-birth rate: A population-based cohort study. Fertil. Steril. 2019, 112, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Abuzeid, M.I.; Bolonduro, O.; La Chance, J.; Abozaid, T.; Urich, M.; Ullah, K.; Ali, T.; Ashraf, M.; Khan, I. Cumulative live birth rate and assisted reproduction: Impact of female age and transfer day. Facts Views Vis. ObGyn 2014, 6, 145–149. [Google Scholar] [PubMed]
- Khalife, D.; Nassar, A.; Khalil, A.; Awwad, J.; Abu Musa, A.; Hannoun, A.; El Taha, L.; Khalifeh, F.; Abiad, M.; Ghazeeri, G. Cumulative Live-Birth Rates by Maternal Age after One or Multiple In Vitro Fertilization Cycles: An Institutional Experience. Int. J. Fertil. Steril. 2020, 14, 34–40. [Google Scholar]
- Hu, K.L.; Liu, F.T.; Xu, H.; Li, R.; Qiao, J. Association of serum anti-Müllerian hormone and other factors with cumulative live birth rate following IVF. Reprod. Biomed. Online 2020, 40, 675–683. [Google Scholar] [CrossRef]
- Zhang, B.; Meng, Y.; Jiang, X.; Liu, C.; Zhang, H.; Cui, L.; Chen, Z.J. IVF outcomes of women with discrepancies between age and serum anti-Müllerian hormone levels. Reprod. Biol. Endocrinol. 2019, 17, 58. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil. Steril. 2014, 101, 633–634. [Google Scholar] [CrossRef]
- Sahmay, S.; Atakul, N.; Oncul, M.; Tuten, A.; Aydogan, B.; Seyisoglu, H. Serum anti-Mullerian hormone levels in the main phenotypes of polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Kotanidis, L.; Nikolettos, K.; Petousis, S.; Asimakopoulos, B.; Chatzimitrou, E.; Kolios, G.; Nikolettos, N. The use of serum anti-Mullerian hormone (AMH) levels and antral follicle count (AFC) to predict the number of oocytes collected and availability of embryos for cryopreservation in IVF. J. Endocrinol. Investig. 2016, 39, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- Pankhurst, M.W. A putative role for anti-Müllerian hormone (AMH) in optimising ovarian reserve expenditure. J. Endocrinol. 2017, 233, R1–R13. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Mensah, V.; Huber, W.J., 3rd; Huang, Y.T.; Alvero, R. Discordant anti-müllerian hormone (AMH) and follicle stimulating hormone (FSH) among women undergoing in vitro fertilization (IVF): Which one is the better predictor for live birth? J. Ovarian Res. 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.; Schorsch, M.; Hahn, T.; Henke, A.; Hoffmann, I.; Seufert, R.; Skala, C. The influence of AMH on IVF success. Arch. Gynecol. Obstet. 2016, 293, 667–673. [Google Scholar] [CrossRef]
- Alson, S.S.E.; Bungum, L.J.; Giwercman, A.; Henic, E. Anti-müllerian hormone levels are associated with live birth rates in ART, but the predictive ability of anti-müllerian hormone is modest. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 199–204. [Google Scholar] [CrossRef]
- Guan, Y.; Kong, P.; Xiao, Z.; Zhang, J.; He, J.; Geng, W.; Yan, J.; Sun, S.; Mu, M.; Du, X.; et al. Independent Variables for Determining the Cumulative Live Birth Rates of Aged Patients with Polycystic Ovary Syndrome or Tubal Factor Infertility: A Retrospective Cohort Study. Front. Endocrinol. 2021, 12, 728051. [Google Scholar] [CrossRef]
- Lukaszuk, K.; Liss, J.; Kunicki, M.; Jakiel, G.; Wasniewski, T.; Woclawek-Potocka, I.; Pastuszek, E. Anti-Müllerian hormone (AMH) is a strong predictor of live birth in women undergoing assisted reproductive technology. Reprod. Biol. 2014, 14, 176–181. [Google Scholar] [CrossRef]
- Peuranpää, P.; Hautamäki, H.; Halttunen-Nieminen, M.; Hydén-Granskog, C.; Tiitinen, A. Low anti-Müllerian hormone level is not a risk factor for early pregnancy loss in IVF/ICSI treatment. Hum. Reprod. 2020, 35, 504–515. [Google Scholar] [CrossRef]
- Reijnders, I.F.; Nelen, W.L.; IntHout, J.; van Herwaarden, A.E.; Braat, D.D.; Fleischer, K. The value of Anti-Müllerian hormone in low and extremely low ovarian reserve in relation to live birth after in vitro fertilization. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 200, 45–50. [Google Scholar] [CrossRef]
- Keane, K.; Cruzat, V.F.; Wagle, S.; Chaudhary, N.; Newsholme, P.; Yovich, J. Specific ranges of anti-Mullerian hormone and antral follicle count correlate to provide a prognostic indicator for IVF outcome. Reprod. Biol. 2017, 17, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Royston, P. The cost of dichotomising continuous variables. BMJ 2006, 332, 1080. [Google Scholar] [CrossRef]
- Arce, J.C.; La Marca, A.; Mirner Klein, B.; Nyboe Andersen, A.; Fleming, R. Antimüllerian hormone in gonadotropin releasing-hormone antagonist cycles: Prediction of ovarian response and cumulative treatment outcome in good-prognosis patients. Fertil. Steril. 2013, 99, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, Y.; Yang, H.; Gao, T.; Yu, C.; Cao, F.; Xia, X.; Wu, J.; Zhou, X.; Chen, L. AMH has no role in predicting oocyte quality in women with advanced age undergoing IVF/ICSI cycles. Sci. Rep. 2020, 10, 19750. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Y.; Wang, X.; Zhang, Y.; Du, A.; Yao, R.; Lv, J.; Luo, H. Serum anti-Müllerian hormone levels are associated with early miscarriage in the IVF/ICSI fresh cycle. BMC Pregnancy Childbirth 2022, 22, 279. [Google Scholar] [CrossRef]
- Kostrzewa, M.; Żyła, M.; Garnysz, K.; Kaczmarek, B.; Szyłło, K.; Grzesiak, M. Anti-Müllerian hormone as a marker of abortion in the first trimester of spontaneous pregnancy. Int. J. Gynaecol. Obstet. 2020, 149, 66–70. [Google Scholar] [CrossRef]
- Hu, K.L.; Liu, F.T.; Xu, H.; Li, R.; Qiao, J. High antimullerian hormone levels are associated with preterm delivery in patients with polycystic ovary syndrome. Fertil. Steril. 2020, 113, 444–452.e441. [Google Scholar] [CrossRef]
- Kaing, A.; Jaswa, E.A.; Diamond, M.P.; Legro, R.S.; Cedars, M.I.; Huddleston, H.G. Highly elevated level of antimüllerian hormone associated with preterm delivery in polycystic ovary syndrome patients who underwent ovulation induction. Fertil. Steril. 2021, 115, 438–446. [Google Scholar] [CrossRef]
- Valeri, C.; Pappalardo, S.; De Felici, M.; Manna, C. Correlation of oocyte morphometry parameters with woman’s age. J. Assist. Reprod. Genet. 2011, 28, 545–552. [Google Scholar] [CrossRef]
- Ligon, S.; Lustik, M.; Levy, G.; Pier, B. Low antimüllerian hormone (AMH) is associated with decreased live birth after in vitro fertilization when follicle-stimulating hormone and AMH are discordant. Fertil. Steril. 2019, 112, 73–81.e71. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Yates, R.W.; Fleming, R. Serum anti-Müllerian hormone and FSH: Prediction of live birth and extremes of response in stimulated cycles—Implications for individualization of therapy. Hum. Reprod. 2007, 22, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- du Boulet, B.; Ranisavljevic, N.; Mollevi, C.; Bringer-Deutsch, S.; Brouillet, S.; Anahory, T. Individualized luteal phase support based on serum progesterone levels in frozen-thawed embryo transfer cycles maximizes reproductive outcomes in a cohort undergoing preimplantation genetic testing. Front. Endocrinol. 2022, 13, 1051857. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Kasapoglu, I.; Cakir, C.; Avci, B.; Uncu, G. Supernumerary embryos, do they show the cycle success in a fresh embryo transfer? A retrospective analysis. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2021, 37, 1107–1110. [Google Scholar] [CrossRef]
- Hill, M.J.; Richter, K.S.; Heitmann, R.J.; Lewis, T.D.; DeCherney, A.H.; Graham, J.R.; Widra, E.; Levy, M.J. Number of supernumerary vitrified blastocysts is positively correlated with implantation and live birth in single-blastocyst embryo transfers. Fertil. Steril. 2013, 99, 1631–1636. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Stoddard, G.; Johnstone, E. A clinical counseling tool predicting supernumerary embryos after a fresh IVF cycle. J. Assist. Reprod. Genet. 2020, 37, 1137–1145. [Google Scholar] [CrossRef]
- Kirillova, A.; Lysenkov, S.; Farmakovskaya, M.; Kiseleva, Y.; Martazanova, B.; Mishieva, N.; Abubakirov, A.; Sukhikh, G. Should we transfer poor quality embryos? Fertil. Res. Pract. 2020, 6, 2. [Google Scholar] [CrossRef]
- Zhang, M.; Bu, T.; Tian, H.; Li, X.; Wang, D.; Wan, X.; Wang, Q.; Mao, X.; La, X. Use of Cumulative Live Birth Rate per Total Number of Embryos to Calculate the Success of IVF in Consecutive IVF Cycles in Women Aged ≥35 Years. BioMed Res. Int. 2019, 2019, 6159793. [Google Scholar] [CrossRef]
- Vergaro, P.; Tiscornia, G.; Rodríguez, A.; Santaló, J.; Vassena, R. Transcriptomic analysis of the interaction of choriocarcinoma spheroids with receptive vs. non-receptive endometrial epithelium cell lines: An in vitro model for human implantation. J. Assist. Reprod. Genet. 2019, 36, 857–873. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 16,583) | No Live Birth (n = 8616) | Live Birth (n = 7967) | p Value |
|---|---|---|---|---|
| Age | 32.68 ± 5.24 | 34.22 ± 5.65 | 31.00 ± 4.15 | <0.001 |
| BMI | 23.90 ± 3.49 | 24.11 ± 3.47 | 23.67 ± 3.50 | <0.001 |
| Duration of infertility | 3.73 ± 2.98 | 3.84 ± 3.24 | 3.62 ± 2.66 | <0.001 |
| FBG | 5.25 ± 0.80 | 5.27 ± 0.79 | 5.24 ± 0.81 | 0.028 |
| AMH | 3.28 ± 2.75 | 2.74 ± 2.64 | 3.87 ± 2.75 | <0.001 |
| Basal FSH | 7.09 ± 2.64 | 7.54 ± 3.10 | 6.60 ± 1.93 | <0.001 |
| Gravidity ≥ 1 (%) | 57.20 | 62.50 | 51.30 | <0.001 |
| Infertility etiology (%) | ||||
| Tubal factor | 79.40 | 79.10 | 79.70 | 0.327 |
| Uterine factor | 18.60 | 22.30 | 14.60 | <0.001 |
| Male factor | 14.80 | 12.60 | 17.30 | <0.001 |
| Unexplained | 6.50 | 7.60 | 5.30 | <0.001 |
| Endometriosis | 5.90 | 6.10 | 5.70 | 0.276 |
| Ovulatory dysfunction | 0.70 | 0.60 | 0.70 | 0.502 |
| Total Gonadotropin dose (IU) | 2109.92 ± 1104.77 | 2211.21 ± 1207.59 | 2000.39 ± 969.78 | <0.001 |
| No. of retrieved oocytes | 9.37 ± 6.01 | 7.69 ± 5.91 | 11.19 ± 5.57 | <0.001 |
| Cycles with oocytes retrieved (%) | 98.30 | 96.70 | 100.00 | <0.001 |
| No. of cryopreserved embryos | 2.17 ± 2.47 | 1.35 ± 2.12 | 3.05 ± 2.51 | <0.001 |
| Cycles with embryos cryopreserved (%) | 66.50 | 47.90 | 86.60 | <0.001 |
| No. of total transferred embryos | 1.45 ± 1.06 | 1.10 ± 1.14 | 1.83 ± 0.80 | <0.001 |
| Cycles with transferred embryos (%) | 78.80 | 59.30 | 100.00 | <0.001 |
| Parameter | Adjusted OR (95%CI) | p Value |
|---|---|---|
| Total population (n = 16,583) | ||
| Age | 0.910 (0.903–0.917) | <0.001 |
| AMH | 1.021 (1.006–1.037) | 0.005 |
| Basal FSH | 0.967 (0.951–0.983) | <0.001 |
| Uterine infertility | 0.822 (0.747–0.904) | <0.001 |
| Male infertility | 1.185 (1.072–1.309) | 0.001 |
| No. of cryopreserved embryos | 1.252 (1.231–1.274) | <0.001 |
| No. of total transferred embryos | 2.033 (1.954–2.115) | <0.001 |
| Ovulatory Women (n = 16,474) | ||
| Age | 0.909 (0.902–0.917) | <0.001 |
| AMH | 1.023 (1.007–1.038) | 0.003 |
| Basal FSH | 0.966 (0.950–0.982) | <0.001 |
| Uterine infertility | 0.824 (0.748–0.907) | <0.001 |
| Male infertility | 1.181 (1.069–1.306) | 0.001 |
| No. of cryopreserved embryos | 1.252 (1.231–1.274) | <0.001 |
| No. of total transferred embryos | 2.035 (1.955–2.117) | <0.001 |
| Women without endometriosis (n = 15,605) | ||
| Age | 0.910 (0.902–0.917) | <0.001 |
| AMH | 1.020 (1.004–1.036) | 0.012 |
| Basal FSH | 0.968 (0.951–0.985) | <0.001 |
| Uterine infertility | 0.786 (0.711–0.869) | <0.001 |
| Male infertility | 1.175 (1.061–1.300) | 0.002 |
| Total Gonadotropin dose/100 | 0.997 (0.993–1.000) | 0.090 |
| No. of cryopreserved embryos | 1.247 (1.225–1.269) | <0.001 |
| No. of total transferred embryos | 2.027 (1.946–2.112) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Qi, D.; Zhang, L.; Wang, J.; Du, Y.; Lv, H.; Yan, L. Association of the Cumulative Live Birth Rate with the Factors in Assisted Reproductive Technology: A Retrospective Study of 16,583 Women. J. Clin. Med. 2023, 12, 493. https://doi.org/10.3390/jcm12020493
Wang Q, Qi D, Zhang L, Wang J, Du Y, Lv H, Yan L. Association of the Cumulative Live Birth Rate with the Factors in Assisted Reproductive Technology: A Retrospective Study of 16,583 Women. Journal of Clinical Medicine. 2023; 12(2):493. https://doi.org/10.3390/jcm12020493
Chicago/Turabian StyleWang, Qiumin, Dan Qi, Lixia Zhang, Jingru Wang, Yanbo Du, Hong Lv, and Lei Yan. 2023. "Association of the Cumulative Live Birth Rate with the Factors in Assisted Reproductive Technology: A Retrospective Study of 16,583 Women" Journal of Clinical Medicine 12, no. 2: 493. https://doi.org/10.3390/jcm12020493
APA StyleWang, Q., Qi, D., Zhang, L., Wang, J., Du, Y., Lv, H., & Yan, L. (2023). Association of the Cumulative Live Birth Rate with the Factors in Assisted Reproductive Technology: A Retrospective Study of 16,583 Women. Journal of Clinical Medicine, 12(2), 493. https://doi.org/10.3390/jcm12020493






