Comparison of Incidence and Prognosis of Myocardial Injury in Patients with COVID-19-Related Respiratory Failure and Other Pulmonary Infections: A Contemporary Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
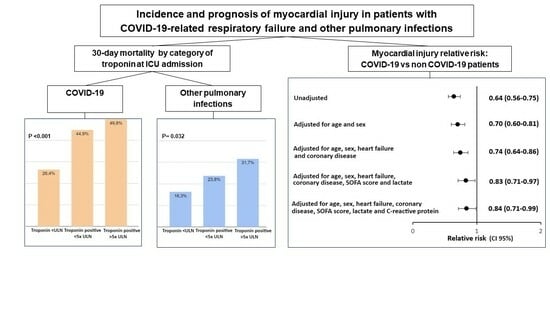
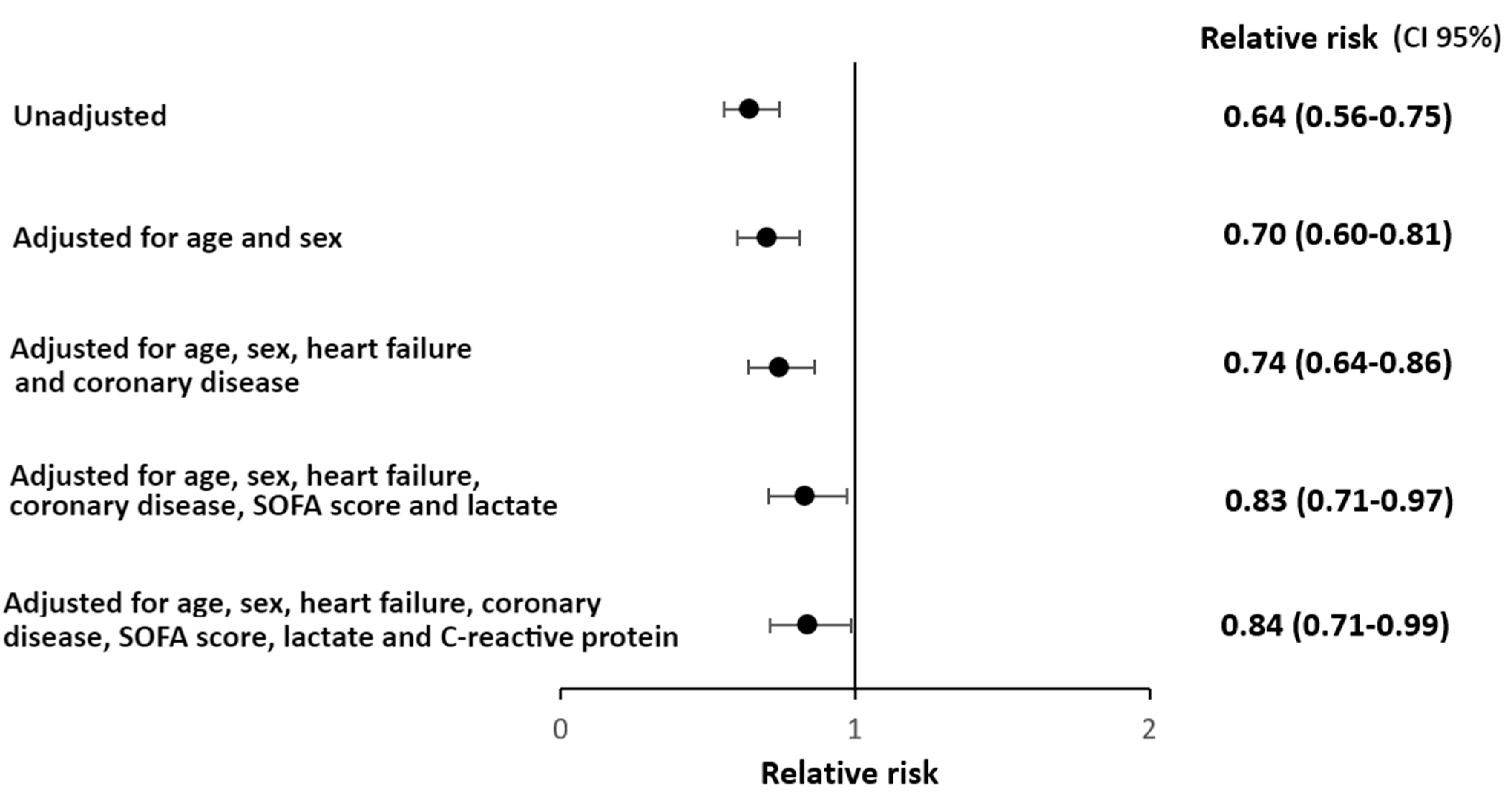
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pei, H.; Zhou, C.; Lou, Y. Myocardial Injury Predicts Risk of Short-Term All-Cause Mortality in Patients with COVID-19: A Dose-Response Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 850447. [Google Scholar] [CrossRef] [PubMed]
- Changal, K.; Veria, S.; Mack, S.; Paternite, D.; Sheikh, S.A.; Patel, M.; Mir, T.; Sheikh, M.; Ramanathan, P.K. Myocardial injury in hospitalized COVID-19 patients: A retrospective study, systematic review, and meta-analysis. BMC Cardiovasc. Disord. 2021, 21, 626. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Patel, N.H.; Somi, S.; Singh, J. Elevated cardiac troponin I as a predictor of outcomes in COVID-19 hospitalizations: A meta-analysis. Infez. Med. 2020, 28, 500–506. [Google Scholar]
- Metkus, T.S.; Sokoll, L.J.; Barth, A.S.; Czarny, M.J.; Hays, A.G.; Lowenstein, C.J.; Michos, E.D.; Nolley, E.P.; Post, W.S.; Resar, J.R.; et al. Myocardial Injury in Severe COVID-19 Compared with Non-COVID-19 Acute Respiratory Distress Syndrome. Circulation 2021, 143, 553–565. [Google Scholar] [CrossRef]
- Jirak, P.; Larbig, R.; Shomanova, Z.; Fröb, E.J.; Dankl, D.; Torgersen, C.; Frank, N.; Mahringer, M.; Butkiene, D.; Haake, H.; et al. Myocardial injury in severe COVID-19 is similar to pneumonias of other origin: Results from a multicentre study. ESC Heart Fail. 2021, 8, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Sewanan, L.R.; Clerkin, K.J.; Tucker, N.R.; Tsai, E.J. How Does COVID-19 Affect the Heart? Curr. Cardiol. Rep. 2023, 25, 171–184. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819. [Google Scholar] [CrossRef]
- Zeng, J.H.; Liu, Y.X.; Yuan, J.; Wang, F.-X.; Wu, W.-B.; Li, J.-X.; Wang, L.-F.; Gao, H.; Wang, Y.; Dong, C.-F.; et al. First case of COVID-19 complicated with fulminant myocarditis: A case report and insights. Infection 2020, 48, 773. [Google Scholar] [CrossRef]
- Hu, H.; Ma, F.; Wei, X.; Fang, Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur. Heart J. 2021, 42, 206. [Google Scholar] [CrossRef]
- Fried, J.A.; Ramasubbu, K.; Bhatt, R.; Topkara, V.K.; Clerkin, K.J.; Horn, E.; Rabbani, L.; Brodie, D.; Jain, S.S.; Kirtane, A.J.; et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation 2020, 141, 1930. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020, 22, 911. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.C.; Kim, J.Y.; Kim, H.A.; Han, S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020, 41, 1859. [Google Scholar] [CrossRef] [PubMed]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheis, M.; et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020, 7, 2440. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Mehta, J.L. Takotsubo cardiomyopathy and COVID-19 infection. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1299. [Google Scholar] [CrossRef]
- Nicol, M.; Cacoub, L.; Baudet, M.; Nahmani, Y.; Cacoub, P.; Cohen-Solal, A.; Henry, P.; Adle-Biassette, H.; Logeart, D. Delayed acute myocarditis and COVID-19-related multisystem inflammatory syndrome. ESC Heart Fail. 2020, 7, 4371–4376. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Quincy Brown, J.; Vander Heide, R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Lim, W.; Qushmaq, I.; Devereaux, P.J.; Heels-Ansdell, D.; Lauzier, F.; Ismaila, A.S.; Crowther, M.A.; Cook, D.J. Elevated cardiac troponin measurements in critically ill patients. Arch. Intern. Med. 2006, 166, 2446–2454. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Ferrari, F.; Martins, V.M.; Fuchs, F.D.; Stein, R. Renin-Angiotensin-Aldosterone System Inhibitors in COVID-19: A Review. Clinics 2021, 76, e2342. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Macedo, A.V.S.; De Barros ESilva, P.G.M.; Moll-Bernardes, R.J.; Dos Santos, T.M.; Mazza, L.; Feldman, A.; D’Andréa Saba Arruda, G.; De Albuquerque, D.C.; Camiletti, A.S.; et al. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted with COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Metkus, T.S.; Guallar, E.; Sokoll, L.; Morrow, D.; Tomaselli, G.; Brower, R.; Schulman, S.; Korley, F.K. Prevalence and Prognostic Association of Circulating Troponin in the Acute Respiratory Distress Syndrome. Crit. Care Med. 2017, 45, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Efros, O.; Soffer, S.; Leibowitz, A.; Fardman, A.; Klempfner, R.; Meisel, E.; Grossman, E. Risk factors and mortality in patients with pneumonia and elevated troponin levels. Sci. Rep. 2020, 10, 21619. [Google Scholar] [CrossRef]
- Vestjens, S.M.T.; Spoorenberg, S.M.C.; Rijkers, G.T.; Grutters, J.C.; Ten Berg, J.M.; Noordzij, P.G.; Van de Garde, E.M.W.; Bos, W.J.W.; Ovidius Study Group. High-sensitivity cardiac troponin T predicts mortality after hospitalization for community-acquired pneumonia. Respirology 2017, 22, 1000–1006. [Google Scholar] [CrossRef]
- La Via, L.; Dezio, V.; Santonocito, C.; Astuto, M.; Morelli, A.; Huang, S.; Vieillard-Baron, A.; Sanfilippo, F. Full and simplified assessment of left ventricular diastolic function in COVID-19 patients admitted to ICU: Feasibility, incidence, and association with mortality. Echocardiography 2022, 39, 1391–1400. [Google Scholar] [CrossRef]
- Huang, S.; Vieillard-Baron, A.; Evrard, B.; Prat, G.; Chew, M.S.; Balik, M.; Clau-Terré, F.; De Backer, D.; Mekontso Dessap, A.; Orde, S.; et al. Echocardiography phenotypes of right ventricular involvement in COVID-19 ARDS patients and ICU mortality: Post-hoc (exploratory) analysis of repeated data from the ECHO-COVID study. Intensive Care Med. 2023, 49, 946–956. [Google Scholar] [CrossRef]
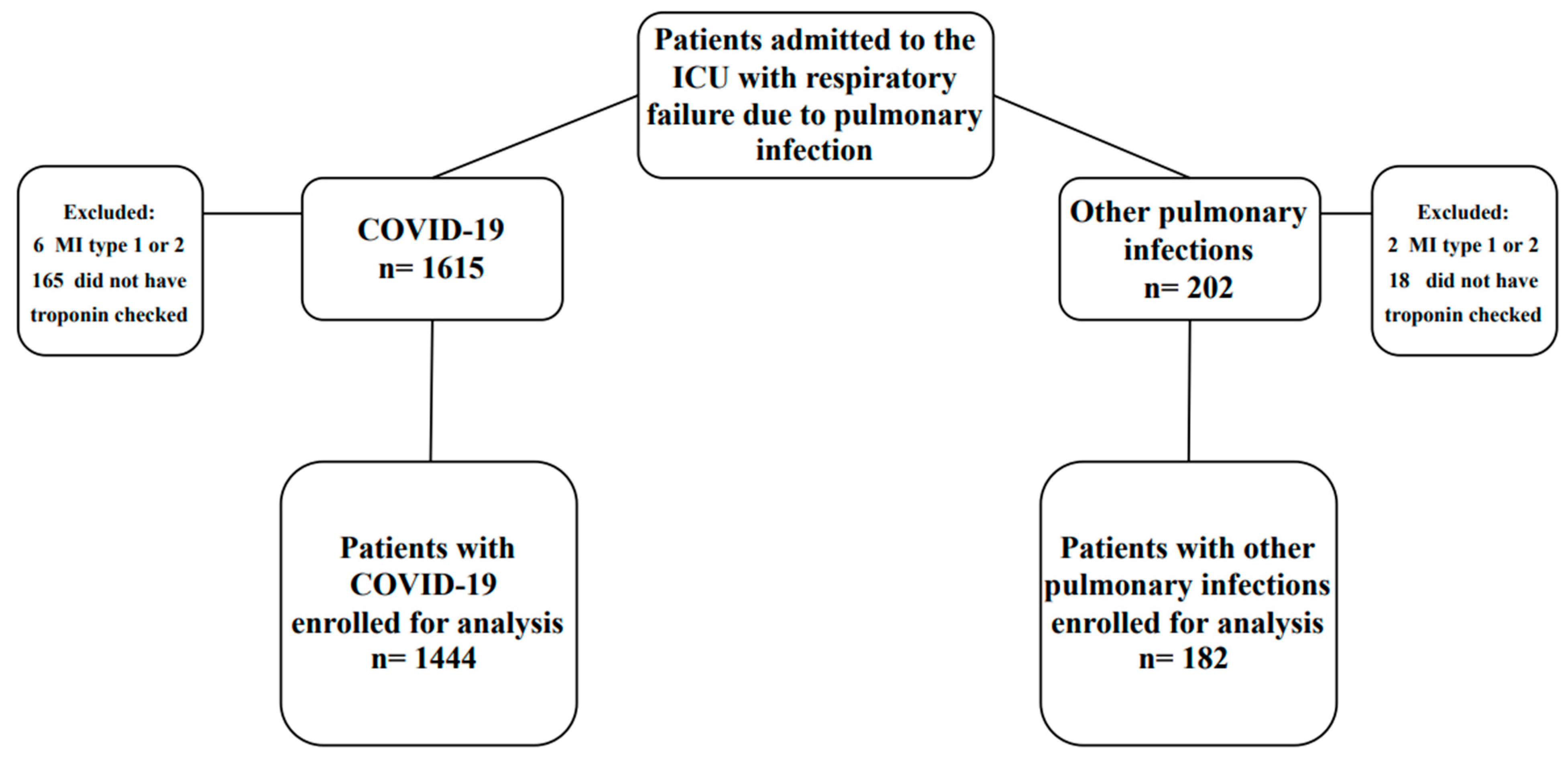


| Features | COVID-19 n = 1444 | Other Pulmonary Infections n = 182 | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 58 (46; 68) | 62 (44; 73) | 0.103 |
| Gender | 0.027 | ||
| Male | 802 (55.5) | 85 (46.7) | |
| Female | 642 (44.5) | 97 (53.3) | |
| Body mass index (kg/m²) | 30.4 (26.5; 35.7) | 26.5 (22.3; 31.3) | <0.001 |
| Comorbidities | |||
| Hypertension | 821 (56.9) | 98 (53.8) | 0.475 |
| Diabetes mellitus | 494 (34.2) | 59 (32.4) | 0.678 |
| Renal replacement therapy | 32 (2.2) | 6 (3.3) | 0.429 |
| Cerebrovascular disease | 78 (5.4) | 25 (13.7) | <0.001 |
| Heart disease | 193 (13.4) | 49 (26.9) | <0.001 |
| Coronary artery disease | 126 (8.7) | 29 (15.9) | 0.003 |
| Heart failure | 147 (10.2) | 37 (20.3) | <0.001 |
| Valvulopathy | 80 (5.5) | 33 (18.1) | <0.001 |
| COPD | 75 (5.2) | 49 (26.9) | <0.001 |
| Smoking (present or past) | 314 (21.7) | 83 (45.6) | <0.001 |
| Malignancy | 87 (6) | 21 (11.5) | 0.010 |
| HIV | 30 (2.1) | 15 (8.2) | <0.001 |
| Laboratory findings at ICU admission | |||
| D-dimer (μg/mL) | 1.5 (0.8; 4.5) | 2.3 (1.1; 4.7) | 0.009 |
| White blood cell count (103/μL) | 9.9 (7.3; 13.7) | 12 (8.9; 15.7) | <0.001 |
| Lactate (mmol/L) | 1.5 (1.2; 2.1) | 1.8 (1.2; 3.2) | <0.001 |
| Prothrombin time (seconds) | 13.8 (13.1; 14.8) | 14.8 (13.9; 16.4) | <0.001 |
| Creatinine (mg/dL) | 0.9 (0.8; 1.6) | 1.2 (0.8; 2) | 0.015 |
| Fibrinogen (mg/L) | 652 (549; 751) | 538 (374; 658) | <0.001 |
| CRP (mg/L) | 162 (100; 241) | 109 (35; 213) | <0.001 |
| Clinical data at ICU admission | |||
| SOFA score | 4 (3; 6) | 5 (3; 8) | 0.035 |
| Ventilatory support | <0.001 | ||
| Non-invasive or HFNC | 352 (24.4) | 18 (9.9) | |
| Invasive mechanical ventilation | 503 (34.8) | 75 (41.2) | |
| Vasopressor | 334 (23.1) | 67 (36.8) | <0.001 |
| PaO2/FiO2 ratio | 122 (86; 194) | 203 (131; 292) | 0.000 |
| All Patients | COVID-19 n = 1444 | Other Pulmonary Infections n = 182 | p-Value | |
|---|---|---|---|---|
| Troponin | 13.2 (9.9; 62.8) | 11.6 (9.9; 53.7) | 35.5 (9.9; 218) | <0.001 |
| Myocardial injury | 627 (38.5) | 525 (36.4) | 102 (56) | <0.001 |
| Troponin positive <5× ULN | 314 (50) | 272 (51.8) | 42 (41.2) | |
| Troponin positive ≥5× ULN | 106 (16.9) | 86 (16.4) | 20 (19.6) | |
| Troponin positive ≥10× ULN | 207 (33.1) | 167 (31.8) | 40 (39.2) |
| COVID-19 n = 1444 | Other Pulmonary Infections n = 182 | p-Value | |
|---|---|---|---|
| Outcomes | |||
| Renal replacement therapy (new) | 339 (23.5) | 21 (11.5) | <0.001 |
| Pulmonary embolism | 298 (20.6) | 10 (5.5) | <0.001 |
| Non-survivor | 592 (41) | 48 (26.4) | <0.001 |
| Composite | 838 (58) | 66 (36.3) | <0.001 |
| Length of hospital stay | 19 (11; 32) | 14 (10; 22) | <0.001 |
| Length of ICU stay | 10 (6; 21) | 4 (1; 12) | <0.001 |
| Length of mechanical ventilation | 13 (7; 24) | 6 (4; 11) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivan, M.A.; Hirakata, V.N.; Arteche, M.A.T.; de Araujo, D.M.; Fuchs, S.C.; Fuchs, F.D. Comparison of Incidence and Prognosis of Myocardial Injury in Patients with COVID-19-Related Respiratory Failure and Other Pulmonary Infections: A Contemporary Cohort Study. J. Clin. Med. 2023, 12, 6403. https://doi.org/10.3390/jcm12196403
Vivan MA, Hirakata VN, Arteche MAT, de Araujo DM, Fuchs SC, Fuchs FD. Comparison of Incidence and Prognosis of Myocardial Injury in Patients with COVID-19-Related Respiratory Failure and Other Pulmonary Infections: A Contemporary Cohort Study. Journal of Clinical Medicine. 2023; 12(19):6403. https://doi.org/10.3390/jcm12196403
Chicago/Turabian StyleVivan, Manoela Astolfi, Vania Naomi Hirakata, Maria Antônia Torres Arteche, Débora Marques de Araujo, Sandra C. Fuchs, and Flávio D. Fuchs. 2023. "Comparison of Incidence and Prognosis of Myocardial Injury in Patients with COVID-19-Related Respiratory Failure and Other Pulmonary Infections: A Contemporary Cohort Study" Journal of Clinical Medicine 12, no. 19: 6403. https://doi.org/10.3390/jcm12196403
APA StyleVivan, M. A., Hirakata, V. N., Arteche, M. A. T., de Araujo, D. M., Fuchs, S. C., & Fuchs, F. D. (2023). Comparison of Incidence and Prognosis of Myocardial Injury in Patients with COVID-19-Related Respiratory Failure and Other Pulmonary Infections: A Contemporary Cohort Study. Journal of Clinical Medicine, 12(19), 6403. https://doi.org/10.3390/jcm12196403