Bronchiectasis-COPD Overlap Syndrome: Role of Peripheral Eosinophil Count and Inhaled Corticosteroid Treatment
Abstract
:1. Introduction
2. Methods
2.1. Study Design, Participants, and Ethics
2.2. Clinical Characterization
2.3. High-Resolution CT Scan (HRCT Scan)
2.4. Microbiology
2.5. Data Analysis
3. Results
3.1. Blood Eosinophil Count and Follow-Up Variables
3.2. Effects of Treatment with ICs According to the BEC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soriano, J.B.; Alfageme, I.; Miravitlles, M.; de Lucas, P.; Soler-Cataluña, J.J.; García-Río, F.; Casanova, C.; Gonzalez-Moro, J.M.R.; Cosío, B.G.; Sanchez, G.; et al. Prevalence and Determinants of COPD in Spain: EPISCAN II. Arch. Bronconeumol. 2021, 57, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Celli, B.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; de Oca, M.M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2023, 207, 819–837. [Google Scholar] [PubMed]
- Miravitlles, M.; Calle, M.; Molina, J.; Almagro, P.; Gómez, J.T.; Trigueros, J.A.; Cosío, B.G.; Casanova, C.; López-Campos, J.L.; Riesco, J.A.; et al. Spanish COPD Guidelines (GesEPOC) 2021: Updated Pharmacological treatment of stable COPD. Arch. Bronconeumol. 2022, 58, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Martinez-García, M.A.; Villa, C.; Dobarganes, Y.; Girón, R.; Maíz, L.; García-Clemente, M.; Sibila, O.; Golpe, R.; Barreiro, E.; Rodriguez, J.L.; et al. RIBRON: The spanish Online Bronchiectasis Registry. Characterization of the First 1912 Patients. Arch. Bronconeumol. 2021, 57, 28–35. [Google Scholar] [CrossRef]
- Huang, J.T.; Cant, E.; Keir, H.R.; Barton, A.K.; Kuzmanova, E.; Shuttleworth, M.; Pollock, J.; Finch, S.; Polverino, E.; Bottier, M.; et al. Endotyping Chronic Obstructive Pulmonary Disease, Bronchiectasis, and the “Chronic Obstructive Pulmonary Disease-Bronchiectasis Association”. Am. J. Respir. Crit Care Med. 2022, 206, 417–426. [Google Scholar] [CrossRef]
- Martínez-García, M.Á. Bronchiectasis and Eosinophils. Arch. Bronconeumol. 2021, 57, 671–672. [Google Scholar] [CrossRef]
- Fuschillo, S.; De Felice, A.; Balzano, G. Mucosal inflammation in idiopathic bronchiectasis: Cellular and molecular mechanisms. Eur. Respir. J. 2008, 31, 396–406. [Google Scholar] [CrossRef]
- Long, M.B.; Chalmers, J.D. Treating Neutrophilic Inflammation in Airways Diseases. Arch. Bronconeumol. 2022, 58, 463–465. [Google Scholar]
- Guan, W.J.; Oscullo, G.; He, M.Z.; Xu, D.Y.; Gómez-Olivas, J.D.; Martinez-Garcia, M.A. Significance and Potential Role of Eosinophils in Non-Cystic Fibrosis Bronchiectasis. J. Allergy Clin. Immunol. Pract. 2022, 11, 1089–1099. [Google Scholar] [CrossRef]
- Leung, J.M.; Tiew, P.Y.; Mac Aogain, M.; Budden, K.F.; Yong, V.F.; Thomas, S.S.; Pethe, K.; Hansbro, P.M.; Chotirmall, S.H. The role of acute and chronic respiratory colonization and infections in the pathogenesis of copd. Respirology 2017, 22, 634–650. [Google Scholar] [CrossRef]
- Patel, I.S.; Seemungal, T.A.; Wilks, M.; Lloyd-Owen, S.J.; Donaldson, G.C.; Wedzicha, J.A. Relationship between bacterial colonisation and the frequency, character, and severity of copd exacerbations. Thorax 2002, 57, 759–764. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa Carrillo, D.; Martínez-García, M.Á.; Barreiro, E.; Tabernero Huguet, E.; Costa Sola, R.; García-Clemente, M.M.; Jiménez, N.C.; Pons, L.R.; Acuña, C.C.; Hermosa, J.L.R.; et al. Effectiveness and Safety of Inhaled Antibiotics in Patients With Chronic Obstructive Pulmonary Disease. A Multicentre Observational Study. Arch. Bronconeumol. 2022, 58, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kunadharaju, R.; Rudraraju, A.; Sethi, S. Pseudomonas aeruginosa Colonization and COPD: The Chicken or the Egg? Arch. Bronconeumol. 2022, 58, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Aogáin, M.M.; Jaggi, T.K.; Chotirmall, S.H. The Airway Microbiome: Present and Future Applications. Arch. Bronconeumol. 2022, 58, 8–10. [Google Scholar] [CrossRef]
- Flume, P.A.; Chalmers, J.D.; Olivier, K.N. Advances in bronchiectasis: Endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018, 392, 880–890. [Google Scholar] [CrossRef] [PubMed]
- García-Río, F.; Alcázar-Navarrete, B.; Castillo-Villegas, D.; Cilloniz, C.; García-Ortega, A.; Leiro-Fernández, V.; Lojo-Rodriguez, I.; Padilla-Galo, A.; Quezada-Loaiza, C.A.; Rodriguez-Portal, J.A.; et al. Biological Biomarkers in Respiratory Diseases. Arch. Bronconeumol. 2022, 58, 323–333. [Google Scholar] [CrossRef]
- Miravitlles, M.; Monteagudo, M.; Solntseva, I.; Alcázar, B. Blood Eosinophil Counts and Their Variability and Risk of Exacerbations in COPD: A Population-Based Study. Arch. Bronconeumol. 2021, 57, 13–20. [Google Scholar] [CrossRef]
- Siddharthan, T.; Gupte, A.; Barnes, P.J. Chronic Obstructive Pulmonary Disease Endotypes in Low- and Middle-Income Country Settings: Precision Medicine for All. Am. J. Respir. Crit. Care Med. 2020, 202, 171–172. [Google Scholar] [CrossRef]
- Alcázar-Navarrete, B.; Díaz-Lopez, J.M.; García-Flores, P.; Ortega-Antelo, M.; Aguilar-Cruz, I.; Ruiz-Rodríguez, O.; Santiago-Diaz, P.; Palacios, P.J.R. T2 Biomarkers as Predictors of Exacerbations of Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2022, 58, 595–600. [Google Scholar] [CrossRef]
- Posadas, T.; Oscullo, G.; Zaldivar, E.; Villa, C.; Dobarganes, Y.; Girón, R.; Olveira, C.; Maíz, L.; García-Clemente, M.; Sibila, O.; et al. C-Reactive Protein Concentration in Steady-State Bronchiectasis: Prognostic Value of Future Severe Exacerbations. Data From the Spanish Registry of Bronchiectasis (RIBRON). Arch. Bronconeumol. 2021, 57, 21–27. [Google Scholar] [CrossRef]
- Shoemark, A.; Shteinberg, M.; De Soyza, A.; Haworth, C.; Richardson, H.; Gao, Y.; Perea, L.; Dicker, A.J.; Goeminne, P.C.; Cant, E.; et al. Characterisation of Eosinophilic Bronchiectasis: A European Multicohort Study. Am. J. Respir. Crit. Care Med. 2022, 205, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Mendez, R.; Olveira, C.; Girón, R.; Garcia-Clemente, M.; Maiz, L.; Sibila, O.; Golpe, R.; Rodríguez-Hermosa, J.L.; Barreiro, E.; et al. The U-shaped relationship between eosinophil count and bronchiectasis severity: The effect of inhaled corticosteroids. Chest 2023, 164, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Cosío, B.G.; Shafiek, H.; Martínez-García, M.Á. Is it Time to Readjust the Doses of Inhaled Corticosteroids in COPD? Arch. Bronconeumol. 2022, 58, 593–594. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Zhou, W.; Yang, X.; Li, J.; Cao, J. Risk of Pneumonia with Different Inhaled Corticosteroids in COPD Patients: A Meta-Analysis. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á.; Faner, R.; Oscullo, G.; la Rosa-Carrillo, D.; Soler-Cataluña, J.J.; Ballester, M.; Muriel, A.; Agusti, A. Chronic Bronchial Infection Is Associated with More Rapid Lung Function Decline in Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2022, 19, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Dimakou, K.; Hurst, J.; Martinez-Garcia, M.A.; Miravitlles, M.; Paggiaro, P.; Shteinberg, M.; Aliberti, S.; Chalmers, J.D. The overlap between bronchiectasis and chronic airway diseases: State of the art and future directions. Eur. Respir. J. 2018, 52, 1800328. [Google Scholar] [CrossRef]
- Solarat, B.; Perea, L.; Faner, R.; de La Rosa, D.; Martínez-García, M.Á.; Sibila, O. Pathophysiology of Chronic Bronchial Infection in Bronchiectasis. Arch. Bronconeumol. 2023, 59, 101–108. [Google Scholar] [CrossRef]
- Gomez-Olivas, J.D.S.; Oscullo, G.; Martinez-Garcia, M.A. Etiology of Bronchiectasis in the World: Data from the Published National and International Registries. J. Clin. Med. 2023, 12, 5782. [Google Scholar] [CrossRef]
- Hurst, J.; Elborns, S.; De Soyza, A. COPD-bronchiectasis overlap syndrome. Eur. Respir. J. 2015, 45, 310–313. [Google Scholar] [CrossRef]
- Aliberti, S.; Goeminne, P.C.; O’Donnell, A.; Aksamit, T.R.; Al-Jahdali, H.; Barker, A.; Blasi, F.; Boersma, W.G.; Crichton, M.L.; De Soyza, A.; et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: International consensus recommendations. Lancet Respir. Med. 2022, 10, 298–306. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; de la Rosa Carrillo, D.; Soler-Cataluna, J.J.; Donat-Sanz, Y.; Serra, P.C.; Lerma, M.A.; Ballestín, J.; Sánchez, I.V.; Ferrer, M.J.S.; Dalfo, A.R.; et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á.; Máiz, L.; Olveira, C.; Girón, R.M.; de la Rosa, D.; Blanco, M.; Cantón, R.; Vendrell, M.; Polverino, E.; De Gracia, J.; et al. Spanish Guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch. Bronconeumol. 2018, 54, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Sanchis, J.; Agusti-Vidal, A.; Segarra, F.; Navajas, D.; Rodriguez-Roisin, R.; Casan, P.; Sans, S. Spirometric reference values from a Mediterranean population. Bull. Eur. Physiopathol. Respir. 1986, 22, 217–224. [Google Scholar] [PubMed]
- Soler-Cataluña, J.J.; Piñera, P.; Trigueros, J.A.; Calle, M.; Casanova, C.; Cosío, B.G.; López-Campos, J.L.; Molina, J.; Almagro, P.; Gómez, J.T.; et al. Spanish COPD Guidelines (GesEPOC) 2021 Update Diagnosis and Treatment of COPD Exacerbation Syndrome. Arch. Bronconeumol. 2022, 58, 159–170. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Oscullo, G.; García-Ortega, A.; Matera, M.G.; Rogliani, P.; Cazzola, M. Inhaled Corticosteroids in Adults with Non-cystic Fibrosis Bronchiectasis: From Bench to Bedside. A Narrative Review. Drugs 2022, 82, 1453–1468. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Faner, R.; Oscullo, G.; de la Rosa, D.; Soler-Cataluña, J.J.; Ballester, M.; Agusti, A. Inhaled Steroids, Circulating Eosinophils, Chronic Airway Infection, and Pneumonia Risk in Chronic Obstructive Pulmonary Disease. A Network Analysis. Am. J. Respir. Crit. Care Med. 2020, 201, 1078–1085. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Máiz, L.; Olveira, C.; Girón, R.M.; de la Rosa, D.; Blanco, M.; Cantón, R.; Vendrell, M.; Polverino, E.; de Gracia, J.; et al. Spanish Guidelines on Treatment of Bronchiectasis in Adults. Arch. Bronconeumol. 2018, 54, 88–98. [Google Scholar] [CrossRef]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, J.S.; Floto, R.A.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British Thoracic Society guideline for bronchiectasis in adults. Thorax 2019, 74, 1–69. [Google Scholar] [CrossRef]
- Pascoe, S.; Barnes, N.; Brusselle, G.; Compton, C.; Criner, G.J.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Hartley, B.; Lange, P.; et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: Analysis of the IMPACT trial. Lancet Respir. Med. 2019, 7, 745–756. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Munitz, A.; Ackerman, S.J.; Drake, M.G.; Jackson, D.J.; Wardlaw, A.J.; Dougan, S.K.; Berdnikovs, S.; Schleich, F.; Matucci, A.; et al. Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clin. Proc. 2021, 96, 2694–2707. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.I.; Singayagam, A.; Mitchell, S.; Wedzicha, J.A.; Shah, A.; Bloom, C.I. The Effect of Inhaled Corticosteroids on Pneumonia Risk in Patients With COPD-Bronchiectasis Overlap. A UK Population-Based Case-Control Study. Chest 2023, 164, 875–884. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.M.; Gibson, P.G. Treatable Traits in Asthma and COPD. Arch. Bronconeumol. 2022, 58, 583–585. [Google Scholar] [CrossRef]
- Navas-Bueno, B.; Casas-Maldonado, F.; Padilla-Galo, A.; González-Moya-Mondelo, E.; Arenas-Gordillo, M.; Bioque-Rivera, J.C.; Galván, R.J.; Cano-Gómez, M.S.; López-Campos, J.L.; Merlos-Navarro, S.; et al. High Adherence, Microbiological Control and Reduced Exacerbations in Patients With Non-Cystic Fibrosis Bronchiectasis Treated With Nebulised Colistin. A Prospective Observational Study. Arch. Bronconeumol. 2022, 58, 834–836. [Google Scholar] [CrossRef]
- Soler-Cataluña, J.J.; Miralles, C. Exacerbation Syndrome in COPD: A Paradigm Shift. Arch. Bronconeumol. 2021, 57, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Scioscia, G.; Amaro, R.; Alcaraz-Serrano, V.; Gabarrus, A.; Fernandez-Barat, L.; Huamán, P.O.; Menendez, R.; Mendez, R.; Barbaro, M.P.F.; Torres, A. Factors Associated With One-Year Mortality in Hospitalised Patients With Exacerbated Bronchiectasis. Arch. Bronconeumol. 2022, 58, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Sabroe, I.; Postma, D.; Heijink, I.; Dockrell, D.H. The yin and the yang of immunosuppression with inhaled corticosteroids. Thorax 2013, 68, 1085–1087. [Google Scholar] [CrossRef]
- Laorden, D.; Zamarrón, E.; Domínguez-Ortega, J.; Romero, D.; Quirce, S.; Álvarez-Sala, R. Successful Long-Term Treatment Combining Omalizumab and Anti-IL-5 Biologics in Allergic Bronchopulmonary Aspergillosis. Arch. Bronconeumol. 2022, 58, 624–626. [Google Scholar] [CrossRef]
- Rademacher, J.; Konwert, S.; Fuge, J.; Dettmer, S.; Welte, T.; Ringshausen, F.C. Anti-IL5 and anti-IL5Rα therapy for clinically significant bronchiectasis with eosinophilic endotype: A case series. Eur. Respir. J. 2020, 55, 1901333. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Cole, J.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N. Engl. J. Med. 2023, 389, 205–214. [Google Scholar] [CrossRef]

| Variables | Less than 100 Eosinophils/µL | Between 101–300 Eosinophils/µL | More than 300 Eosinophils/µL | p Value (ANOVA) |
|---|---|---|---|---|
| n = 27 (23.5%) | n = 63 (54.8%) | n = 25 (21.7%) | ||
| Age, yrs. | 72.2 (9.2) | 71.6 (8.5) | 70.6 (8.3) | 0.801 |
| Males, % | 89% | 94% | 96% | 0.586 |
| Body mass index, kg/m2 | 27.2 (3.9) | 25.8 (4.8) | 27.7 (5.6) | 0.182 |
| Smoking habit, pack.years | 55.5 (29.2) | 66.3 (30.4) | 58.6 (37.7) | 0.285 |
| Charlson index | 2.6 (1.4) | 2.6 (1.5) | 2.2 (1.4) | 0.481 |
| Dyspnoea (MRC) | 1.9 (0.9) | 1.6 (0.9) | 1.6 (0.9) | 0.356 |
| Daily sputum production, % | 77.8% | 74.6% | 64% | 0.567 |
| Purulent sputum, % | 22.2% | 25% | 33.3% | 0.858 |
| Time from symptoms | 14 (16.6) | 13.4 (10.7) | 10.7 (10.6) | 0.578 |
| Post-bd FEV1, % ref. | 46.6 (13.7) | 45.1 (13.1) | 45.9 (11.7) | 0.879 |
| FEV1/FVC, % | 52.7 (13.8) | 48.8 (13.2) | 52.7 (11.9) | 0.291 |
| Fibrinogen, mg/dL | 327 (145) | 430 (172) | 338 (221) | 0.132 |
| CRP, IU/mL | 8.2 (6.9) | 8.1 (8.5) | 9.1 (10) | 0.947 |
| Long-acting bronchodilators, % | 97% | 98% | 96% | 0.956 |
| Inhaled corticosteroids, % | 64% | 69% | 66% | 0.879 |
| Triple therapy, % | 51.8% | 58.7% | 64% | 0.676 |
| Long-term antibiotics | 26% | 23% | 15% | 0.064 |
| Macrolides | 28% | 25.4% | 16% | 0.086 |
| Variables | Less than 100 Eosinophils/µL | Between 101–300 Eosinophils/µL | More than 300 Eosinophils/µL | p Value (ANOVA) |
|---|---|---|---|---|
| n = 27 (23.5%) | n = 63 (54.8%) | n = 25 (21.7%) | ||
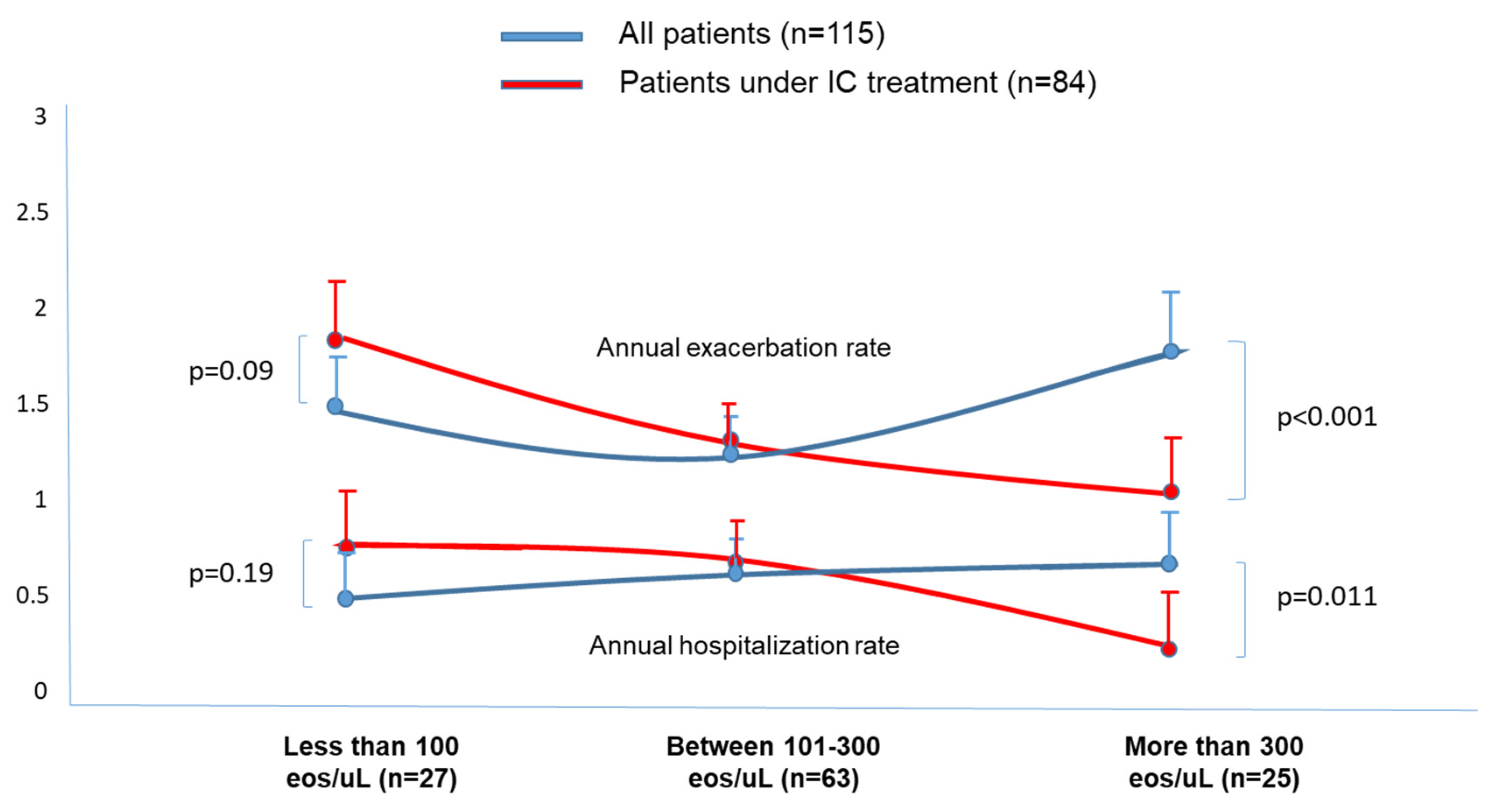
Annual exacerbation rate
| 1.48 (1.1) | 1.27 (1.1) | 1.77 (1.2) | 0.031 * |
| 1.71 (1.1) | 1.31 (1.1) | 1.08 (0.6) | 0.011 ** | |
Annual hospitalization rate
| 0.48 (0.7) | 0.58 (0.9) | 0.67 (0.8) | 0.041 ** |
| 0.69 (0.8) | 0.61 (0.9) | 0.35 (0.5) | 0.033 ** | |
Chronic bronchial infection
| 51.8% | 31.7% | 24% | 0.012 ** |
| 50% | 34% | 24.3% | 0.032 ** | |
CBI by P. aeruginosa
| 33.3% | 17.4% | 12% | 0.043 *** |
| 35% | 20% | 7.1% | 0.047 *** | |
At least one pneumonia
| 74.1% | 44.4% | 12% | <0.001 *** |
| 70% | 50% | 7.1% | 0.001 *** | |
Death
| 59.2% | 57.1% | 64% | 0.844 |
| 55% | 64% | 57.1% | 0.757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oscullo, G.; Gómez-Olivas, J.D.; Ingles, M.; Mompean, S.; Martinez-Perez, R.; Suarez-Cuartin, G.; Rosa-Carrillo, D.l.; Martinez-Garcia, M.A. Bronchiectasis-COPD Overlap Syndrome: Role of Peripheral Eosinophil Count and Inhaled Corticosteroid Treatment. J. Clin. Med. 2023, 12, 6417. https://doi.org/10.3390/jcm12196417
Oscullo G, Gómez-Olivas JD, Ingles M, Mompean S, Martinez-Perez R, Suarez-Cuartin G, Rosa-Carrillo Dl, Martinez-Garcia MA. Bronchiectasis-COPD Overlap Syndrome: Role of Peripheral Eosinophil Count and Inhaled Corticosteroid Treatment. Journal of Clinical Medicine. 2023; 12(19):6417. https://doi.org/10.3390/jcm12196417
Chicago/Turabian StyleOscullo, Grace, Jose Daniel Gómez-Olivas, Marina Ingles, Sergio Mompean, Rosalia Martinez-Perez, Guillermo Suarez-Cuartin, David la Rosa-Carrillo, and Miguel Angel Martinez-Garcia. 2023. "Bronchiectasis-COPD Overlap Syndrome: Role of Peripheral Eosinophil Count and Inhaled Corticosteroid Treatment" Journal of Clinical Medicine 12, no. 19: 6417. https://doi.org/10.3390/jcm12196417
APA StyleOscullo, G., Gómez-Olivas, J. D., Ingles, M., Mompean, S., Martinez-Perez, R., Suarez-Cuartin, G., Rosa-Carrillo, D. l., & Martinez-Garcia, M. A. (2023). Bronchiectasis-COPD Overlap Syndrome: Role of Peripheral Eosinophil Count and Inhaled Corticosteroid Treatment. Journal of Clinical Medicine, 12(19), 6417. https://doi.org/10.3390/jcm12196417






