Contrast-Enhanced Ultrasound Feasibility in Assessing Carotid Plaque Vulnerability—Narrative Review
Abstract
1. Introduction
2. Aim of the Review and Search Strategy
3. Vulnerable Plaques
4. Need for Early Identification
5. Screening for Vulnerable Plaques
6. B-Mode US with Doppler
7. Gray–Weale–Nicolaides Scale
8. Intima–Media Thickness
9. Ulceration
10. Doppler US
11. Contrast-Enhanced Ultrasound
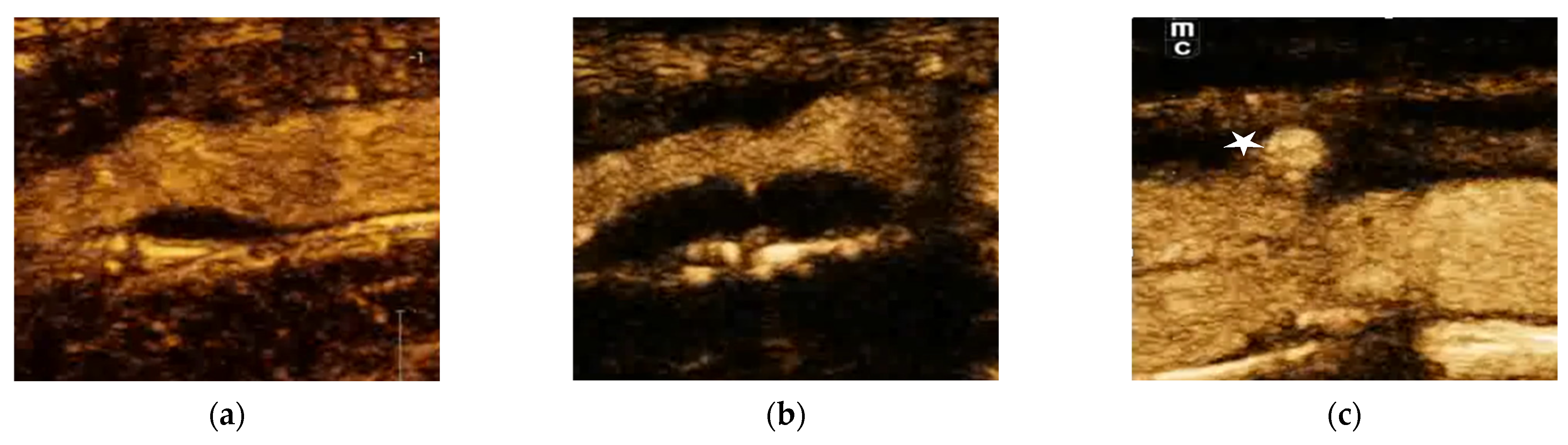
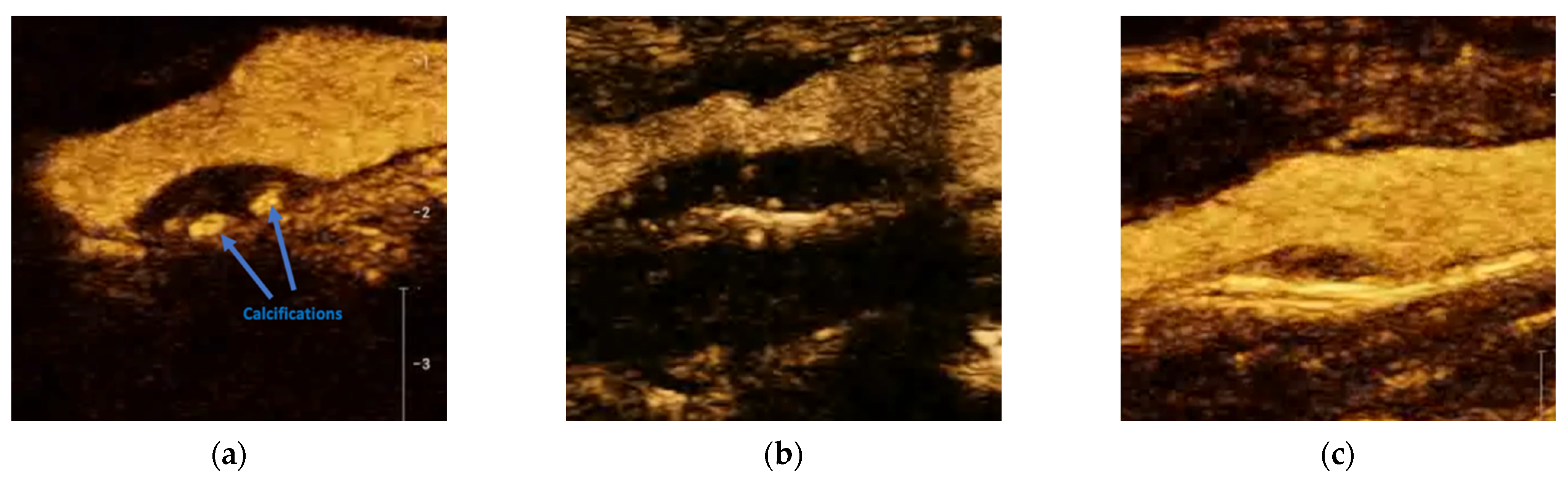
12. CEUS Assessment of Intraplaque Neovascularization
13. CEUS Assessment of Plaque Surfaces
- Smooth refers to a regular surface with no notable irregularities.
- Irregular plaques have fluctuations between 0.3 and 0.9 mm.
14. Quantification of CEUS
15. Clinical Applications
16. CEUS versus Other Imaging Methods
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.J.; Werring, D.J. Stroke: Causes and clinical features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef]
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.M.V. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr. Diabetes Rep. 2019, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Korantzopoulos, P.; Liu, T. Correction to: Carotid Atherosclerosis in Patients with Atrial Fibrillation. Curr. Atheroscler. Rep. 2019, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.B.; Fletcher, A.J.; Forsythe, O.R.; Kaczynski, J.; Newby, E.D.; Dweck, M.R.; van Beek, E.J. Emerging techniques in atherosclerosis imaging. Br. J. Radiol. 2019, 92, 20180309. [Google Scholar] [CrossRef]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Avgerinos, N.A.; Neofytou, P. Mathematical Modelling and Simulation of Atherosclerosis Formation and Progress: A Review. Ann. Biomed. Eng. 2019, 47, 1764–1785. [Google Scholar] [CrossRef]
- Kotlyarov, S. Diversity of Lipid Function in Atherogenesis: A Focus on Endothelial Mechanobiology. Int. J. Mol. Sci. 2021, 22, 11545. [Google Scholar] [CrossRef]
- Deng, W.; Tang, T.; Hou, Y.; Zeng, Q.; Wang, Y.; Fan, W.; Qu, S. Extracellular vesicles in atherosclerosis. Clin. Chim. Acta 2019, 495, 109–117. [Google Scholar] [CrossRef]
- Singh, R.B.; Mengi, A.S.; Xu, Y.-J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar]
- Logan, J.K.; Ayers, M.P. Noninvasive Imaging for the Asymptomatic Patient. Med. Clin. N. Am. 2022, 106, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kovacic, J.C. Endothelial to Mesenchymal Transition in Health and Disease. Annu. Rev. Physiol. 2023, 85, 245–267. [Google Scholar] [CrossRef]
- Keeter, W.C.; Ma, S.; Stahr, N.; Moriarty, A.K.; Galkina, E.V. Atherosclerosis and multi-organ-associated pathologies. Semin. Immunopathol. 2022, 44, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Raitakari, O.; Pahkala, K.; Magnussen, C.G. Prevention of atherosclerosis from childhood. Nat. Rev. Cardiol. 2022, 19, 543–554. [Google Scholar] [CrossRef] [PubMed]
- da Luz, P.L.; Chagas, A.C.P.; Dourado, P.M.M.; Laurindo, F.R. Endothelium in Atherosclerosis: Plaque Formation and Its Complications. In Endothelium and Cardiovascular Diseases: Vascular Biology and Clinical Syndromes; Academic Press: Cambridge, MA, USA, 2018; pp. 493–512. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019, 5, 70. [Google Scholar] [CrossRef]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, A.B.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef] [PubMed]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on clinical indications for cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef]
- Kim, H.W.; Regenhardt, R.W.; D’Amato, A.S.; Nahhas, M.I.; Dmytriw, A.A.; Hirsch, A.J.; Silverman, S.B.; Martinez-Gutierrez, J.C. Asymptomatic carotid artery stenosis: A summary of current state of evidence for revascularization and emerging high-risk features. J. NeuroInterventional Surg. 2022, 15, 717–722. [Google Scholar] [CrossRef]
- Brinjikji, W.; Rabinstein, A.A.; Lanzino, G.; Murad, M.H.; Williamson, E.E.; DeMarco, J.K.; Iii, J.H. Ultrasound Characteristics of Symptomatic Carotid Plaques: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2015, 40, 165–174. [Google Scholar] [CrossRef]
- Casscells, W.; Naghavi, M.; Willerson, J.T. Vulnerable Atherosclerotic Plaque. Circulation 2003, 107, 2072–2075. [Google Scholar] [CrossRef]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From Vulnerable Plaque to Vulnerable Patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.; Sillesen, H.; et al. Editor’s Choice–European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2022, 65, 7–111. [Google Scholar] [CrossRef] [PubMed]
- Heck, D.; Jost, A. Carotid stenosis, stroke, and carotid artery revascularization. Prog. Cardiovasc. Dis. 2021, 65, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.-K. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef]
- Mury, P.; Chirico, E.N.; Mura, M.; Millon, A.; Canet-Soulas, E.; Pialoux, V. Oxidative Stress and Inflammation, Key Targets of Ather-osclerotic Plaque Progression and Vulnerability: Potential Impact of Physical Activity. Sports Med. 2018, 48, 2725–2741. [Google Scholar] [CrossRef]
- Nielsen, S.H.; Jonasson, L.; Kalogeropoulos, K.; Karsdal, M.A.; Reese-Petersen, A.L.; Keller, U.A.D.; Genovese, F.; Nilsson, J.; Goncalves, I. Exploring the role of extracellular matrix proteins to develop biomarkers of plaque vulnerability and outcome. J. Intern. Med. 2020, 287, 493–513. [Google Scholar] [CrossRef]
- Chiorescu, R.M.; Mocan, M.; Inceu, A.I.; Buda, A.P.; Blendea, D.; Vlaicu, S.I. Vulnerable Atherosclerotic Plaque: Is There a Molecular Signature? Int. J. Mol. Sci. 2022, 23, 13638. [Google Scholar] [CrossRef]
- Cai, Y.; Pan, J.; Li, Z. Mathematical modeling of intraplaque neovascularization and hemorrhage in a carotid atherosclerotic plaque. Biomed. Eng. Online 2021, 20, 42. [Google Scholar] [CrossRef]
- Liberale, L. The Role of Vascular Aging in Atherosclerotic Plaque Development and Vulnerability. Curr. Pharm. Des. 2019, 25, 3098–3111. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Miao, X.-H.; Guo, J.-D.; Li, D.-W. Neovascularization and tissue regeneration by endothelial progenitor cells in ischemic stroke. Neurol. Sci. 2021, 42, 3585–3593. [Google Scholar] [CrossRef] [PubMed]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.F.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, X.; Zuo, G.; Pu, J.; Wu, X.; Chen, S. The Role of Angiogenesis in Coronary Artery Disease: A Double-Edged Sword: Intraplaque Angiogenesis in Physiopathology and Therapeutic Angiogenesis for Treatment. Curr. Pharm. Des. 2018, 24, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.K.P.; Solly, E.L.; Bursill, C.A.; Tan, J.T.M.; Ng, M.K.C. Pathophysiology of Angiogenesis and Its Role in Vascular Disease. In Mechanisms of Vascular Disease: A Textbook for Vascular Specialists; Springer: Berlin/Heidelberg, Germany, 2020; pp. 89–116. [Google Scholar] [CrossRef]
- Bonafiglia, Q.A.; Bendeck, M.; Gotlieb, A.I. Vascular Pathobiology: Atherosclerosis and Large Vessel Disease. In Cardiovascular Pathology; Academic Press: Cambridge, MA, USA, 2022; pp. 265–306. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Nikiforov, N.G.; Melnichenko, A.A.; Kalmykov, V.; Shakhpazyan, N.K.; Orekhova, V.A.; Orekhov, A.N. Functional Phenotypes of Intraplaque Macrophages and Their Distinct Roles in Atherosclerosis Development and Atheroinflammation. Biomedicines 2022, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Nasir, U.; Segal, J.; Waheed, T.A.; Ameen, M.; Hafeez, H. The utility of ultrasound and computed tomography in the as-sessment of carotid artery plaque vulnerability–A mini review. Front. Cardiovasc. Med. 2022, 9, 1023562. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.; Della Schiava, N.; Long, A.; Chirico, E.N.; Pialoux, V.; Millon, A. Carotid intraplaque haemorrhage: Pathogenesis, histological classification, imaging methods and clinical value. Ann. Transl. Med. 2020, 8, 1273. [Google Scholar] [CrossRef]
- Porcu, M.; Mannelli, L.; Melis, M.; Suri, J.S.; Gerosa, C.; Cerrone, G.; Defazio, G.; Faa, G.; Saba, L. Carotid plaque imaging profiling in subjects with risk factors (diabetes and hypertension). Cardiovasc. Diagn. Ther. 2020, 10, 1005–1018. [Google Scholar] [CrossRef]
- Zhu, G.; Hom, J.; Li, Y.; Jiang, B.; Rodriguez, F.; Fleischmann, D.; Saloner, D.; Porcu, M.; Zhang, Y.; Saba, L.; et al. Carotid plaque imaging and the risk of atherosclerotic cardiovascular disease. Cardiovasc. Diagn. Ther. 2020, 10, 1048–1067. [Google Scholar] [CrossRef]
- Zamani, M.; Skagen, K.; Scott, H.; Russell, D.; Skjelland, M. Advanced ultrasound methods in assessment of carotid plaque instability: A prospective multimodal study. BMC Neurol. 2020, 20, 39. [Google Scholar] [CrossRef]
- Saba, L.; Antignani, P.L.; Gupta, A.; Cau, R.; Paraskevas, K.I.; Poredos, P.; Wasserman, B.A.; Kamel, H.; Avgerinos, E.D.; Salgado, R.; et al. International Union of Angiology (IUA) consensus paper on imaging strategies in atherosclerotic carotid artery imaging: From basic strategies to advanced approaches. Atherosclerosis 2022, 354, 23–40. [Google Scholar] [CrossRef]
- Golemati, S.; Cokkinos, D.D. Recent advances in vascular ultrasound imaging technology and their clinical implications. Ultrasonics 2021, 119, 106599. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-T.; Lai, Q.-L.; Cai, M.-T.; Wang, J.-J.; Zhuang, L.-Y.; Cheng, L.; Mo, Y.-J.; Liu, L.; Zang, Y.-X.; Qiao, S. Detecting vulnerable carotid plaque and its component characteristics: Progress in related imaging techniques. Front. Neurol. 2022, 13, 982147. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, D.E.; Stultz, C.M. Deep Learning for Cardiovascular Risk Stratification. Curr. Treat. Options Cardiovasc. Med. 2020, 22, 15. [Google Scholar] [CrossRef]
- Fabiani, I.; Palombo, C.; Caramella, D.; Nilsson, J.; De Caterina, R. Imaging of the vulnerable carotid plaque. Neurology 2020, 94, 922–932. [Google Scholar] [CrossRef]
- Schinkel, A.F.; Bosch, J.G.; Staub, D.; Adam, D.; Feinstein, S.B. Contrast-Enhanced Ultrasound to Assess Carotid Intraplaque Neovascularization. Ultrasound Med. Biol. 2019, 46, 466–478. [Google Scholar] [CrossRef]
- Cismaru, G.; Serban, T.; Tirpe, A. Ultrasound Methods in the Evaluation of Atherosclerosis: From Pathophysiology to Clinic. Biomedicines 2021, 9, 418. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Lyu, Q.; Shi, G. Imaging diagnosis and research progress of carotid plaque vulnerability. J. Clin. Ultrasound 2022, 50, 905–912. [Google Scholar] [CrossRef]
- Rafailidis, V.; Charitanti, A.; Tegos, T.; Rafailidis, D.; Chryssogonidis, I. Swirling of microbubbles: Demonstration of a new finding of carotid plaque ulceration on contrast-enhanced ultrasound explaining the arterio-arterial embolism mechanism. Clin. Hemorheol. Microcirc. 2016, 64, 245–250. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Georgakopoulos, C.; Koutagiar, I.; Tousoulis, D. Diagnostic modalities in peripheral artery disease. Curr. Opin. Pharmacol. 2018, 39, 68–76. [Google Scholar] [CrossRef]
- Yonetsu, T.; Jang, I.-K. Advances in Intravascular Imaging: New Insights into the Vulnerable Plaque from Imaging Studies. Korean Circ. J. 2018, 48, 1–15. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Summerhill, V.I.; Zhang, D.; Romanenko, E.B.; Grechko, A.V.; Orekhov, A.N. Current Advances in the Diagnostic Imaging of Atherosclerosis: Insights into the Pathophysiology of Vulnerable Plaque. Int. J. Mol. Sci. 2020, 21, 2992. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.P.; Fayad, Z.A.; Dweck, M.R. New methods to image unstable atherosclerotic plaques. Atherosclerosis 2018, 272, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Brandt, V.; Bekeredjian, R.; Schoepf, U.J.; Varga-Szemes, A.; Emrich, T.; Aquino, G.J.; Decker, J.; Bayer, R.R.; Ellis, L.; Ebersberger, U.; et al. Prognostic value of epicardial adipose tissue volume in combination with coronary plaque and flow assessment for the prediction of major adverse cardiac events. Eur. J. Radiol. 2022, 148, 110157. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Li, X.; Sidhu, P.S.; Partovi, S.; Staub, D. Contrast imaging ultrasound for the detection and characterization of carotid vulnerable plaque. Cardiovasc. Diagn. Ther. 2020, 10, 965–981. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L.; Wang, H.; Tan, J.; Wei, L.; Weng, Y.; Chen, J. Recent Advances: From Cell Biology to Cell Therapy in Atherosclerosis Plaque via Stent Implantation. Curr. Med. Chem. 2022, 30, 3582–3613. [Google Scholar] [CrossRef]
- Shishikura, D.; Octavia, Y.; Hayat, U.; Thondapu, V.; Barlis, P. Atherogenesis and Inflammation. Eur. Heart J. 1993, 14, 2–6. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, M.; Majdoubi, J.; Rahimi-Gorji, M.; Srivastav, V.K. A review study on blood in human coronary artery: Numerical approach. Comput. Methods Programs Biomed. 2019, 187, 105243. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef]
- Saito, Y.; Kobayashi, Y.; Fujii, K.; Sonoda, S.; Tsujita, K.; Hibi, K.; Morino, Y.; Okura, H.; Ikari, Y.; Honye, J. Clinical expert consensus document on standards for measurements and assessment of intravascular ultrasound from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc. Interv. Ther. 2020, 35, 1–12. [Google Scholar] [CrossRef]
- Alexandratou, M.; Papachristodoulou, A.; Li, X.; Partovi, S.; Davidhi, A.; Rafailidis, V.; Prassopoulos, P.; Kamperidis, V.; Koutroulou, I.; Tsivgoulis, G.; et al. Advances in Noninvasive Carotid Wall Imaging with Ultrasound: A Narrative Review. J. Clin. Med. 2022, 11, 6196. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.-B. Phylogenic Determinants of Cardiovascular Frailty, Focus on Hemodynamics and Arterial Smooth Muscle Cells. Physiol. Rev. 2020, 100, 1779–1837. [Google Scholar] [CrossRef]
- Syed, M.B.; Doris, M.; Dweck, M.; Forsythe, R.; Newby, D.E. Imaging vascular calcification: Where are we headed. In Coronary Calcium: A Comprehensive Understanding of Its Biology, Use in Screening, and Interventional Management; Academic Press: Cambridge, MA, USA, 2019; pp. 203–246. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.; Linares Sánchez, M.; López Alvarez, F.; Suárez Nieto, C.; Mäkitie, A.A.; Olsen, K.D.; Ferlito, A. Evaluation of Intima-Media Thickness and Arterial Stiffness as Early Ultrasound Biomarkers of Carotid Artery Atherosclerosis. Cardiol. Ther. 2022, 11, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-W.; Guo, Y.-C.; Li, C.-I.; Liu, C.-S.; Lin, C.-H.; Liu, C.-H.; Wang, M.-C.; Yang, S.-Y.; Li, T.-C.; Lin, C.-C. Subclinical Atherosclerosis Markers of Carotid Intima-Media Thickness, Carotid Plaques, Carotid Stenosis, and Mortality in Community-Dwelling Adults. Int. J. Environ. Res. Public Health 2020, 17, 4745. [Google Scholar] [CrossRef]
- Johri, A.M.; Nambi, V.; Naqvi, T.Z.; Feinstein, S.B.; Kim, E.S.; Park, M.M.; Becher, H.; Sillesen, H. Recommendations for the Assessment of Carotid Arterial Plaque by Ultrasound for the Characterization of Atherosclerosis and Evaluation of Cardiovascular Risk: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 917–933. [Google Scholar] [CrossRef]
- Kim, H.-L.; Kim, S.-H. Pulse Wave Velocity in Atherosclerosis. Front. Cardiovasc. Med. 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Barbato, E.; Capodanno, D.; Edvardsen, T.; Prescott, E.; Achenbach, S.; Bax, J.J.; Wijns, W.; Knuuti, J. Imaging in ESC clinical guidelines: Chronic coronary syndromes. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.S.G.; Nahar, T.; Kalashyan, H.; Becher, H.; Nanda, N.C. Ultrasound assessment of carotid arteries: Current concepts, methodologies, diagnostic criteria, and technological advancements. Echocardiography 2018, 35, 2079–2091. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Matsuoka, T.; Shimomura, I. Clinical utility of carotid ultrasonography: Application for the management of patients with diabetes. J. Diabetes Investig. 2019, 10, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.A.; Flumignan, R.L.G.; Sobreira, M.L.; Avelar, W.M.; Fingerhut, C.; Stein, S.; Guillaumon, A.T. Carotid Plaque Composition and the Im-portance of Non-Invasive in Imaging Stroke Prevention. Front. Cardiovasc. Med. 2022, 9, 885483. [Google Scholar] [CrossRef]
- Fedak, A.; Ciuk, K.; Urbanik, A. Ultrasonography of vulnerable atherosclerotic plaque in the carotid arteries: B-mode imaging. J. Ultrason. 2020, 20, e135–e145. [Google Scholar] [CrossRef]
- Lee, W. General principles of carotid Doppler ultrasonography. Ultrasonography 2013, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.J.; Carter, S.E.; Green, D.J. Arterial structure and function in vascular ageing: Are you as old as your arteries? J. Physiol. 2015, 594, 2275–2284. [Google Scholar] [CrossRef]
- Chiha, J.; Mitchell, P.; Gopinath, B.; Burlutsky, G.; Plant, A.; Kovoor, P.; Thiagalingam, A. Prediction of Coronary Artery Disease Extent and Severity Using Pulse Wave Velocity. PLoS ONE 2016, 11, e0168598. [Google Scholar] [CrossRef]
- Staub, D.; Schinkel, A.F.; Coll, B.; Coli, S.; van der Steen, A.F.; Reed, J.D.; Krueger, C.; Thomenius, K.E.; Adam, D.; Sijbrands, E.J.; et al. Contrast-Enhanced Ultrasound Imaging of the Vasa Vasorum: From Early Atherosclerosis to the Identification of Unstable Plaques. JACC Cardiovasc. Imaging 2010, 3, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Fetzer, D.T.; Rafailidis, V.; Peterson, C.; Grant, E.G.; Sidhu, P.; Barr, R.G. Artifacts in contrast-enhanced ultrasound: A pictorial essay. Abdom. Imaging 2017, 43, 977–997. [Google Scholar] [CrossRef] [PubMed]
- Averkiou, M.A.; Bruce, M.F.; Powers, J.E.; Sheeran, P.S.; Burns, P.N. Imaging Methods for Ultrasound Contrast Agents. Ultrasound Med. Biol. 2019, 46, 498–517. [Google Scholar] [CrossRef] [PubMed]
- Greis, C. Technology overview: SonoVue (Bracco, Milan). Eur. Radiol. Suppl. 2004, 14 (Suppl. 8), P11–P15. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.-A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. Eur. J. Ultrasound 2018, 39, e2–e44. [Google Scholar] [CrossRef]
- Morel, D.R.; Schwieger, I.; Hohn, L.; Terrettaz, J.; Llull, J.B.; Cornioley, Y.A.; Schneider, M. Human Pharmacokinetics and Safety Evaluation of SonoVue™, a New Contrast Agent for Ultrasound Imaging. Investig. Radiol. 2000, 35, 80–85. [Google Scholar] [CrossRef]
- Hu, C.; Feng, Y.; Huang, P.; Jin, J. Adverse reactions after the use of SonoVue contrast agent: Characteristics and nursing care experience. Medicine 2019, 98, e17745. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Averkiou, M.; Nielsen, M.B.; Barr, R.G.; Burns, P.N.; Calliada, F.; Cantisani, V.; Choi, B.; Chammas, M.C.; Clevert, D.-A.; et al. How to perform Contrast-Enhanced Ultrasound (CEUS). Ultrasound Int. Open 2018, 04, E2–E15. [Google Scholar] [CrossRef] [PubMed]
- Greis, C. Technical aspects of contrast-enhanced ultrasound (CEUS) examinations: Tips and tricks. Clin. Hemorheol. Microcirc. 2014, 58, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Contribution of neovascularization and intraplaque haemorrhage to atherosclerotic plaque progression and instability. Acta Physiol. 2015, 213, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Greis, C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin. Hemorheol. Microcirc. 2011, 49, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, T.; Sakata, N.; Ogata, T.; Shimada, H.; Inoue, T. Intra-Plaque Vessels on Contrast-Enhanced Ultrasound Sonography Predict Carotid Plaque Histology. Cerebrovasc. Dis. 2018, 46, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, W.; Guo, D.; Chen, L.; Jin, X.; Huang, B.; Wang, W. Quantification of Carotid Plaque Neovascularization Using Contrast-Enhanced Ultrasound with Histopathologic Validation. Ultrasound Med. Biol. 2014, 40, 1827–1833. [Google Scholar] [CrossRef]
- Vavuranakis, M.; Sigala, F.; Vrachatis, D.A.; Papaioannou, T.G.; Filis, K.; Kavantzas, N.; Kalogeras, K.I.; Massoura, C.; Toufektzian, L.; Kariori, M.G.; et al. Quantitative analysis of carotid plaque vasa vasorum by CEUS and correlation with histology after endarterectomy. Vasa 2013, 42, 184–195. [Google Scholar] [CrossRef]
- Iezzi, R.; Petrone, G.; Ferrante, A.; Lauriola, L.; Vincenzoni, C.; la Torre, M.F.; Snider, F.; Rindi, G.; Bonomo, L. The role of contrast-enhanced ultrasound (CEUS) in visualizing atherosclerotic carotid plaque vulnerability: Which injection protocol? Which scanning technique? Eur. J. Radiol. 2015, 84, 865–871. [Google Scholar] [CrossRef]
- Staub, D.; Partovi, S.; Schinkel, A.F.L.; Coll, B.; Uthoff, H.; Aschwanden, M.; Jaeger, K.A.; Feinstein, S.B. Correlation of Carotid Artery Atherosclerotic Lesion Echogenicity and Severity at Standard US with Intraplaque Neovascularization Detected at Contrast-enhanced US. Radiology 2011, 258, 618–626. [Google Scholar] [CrossRef]
- Fleiner, M.; Kummer, M.; Mirlacher, M.; Sauter, G.; Cathomas, G.; Krapf, R.; Biedermann, B.C. Arterial Neovascularization and Inflammation in Vulnerable Patients. Circulation 2004, 110, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.-B.; Martin-Ventura, J.L.; Nicoletti, A.; Ho-Tin-Noé, B. Pathology of human plaque vulnerability: Mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis 2014, 234, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, M.; Vicenzini, E.; Citone, M.; Ricciardi, M.; Irace, L.; Laurito, A.; Scucchi, L.; Di Piero, V.; Gossetti, B.; Mauriello, A.; et al. Contrast Carotid Ultrasound for the Detection of Unstable Plaques with Neoangiogenesis: A Pilot Study. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 722–727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oord, S.C.v.D.; Akkus, Z.; Renaud, G.; Bosch, J.G.; van der Steen, A.F.; Sijbrands, E.J.; Schinkel, A.F. Assessment of carotid atherosclerosis, intraplaque neovascularization, and plaque ulceration using quantitative contrast-enhanced ultrasound in asymptomatic patients with diabetes mellitus. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1213–1218. [Google Scholar] [CrossRef]
- Rafailidis, V.; Chryssogonidis, I.; Tegos, T.; Kouskouras, K.; Charitanti-Kouridou, A. Imaging of the ulcerated carotid atherosclerotic plaque: A review of the literature. Insights Imaging 2017, 8, 213–225. [Google Scholar] [CrossRef]
- Rafailidis, V.; Chryssogonidis, I.; Xerras, C.; Nikolaou, I.; Tegos, T.; Kouskouras, K.; Rafailidis, D.; Charitanti-Kouridou, A. A comparative study of color Doppler imaging and contrast-enhanced ultrasound for the detection of ulceration in patients with carotid atherosclerotic disease. Eur. Radiol. 2018, 29, 2137–2145. [Google Scholar] [CrossRef]
- Saba, L.; Yuan, C.; Hatsukami, T.; Balu, N.; Qiao, Y.; DeMarco, J.; Saam, T.; Moody, A.; Li, D.; Matouk, C.; et al. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. Am. J. Neuroradiol. 2018, 39, E9–E31. [Google Scholar] [CrossRef]
- Piscaglia, F.; Nolsøe, C.; Dietrich, C.; Cosgrove, D.; Gilja, O.; Nielsen, M.B.; Albrecht, T.; Barozzi, L.; Bertolotto, M.; Catalano, O.; et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Med. Eur. J. Ultrasound 2011, 33, 33–59. [Google Scholar] [CrossRef]
- Imbesi, S.; Kerber, C. An Experimental and Angiographic Explanation of Why Ulcerated Carotid Bulbs Embolize. Interv. Neuroradiol. 1999, 5, 11–18. [Google Scholar] [CrossRef]
- Kate, G.L.T.; van Dijk, A.C.; Oord, S.C.v.D.; Hussain, B.; Verhagen, H.J.; Sijbrands, E.J.; van der Steen, A.F.; van der Lugt, A.; Schinkel, A.F. Usefulness of Contrast-Enhanced Ultrasound for Detection of Carotid Plaque Ulceration in Patients with Symptomatic Carotid Atherosclerosis. Am. J. Cardiol. 2013, 112, 292–298. [Google Scholar] [CrossRef]
- Menon, B.K.; Singh, J.; Al-Khataami, A.; Demchuk, A.M.; Goyal, M.; Calgary CTA Study Group. The donut sign on CT angiography: An indicator of reversible intraluminal carotid thrombus? Neuroradiology 2010, 52, 1055–1056. [Google Scholar] [CrossRef]
- Hoogi, A.; Adam, D.; Hoffman, A.; Kerner, H.; Reisner, S.; Gaitini, D. Carotid Plaque Vulnerability: Quantification of Neovascularization on Contrast-Enhanced Ultrasound with Histopathologic Correlation. Am. J. Roentgenol. 2011, 196, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Akkus, Z.; Hoogi, A.; Renaud, G.; Oord, S.C.v.D.; Kate, G.L.T.; Schinkel, A.F.; Adam, D.; de Jong, N.; van der Steen, A.F.; Bosch, J.G. New Quantification Methods for Carotid Intra-plaque Neovascularization Using Contrast-Enhanced Ultrasound. Ultrasound Med. Biol. 2014, 40, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Oord, S.C.H.v.D.; Akkus, Z.; Bosch, J.G.; Hoogi, A.; Kate, G.L.T.; Renaud, G.; Sijbrands, E.J.G.; Verhagen, H.J.; van der Lugt, A.; Adam, D.; et al. Quantitative Contrast-Enhanced Ultrasound of Intraplaque Neovascularization in Patients with Carotid Atherosclerosis. Ultraschall Med. Eur. J. Ultrasound 2014, 36, 154–161. [Google Scholar] [CrossRef]
- Wiesinger, I.; Jung, F.; Jung, E.M. Contrast-enhanced ultrasound (CEUS) and perfusion imaging using VueBox®. Clin. Hemorheol. Microcirc. 2021, 78, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Kanber, B.; Hartshorne, T.C.; Horsfield, M.A.; Naylor, A.R.; Robinson, T.G.; Ramnarine, K.V. A Novel Ultrasound-Based Carotid Plaque Risk Index Associated with the Presence of Cerebrovascular Symptoms. Ultraschall Med. Eur. J. Ultrasound 2014, 36, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Deng, Y.-B.; Zhu, Y.; Liu, Y.-N.; Bi, X.-J. Correlation of Carotid Plaque Neovascularization Detected by Using Contrast-enhanced US with Clinical Symptoms. Radiology 2009, 251, 583–589. [Google Scholar] [CrossRef]
- Staub, D.; Patel, M.B.; Tibrewala, A.; Ludden, D.; Johnson, M.; Espinosa, P.; Coll, B.; Jaeger, K.A.; Feinstein, S.B. Vasa Vasorum and Plaque Neovascularization on Contrast-Enhanced Carotid Ultrasound Imaging Correlates with Cardiovascular Disease and Past Cardiovascular Events. Stroke 2010, 41, 41–47. [Google Scholar] [CrossRef]
- Xu, R.; Yin, X.; Xu, W.; Jin, L.; Lu, M.; Wang, Y. Assessment of carotid plaque neovascularization by contrast-enhanced ultrasound and high sensitivity C-reactive protein test in patients with acute cerebral infarction: A comparative study. Neurol. Sci. 2016, 37, 1107–1112. [Google Scholar] [CrossRef]
- Huang, R.; Abdelmoneim, S.S.; Ball, C.A.; Nhola, L.F.; Farrell, A.M.; Feinstein, S.; Mulvagh, S.L. Detection of Carotid Atherosclerotic Plaque Neovascularization Using Contrast Enhanced Ultrasound: A Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies. J. Am. Soc. Echocardiogr. 2016, 29, 491–502. [Google Scholar] [CrossRef]
- Faggioli, G.; Pini, R.; Mauro, R.; Pasquinelli, G.; Fittipaldi, S.; Freyrie, A.; Serra, C.; Stella, A. Identification of Carotid ‘Vulnerable Plaque’ by Contrast-enhanced Ultrasonography: Correlation with Plaque Histology, Symptoms and Cerebral Computed Tomography. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Van der Veken, B.; De Meyer, G.R.; Martinet, W. Intraplaque neovascularization as a novel therapeutic target in advanced atherosclerosis. Expert. Opin. Ther. Targets. 2016, 20, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Grazhdani, H.; Clevert, D.A.; Iezzi, R.; Aiani, L.; Martegani, A.; Fanelli, F.; Di Marzo, L.; Wlderk, A.; Cirelli, C.; et al. EVAR: Benefits of CEUS for monitoring stent-graft status. Eur. J. Radiol. 2015, 84, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Martinelli, O.; Pacini, P.; Di Serafino, M.; Huang, P.; Dolcetti, V.; Cantisani, V. New Technologies in the Assessment of Carotid Stenosis: Beyond the Color-Doppler Ultrasound—High Frame Rate Vector-Flow and 3D Arterial Analysis Ultrasound. Diagnostics 2023, 13, 1478. [Google Scholar] [CrossRef] [PubMed]
- Kwee, R.M.; Van Oostenbrugge, R.J.; Hofstra, L.; Teule, G.J.; van Engelshoven, J.M.A.; Mess, W.H.; Kooi, M.E. Identifying vulnerable carotid plaques by noninvasive imaging. Neurology 2008, 70, 2401–2409. [Google Scholar] [CrossRef]
- Benson, J.C.; Cheek, H.; Aubry, M.C.; Lanzino, G.; Huston, J., III; Rabinstein, A.; Brinjikji, W. Cervical carotid plaque MRI: Review of atherosclerosis imaging features and their histologic underpinnings. Clin. Neuroradiol. 2021, 31, 295–306. [Google Scholar] [CrossRef]
- Den Hartog, A.G.; Bovens, S.M.; Koning, W.; Hendrikse, J.; Luijten, P.R.; Moll, F.L.; De Borst, G.J. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 7–21. [Google Scholar] [CrossRef]
- Saam, T.; Ferguson, M.S.; Yarnykh, V.L.; Takaya, N.; Xu, D.; Polissar, N.L.; Yuan, C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 234–239. [Google Scholar] [CrossRef]
- Trivedi, R.; U-King-Im, J.; Gillard, J. Accumulation of Ultrasmall Superparamagnetic Particles of Iron Oxide in Human Atherosclerotic Plaques. Circulation 2003, 108, e140. [Google Scholar] [CrossRef]
- Kooi, M.E.; Cappendijk, V.C.; Cleutjens, K.B.J.M.; Kessels, A.G.H.; Kitslaar, P.J.E.H.M.; Borgers, M.; Van Engelshoven, J.M.A. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 2003, 107, 2453–2458. [Google Scholar] [CrossRef]
- Qiao, Y.; Etesami, M.; Malhotra, S.; Astor, B.C.; Virmani, R.; Kolodgie, F.D.; Wasserman, B.A. Identification of intraplaque hemorrhage on MR angiography images: A comparison of contrast-enhanced mask and time-of-flight techniques. Am. J. Neuroradiol. 2011, 32, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Ferguson, M.S.; Chen, H.; Hippe, D.S.; Kerwin, W.S.; Canton, G.; Hatsukami, T.S. Magnetic Resonance Features of the Disruption-Prone and the Disrupted Carotid Plaque. J. Am. Coll. Cardiol. Imaging 2009, 2, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Hermus, L.; Lefrandt, J.D.; Tio, R.A.; Breek, J.C.; Zeebregts, C.J. Carotid plaque formation and serum biomarkers. Atherosclerosis 2010, 213, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Murgia, A.; Erta, M.; Suri, J.S.; Gupta, A.; Wintermark, M.; Saba, L. CT imaging features of carotid artery plaque vulnerability. Ann. Transl. Med. 2020, 8, 1261. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, J.K.; Huston, J. Imaging of high-risk carotid artery plaques: Current status and future directions. Neurosurg. Focus 2014, 36, E1. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Li, Y.; Ding, V.; Jiang, B.; Ball, R.L.; Rodriguez, F.; Wintermark, M. Semiautomated characterization of carotid artery plaque features from computed tomography angiography to predict atherosclerotic cardiovascular disease risk score. J. Comput. Assist. Tomogr. 2019, 43, 452. [Google Scholar] [CrossRef]

| Carotid CEUS Workflow | Reference/Setting |
|---|---|
| Ultrasound machine setup |
|
| Ultrasonographic contrast agent administration |
|
| Image acquisition |
|
| Image analysis |
|
| Imaging Technique | Application Scenarios | Advantages | Limitations |
|---|---|---|---|
| CEUS |
|
|
|
| CT |
|
|
|
| MRI |
|
|
|
| Marker | Classification | Details |
|---|---|---|
| Plaque surface | Visual-based qualitative system | Smooth plaque surface Plaque surface irregularities (0.3 and 0.9 mm) Plaque ulceration (>1 × 1 mm) |
| Surface irregularity index | Index obtained by dividing the sum of angular deviations of the plaque surface from the straight line by the length of the plaque | |
| Intraplaque neovascularization | Visual-based qualitative system | Grade 1: no vascularization Grade 2: moderate vascularization Grade 3: extensive vascularization |
| Quantitative assessment with dedicated software, e.g., VueBox | Plaque region of interest compared with the lumen of the carotid artery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopyto, E.; Czeczelewski, M.; Mikos, E.; Stępniak, K.; Kopyto, M.; Matuszek, M.; Nieoczym, K.; Czarnecki, A.; Kuczyńska, M.; Cheda, M.; et al. Contrast-Enhanced Ultrasound Feasibility in Assessing Carotid Plaque Vulnerability—Narrative Review. J. Clin. Med. 2023, 12, 6416. https://doi.org/10.3390/jcm12196416
Kopyto E, Czeczelewski M, Mikos E, Stępniak K, Kopyto M, Matuszek M, Nieoczym K, Czarnecki A, Kuczyńska M, Cheda M, et al. Contrast-Enhanced Ultrasound Feasibility in Assessing Carotid Plaque Vulnerability—Narrative Review. Journal of Clinical Medicine. 2023; 12(19):6416. https://doi.org/10.3390/jcm12196416
Chicago/Turabian StyleKopyto, Ewa, Marcin Czeczelewski, Eryk Mikos, Karol Stępniak, Maja Kopyto, Małgorzata Matuszek, Karolina Nieoczym, Adam Czarnecki, Maryla Kuczyńska, Mateusz Cheda, and et al. 2023. "Contrast-Enhanced Ultrasound Feasibility in Assessing Carotid Plaque Vulnerability—Narrative Review" Journal of Clinical Medicine 12, no. 19: 6416. https://doi.org/10.3390/jcm12196416
APA StyleKopyto, E., Czeczelewski, M., Mikos, E., Stępniak, K., Kopyto, M., Matuszek, M., Nieoczym, K., Czarnecki, A., Kuczyńska, M., Cheda, M., Drelich-Zbroja, A., & Jargiełło, T. (2023). Contrast-Enhanced Ultrasound Feasibility in Assessing Carotid Plaque Vulnerability—Narrative Review. Journal of Clinical Medicine, 12(19), 6416. https://doi.org/10.3390/jcm12196416





