Trimethylamine N-Oxide Levels Are Associated with Severe Aortic Stenosis and Predict Long-Term Adverse Outcome
Abstract
1. Introduction
2. Methods
2.1. Study Population and TAVR Procedures
2.2. Clinical Characteristics and Laboratory Measurements
2.3. TMAO Measurement
2.4. Statistical Analysis
3. Result
3.1. Participants’ Characteristics
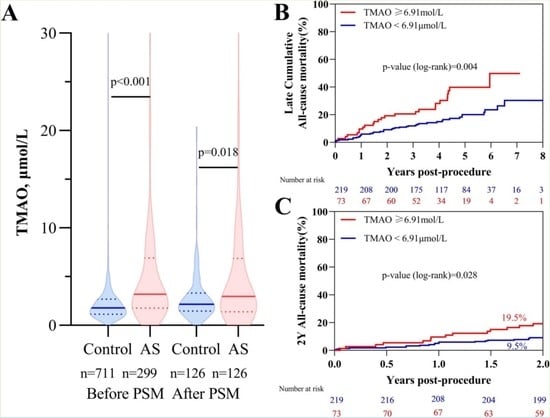
3.2. TMAO Concentrations in Patients with Aortic Stenosis and Control Group
3.3. Baseline Clinical Correlates of TMAO in Aortic Stenosis
3.4. TMAO Levels and Post-TAVR Outcome
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Inohara, T.; Hayashida, K.; Park, D.-W. Transcatheter Aortic Valve Replacement in Asia. JACC Asia 2021, 1, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; OHair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Kobayashi, Y.; Moneghetti, K.J.; Brenner, D.A.; O’Malley, R.; Schnittger, I.; Wu, J.C.; Murtagh, G.; Beshiri, A.; Fischbein, M.; et al. GDF-15 (Growth Differentiation Factor 15) Is Associated With Lack of Ventricular Recovery and Mortality After Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2017, 10, e005594. [Google Scholar] [CrossRef]
- Arain, F.; Abraityte, A.; Bogdanova, M.; Solberg, O.G.; Michelsen, A.E.; Lekva, T.; Aakhus, S.; Holm, S.; Halvorsen, B.; Finsen, A.V.; et al. YKL-40 (Chitinase-3-Like Protein 1) Serum Levels in Aortic Stenosis. Circ. Heart Fail. 2020, 13, e006643. [Google Scholar] [CrossRef] [PubMed]
- Toutouzas, K.; Stathogiannis, K.; Latsios, G.; Synetos, A.; Drakopoulou, M.; Penesopoulou, V.; Michelongona, A.; Tsiamis, E.; Tousoulis, D. Biomarkers in Aortic Valve Stenosis and their Clinical Significance in Transcatheter Aortic Valve Implantation. Curr. Med. Chem. 2019, 26, 864–872. [Google Scholar] [CrossRef]
- Tang, W.; Backhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef]
- Suzuki, T.; Heaney, L.M.; Bhandari, S.S.; Jones, D.J.L.; Ng, L.L. Trimethylamine N-oxide and prognosis in acute heart failure. Heart 2016, 102, 841–848. [Google Scholar] [CrossRef]
- Suzuki, T.; Heaney, L.M.; Jones, D.J.L.; Ng, L.L. Trimethylamine N-oxide and Risk Stratification after Acute Myocardial Infarction. Clin. Chem. 2017, 63, 420–428. [Google Scholar] [CrossRef]
- Suzuki, T.; Yazaki, Y.; Voors, A.A.; Jones, D.; Chan, D.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Hillege, H.L.; et al. Association with outcomes and response to treatment of trimethylamine N-oxide in heart failure (from BIOSTAT-CHF). Eur. J. Heart Fail. 2018, 21, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Karlin, E.T.; Rush, J.E.; Freeman, L.M. A pilot study investigating circulating trimethylamine N -oxide and its precursors in dogs with degenerative mitral valve disease with or without congestive heart failure. J. Vet. Intern. Med. 2019, 33, 46–53. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, H.; Tang, Y.; Zhan, Q.; Lai, W.; Ao, L.; Meng, X.; Ren, H.; Xu, D.; et al. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis 2019, 284, 121–128. [Google Scholar] [CrossRef] [PubMed]
- John, D.; Buellesfeld, L.; Yuecel, S.; Mueller, R.; Latsios, G.; Beucher, H.; Gerckens, U.; Grube, E. Correlation of Device Landing Zone Calcification and Acute Procedural Success in Patients Undergoing Transcatheter Aortic Valve Implantations With the Self-Expanding CoreValve Prosthesis. JACC Cardiovasc. Interv. 2010, 3, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, H.; Wang, Y.; Jin, C.; Lin, X.; Wang, C.; Lu, Y.; Chen, Z.; Wang, J.-A.; Xiang, M. Association of serum ADAMTS7 levels and genetic variant rs1994016 with acute coronary syndrome in a Chinese population: A case control study. Atherosclerosis 2018, 275, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Heaney, L.M.; Jones, D.J.; Mbasu, R.J.; Ng, L.L.; Suzuki, T. High mass accuracy assay for trimethylamine N-oxide using stable-isotope dilution with liquid chromatography coupled to orthogonal acceleration time of flight mass spectrometry with multiple reaction monitoring. Anal. Bioanal. Chem. 2016, 408, 797–804. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Thoemmes, F. Propensity score matching in SPSS. arXiv 2012, arXiv:1201.6385. [Google Scholar]
- Yan, A.T.; Koh, M.; Chan, K.K.; Guo, H.; Alter, D.A.; Austin, P.C.; Tu, J.V.; Wijeysundera, H.C.; Ko, D.T. Association Between Cardiovascular Risk Factors and Aortic Stenosis: The CANHEART Aortic Stenosis Study. J. Am. Coll. Cardiol. 2017, 69, 1523–1532. [Google Scholar] [CrossRef]
- Goody, P.R.; Hosen, M.R.; Christmann, D.; Niepmann, S.T.; Zietzer, A.; Adam, M.; Bönner, F.; Zimmer, S.; Nickenig, G.; Jansen, F. Aortic Valve Stenosis: From Basic Mechanisms to Novel Therapeutic Targets. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 885–900. [Google Scholar] [CrossRef]
- Li, J.; Zeng, Q.; Xiong, Z.; Xian, G.; Liu, Z.; Zhan, Q.; Lai, W.; Ao, L.; Meng, X.; Ren, H.; et al. Trimethylamine N-oxide induces osteogenic responses in human aortic valve interstitial cells in vitro and aggravates aortic valve lesions in mice. Cardiovasc. Res. 2021, 118, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Gupta, T.; Goel, K.; Kolte, D.; Khera, S.; Villablanca, P.A.; Aronow, W.S.; Bortnick, A.E.; Slovut, D.P.; Taub, C.C.; Kizer, J.R.; et al. Association of Chronic Kidney Disease With In-Hospital Outcomes of Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 2050–2060. [Google Scholar] [CrossRef]
- Chan, C.; Law, B.; Waye, M.; Chan, J.; So, W.; Chow, K.M. Trimethylamine-N-oxide as One Hypothetical Link for the Relationship between Intestinal Microbiota and Cancer—Where We Are and Where Shall We Go? J. Cancer 2019, 10, 5874–5882. [Google Scholar] [CrossRef] [PubMed]

| Controls | AS | AS with TMAO | ||
|---|---|---|---|---|
| N = 711 | N = 299 | <6.91 μmol/L N = 220 | ≥6.91 mol/L N = 73 | |
| Clinical Variables | ||||
| Age, years | 59 (52–66) | 77 (72–81) * | 76.5 (72–80.75) | 80 (75–83) † |
| Male (%) | 369 (51.9) | 174 (58.2) | 125 (56.8) | 45 (61.6) |
| BMI, kg/m2 | 24.2 (22.1–26.5) | 22.8 (20.0–24.8) * | 22.9 (20.0–24.8) | 22.6 (20.0–25.0) |
| NYHA III/IV (%) | 43 (7.4) | 266 (89.0) * | 195 (88.6) | 66 (90.4) |
| Smoker (%) | 251 (35.3) | 38 (12.7) * | 26 (11.8) | 11 (15.1) |
| Hyperlipidemia (%) | 86 (12.1) | 68 (22.7) * | 54 (24.5) | 13 (17.8) |
| Diabetes (%) | 85 (12.0) | 66 (22.1) * | 42 (19.1) | 22 (30.1) |
| Hypertension (%) | 345 (48.5) | 163 (54.5) | 113 (51.4) | 46 (63) |
| Prior stroke (%) | 37 (5.2) | 17 (5.7) | 11 (5) | 6 (8.2) |
| PVD (%) | 69 (9.7) | 66 (22.1) * | 45 (20.5) | 20 (27.4) |
| COPD (%) | 36 (5.1) | 68 (22.7) * | 47 (21.4) | 20 (27.4) |
| eGFR, mL/(min × 1.73 m2) | 99 (87–108) | 75 (55–89) * | 79 (60–91) | 60 (33–79) * |
| Biomarkers | ||||
| proBNP, pg/mL | 123 (56–871) | 2945 (987–8863) * | 2598 (787–7595) | 3932 (1542–11,776) ‡ |
| cTn, ng/mL | 0.008 (0.005–0.013) | 0.0275 (0.017–0.050) * | 0.03 (0.01–0.05) | 0.03 (0.02–0.06) ‡ |
| TMAO, μmol/L | 1.78 (1.14–2.68) | 3.18 (1.77–6.91) * | 2.40 (1.37–2.67) | 10.42 (8.58–19.06) * |
| Hemodynamics | ||||
| EF, % | 65.9 (60.1–70.5) | 57.3 (44.2–64.4) * | 57.5 (44.55–64.7) | 57.2 (43.95–62.05) |
| AVA, cm2 | - | 0.58 (0.44–0.72) | 0.59 (0.44–0.73) | 0.55 (0.44–0.68) |
| Mean gradient, mmHg | - | 53 (42–67) | 53 (42–67) | 54 (43–63) |
| Maximum velocity, m/s | - | 4.8 (4.3–5.3) | 4.76 (4.2–5.33) | 4.82 (4.39–5.22) |
| AS- and TAVR-related Variables | ||||
| STS score | - | 5.8 (4.0–9.3) | 5.3 (3.7–8.9) | 8.6 (4.9–12.0) |
| Bicuspid valve | - | 141 (47.2) | 107 (48.6) | 30 (41.1) |
| Calcification > moderate | - | 166 (55.5) | 123 (55.9) | 39 (53.4) |
| Transfemoral route | - | 279 (95.2) | 208 (94.5) | 71 (97.3) |
| Prothesis type | ||||
| BEV | - | 25 (8.5) | 17 (7.7) | 8 (11) |
| SEV | - | 246 (84) | 187 (85) | 59 (80.8) |
| Lotus | - | 22 (7.5) | 16 (7.3) | 6 (8.2) |
| Pre-dilation (%) | - | 281 (95.9) | 208 (94.5) | 73 (100) |
| Post-dilation (%) | - | 133 (45.4) | 106 (48.2) | 27 (37) ‡ |
| Second valve used (%) | - | 22 (7.5) | 18 (8.2) | 4 (5.5) |
| Unadjusted Model | Adjusted Model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age, years | 1.267 | 1.229–1.305 | <0.001 | 1.230 | 1.172–1.291 | <0.001 |
| BMI, kg/m2 | 0.851 | 0.815–0.889 | <0.001 | - | - | - |
| NYHA class | 17.994 | 11.794–24.516 | <0.001 | 20.298 | 11.598–35.523 | <0.001 |
| Male (%) | 1.290 | 0.982–1.695 | 0.067 | - | - | - |
| Smoker (%) | 0.267 | 0.184–0.388 | <0.001 | 0.427 | 0.19–0.962 | 0.040 |
| Hyperlipidemia (%) | 2.139 | 1.504–3.042 | <0.001 | - | - | - |
| Diabetes (%) | 2.086 | 1.463–2.975 | <0.001 | - | - | - |
| Hypertension (%) | 1.271 | 0.97–1.667 | 0.082 | - | - | - |
| PVD (%) | 2.636 | 1.822–3.813 | <0.001 | - | - | - |
| COPD (%) | 5.519 | 3.588–8.491 | <0.001 | - | - | - |
| eGFR, mL/(min × 1.73 m2) | 0.940 | 0.931–0.948 | <0.001 | - | - | - |
| EF, % | 0.942 | 0.931–0.953 | <0.001 | 1.036 | 1.007–1.067 | 0.015 |
| TMAO, μmol/L | 1.217 | 1.157–1.279 | <0.001 | 1.084 | 1.01–1.162 | 0.025 |
| Clinical Outcome | AS Patients | AS with TMAO | p | |
|---|---|---|---|---|
| N = 293 | <6.91 mol/L N = 220 | ≥6.91 mol/L N = 73 | ||
| Follow up duration (months) | 50 (36–65) | 50 (37–66) | 44 (35–60) | 0.045 |
| 30-day mortality (%) | 5 (1.7) | 4 (1.8) | 1 (1.4) | 0.793 |
| Cardiac | 4 (1.4) | 4 (1.8) | 0 (0) | 0.248 |
| Non-cardiac | 1 (0.3) | 0 (0) | 1 (1.4) | 0.085 |
| 1-year mortality (%) | 20 (6.8) | 13 (5.9) | 7 (9.6) | 0.283 |
| Cardiac | 15 (5.1) | 11 (5) | 4 (5.5) | 0.858 |
| Non-cardiac | 5 (1.7) | 2 (0.9) | 3 (4.1) | 0.067 |
| 2-year mortality (%) | 35 (11.9) | 21 (9.5) | 14 (19.2) | 0.028 |
| Cardiac | 16 (5.5) | 11 (5) | 5 (6.8) | 0.520 |
| Non-cardiac | 19 (6.5) | 10 (4.5) | 9 (12.3) | 0.017 |
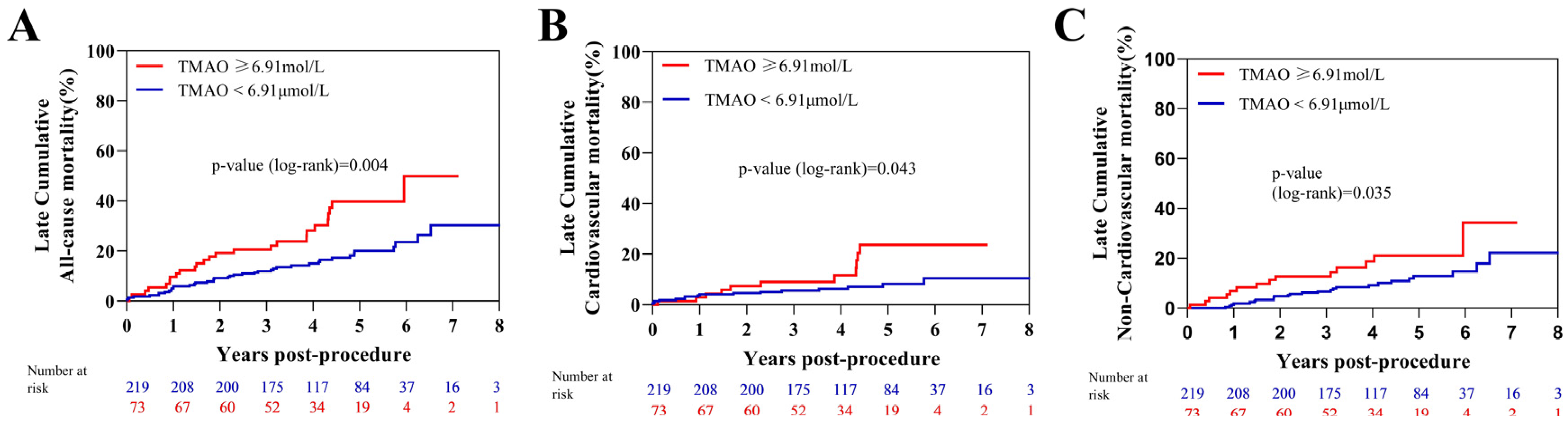
| Late cumulative mortality (%) | 67 (22.9) | 42 (19.1) | 25 (34.2) | 0.004 |
| Cardiac | 28 (9.6) | 17 (7.7) | 11 (15.1) | 0.043 |
| Non-cardiac | 39 (13.3) | 25 (11.4) | 14 (19.2) | 0.035 |
| Univariate Analysis * | Multivariate Analysis ** | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
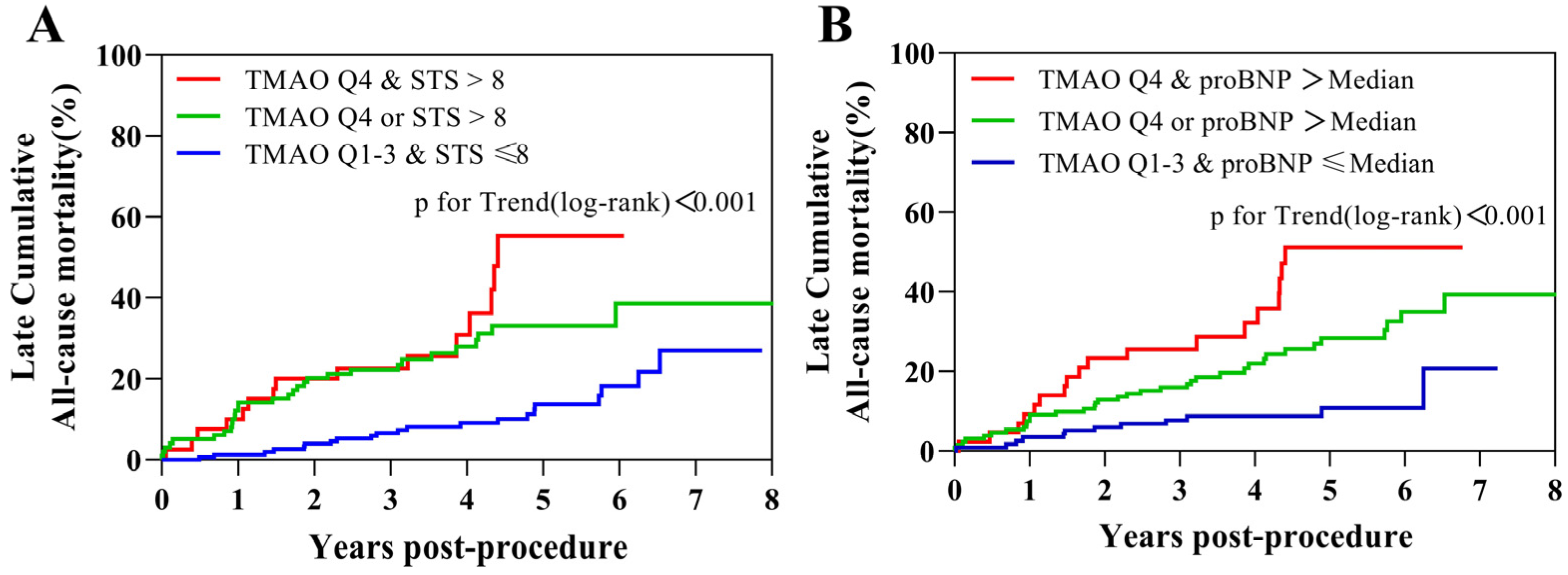
| TMAO ≥ 6.91 mol/L (Q4 vs. Q1–3) | 2.065 (1.255–3.396) | 0.004 | 1.788(1.064–3.005) | 0.028 |
| STS score > 8 | 2.675 (1.645–4.352) | <0.001 | 1.837(1.091–3.093) | 0.022 |
| eGFR < 30 mL/(min × 1.73 m2) | 4.728 (2.466–9.066) | <0.001 | ||
| EF < 50% | 1.793 (1.107–2.903) | 0.018 | ||
| Bicuspid valve | 0.491 (0.29–0.829) | 0.008 | ||
| proBNP > 2871 pg/mL (median) | 2.629 (1.544–4.476) | <0.001 | 2.141(1.230–3.726) | 0.007 |
| Mean gradient > 53 mmHg (median) | 0.559 (0.342–0.916) | 0.021 | ||
| Maximum velocity > 4.78 m/s(median) | 0.557 (0.34–0.912) | 0.020 | 0.556(0.337–0.918) | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Xu, S.; Zhan, H.; Chen, H.; Hu, P.; Zhou, D.; Dai, H.; Liu, X.; Hu, W.; Zhu, G.; et al. Trimethylamine N-Oxide Levels Are Associated with Severe Aortic Stenosis and Predict Long-Term Adverse Outcome. J. Clin. Med. 2023, 12, 407. https://doi.org/10.3390/jcm12020407
Guo Y, Xu S, Zhan H, Chen H, Hu P, Zhou D, Dai H, Liu X, Hu W, Zhu G, et al. Trimethylamine N-Oxide Levels Are Associated with Severe Aortic Stenosis and Predict Long-Term Adverse Outcome. Journal of Clinical Medicine. 2023; 12(2):407. https://doi.org/10.3390/jcm12020407
Chicago/Turabian StyleGuo, Yuchao, Shaojun Xu, Hong Zhan, Han Chen, Po Hu, Dao Zhou, Hanyi Dai, Xianbao Liu, Wangxing Hu, Gangjie Zhu, and et al. 2023. "Trimethylamine N-Oxide Levels Are Associated with Severe Aortic Stenosis and Predict Long-Term Adverse Outcome" Journal of Clinical Medicine 12, no. 2: 407. https://doi.org/10.3390/jcm12020407
APA StyleGuo, Y., Xu, S., Zhan, H., Chen, H., Hu, P., Zhou, D., Dai, H., Liu, X., Hu, W., Zhu, G., Suzuki, T., & Wang, J. (2023). Trimethylamine N-Oxide Levels Are Associated with Severe Aortic Stenosis and Predict Long-Term Adverse Outcome. Journal of Clinical Medicine, 12(2), 407. https://doi.org/10.3390/jcm12020407