Current Management of Diaphyseal Long Bone Defects—A Multidisciplinary and International Perspective
Abstract
:1. Introduction
- -
- traumatic substance loss due to open fracture or debridement
- -
- fracture-associated infection or osteomyelitis
- -
- nonunion
- -
- tumor
1.1. Classic Bone Transport
1.2. Hybrid Techniques of Segmental Bone Transport
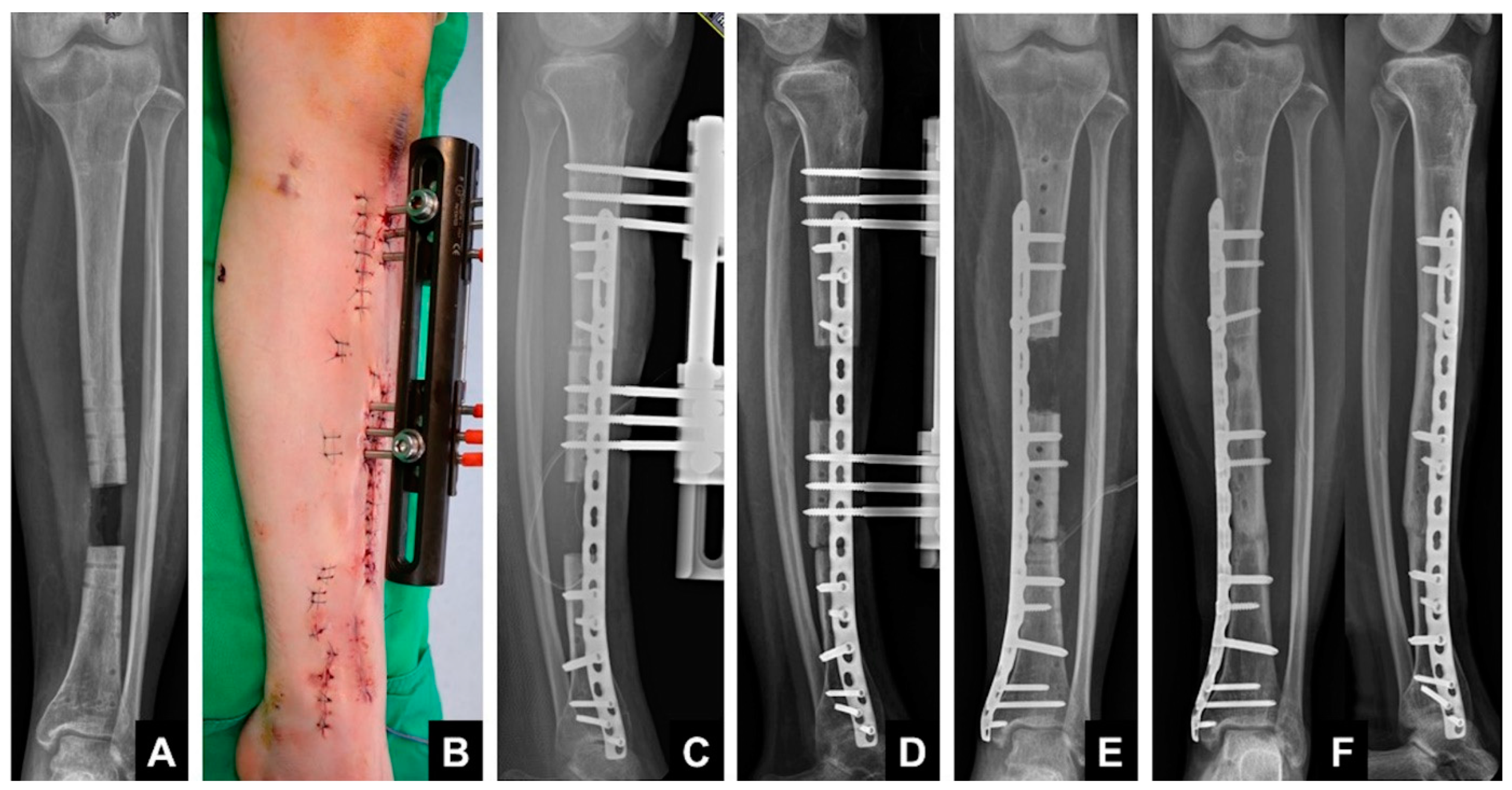
1.3. Induced Membrane Technique—Masquelet
1.4. Principle
1.5. Technique
2. Membrane Characteristics
- Spacer Material
- Bone graft materials
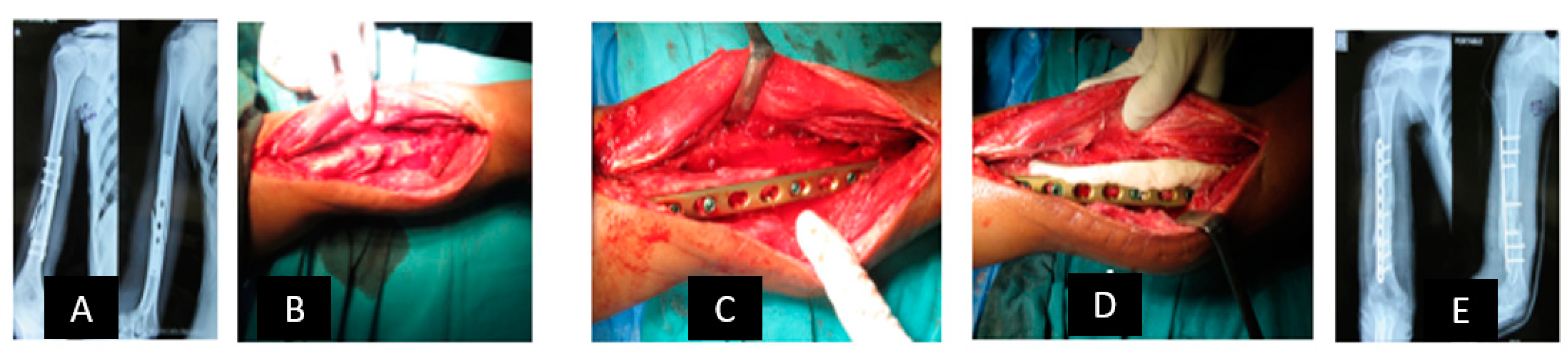

2.1. Reconstruction with Vascularized Bone Graft
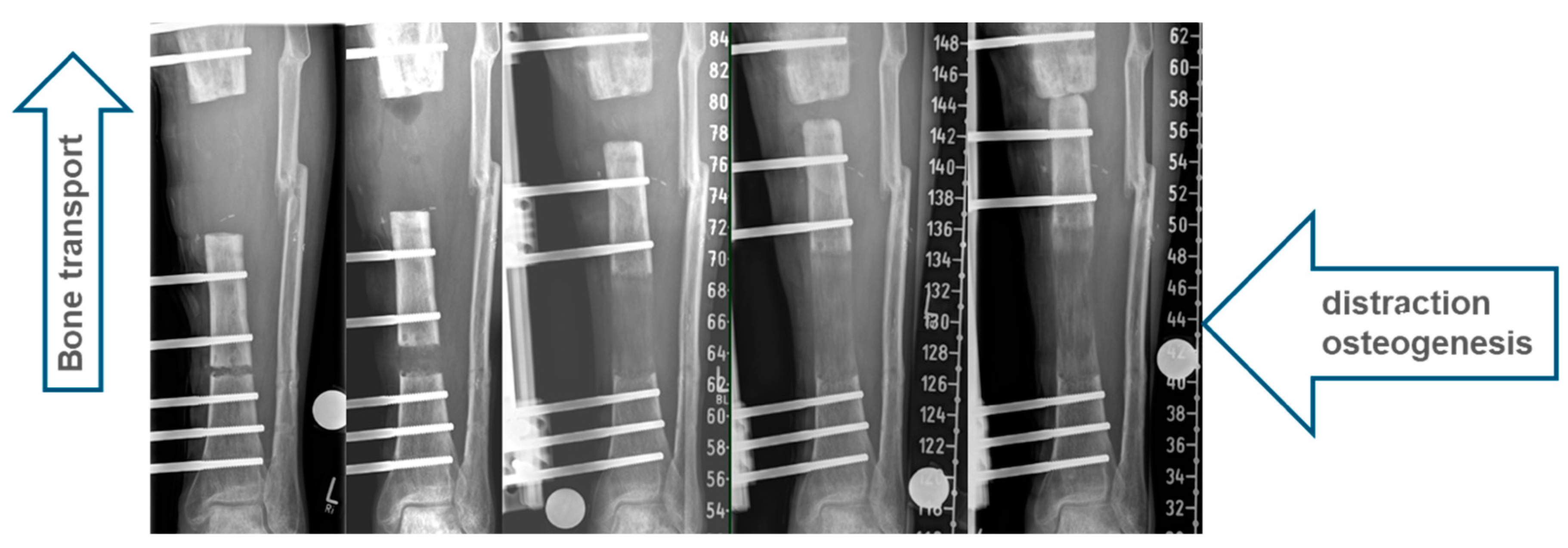
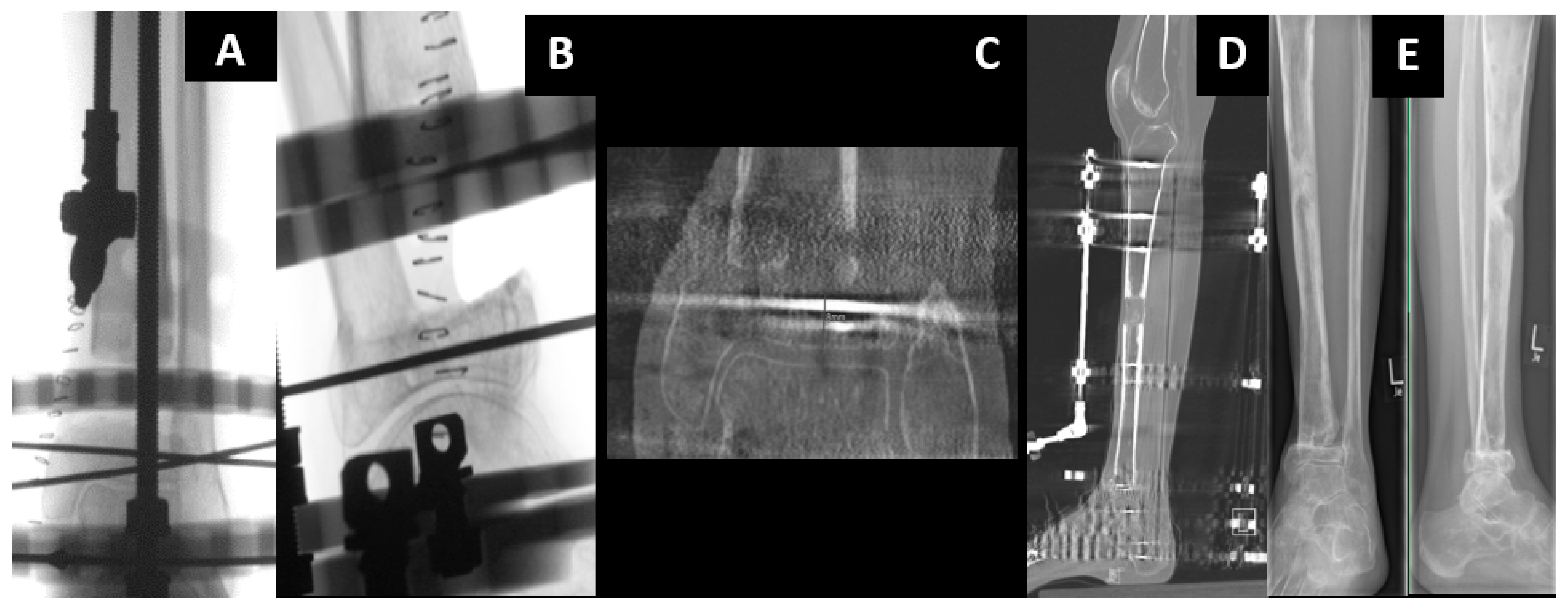
2.2. All-Internal Segmental Bone Transport with Motorized Nails
2.3. Bone Segment Transport Nails (STN)
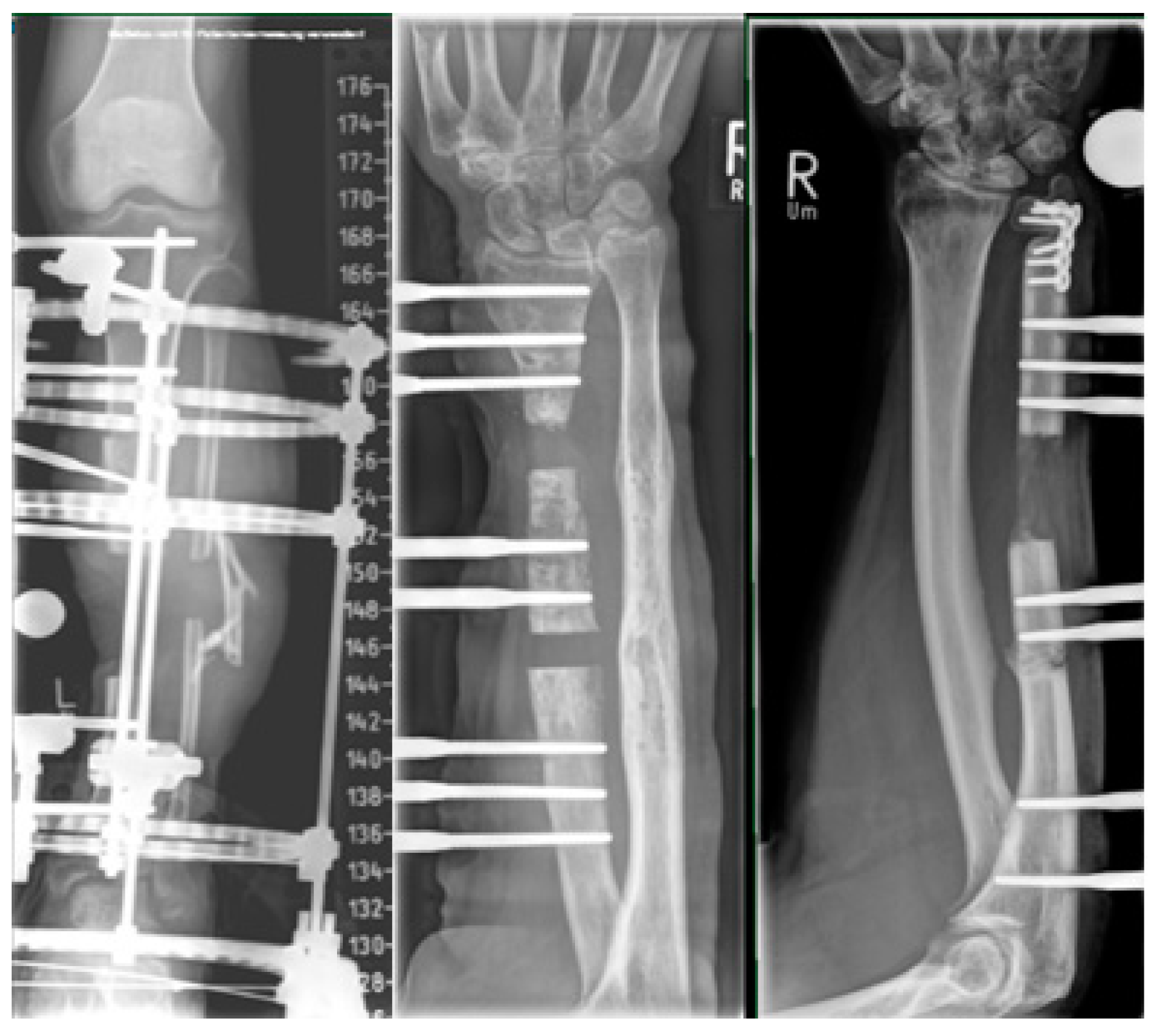
2.4. Plate-Assisted Bone Segment Transport (PABST)
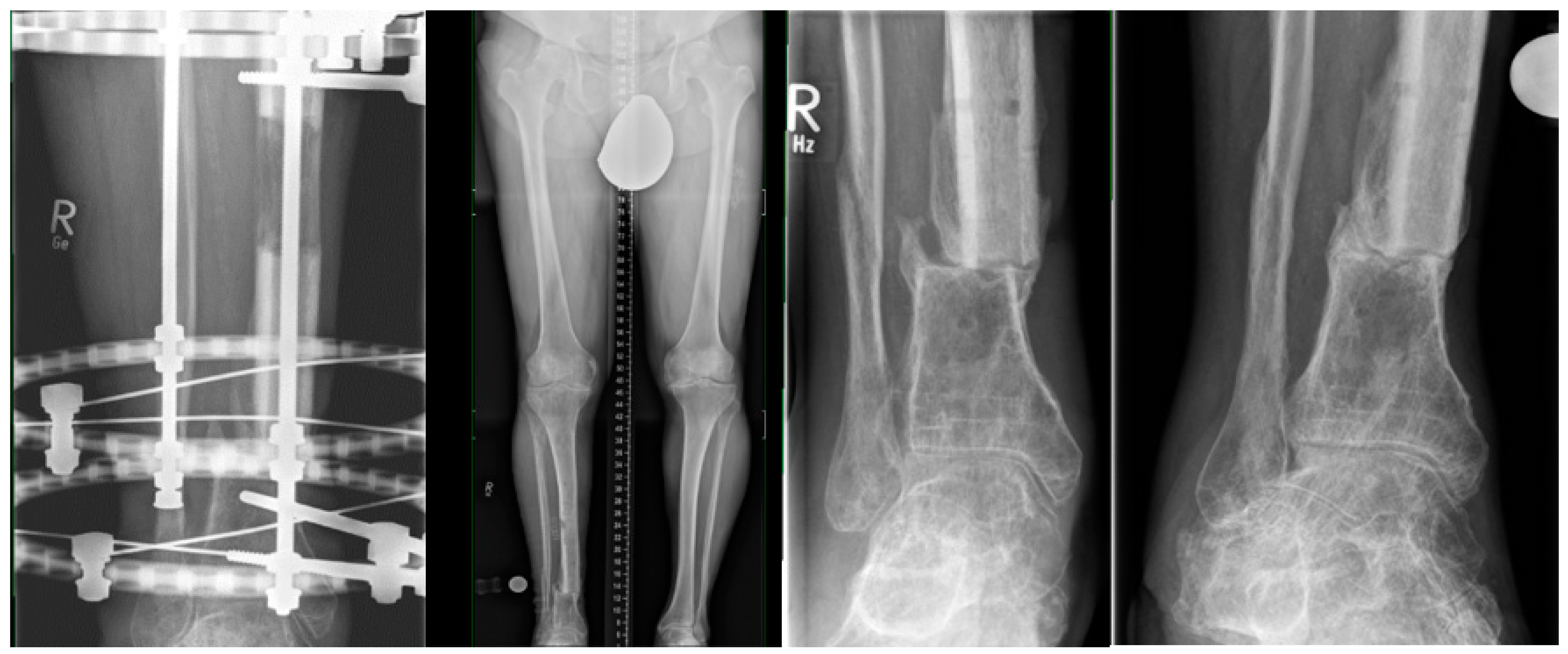
2.5. MagicTube
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Langenbeck, B. Über krankhaftes Längenwachstum der Röhrenknochen und seine Verwertung für die chirurgische Praxis. Berl. Klin. Wochenschr. 1869, 6, 265–270. [Google Scholar]
- Ollier, L. Traité Expérimental et Clinique de la Régénération des os et de la Production Artificielle du Tissu Osseux; Victor Masson Et Fils: Paris, France, 1867; Volume 2. [Google Scholar]
- Codivilla, A. On the Means of Lengthening in the Lower Limbs, the Muscles and the Tissues which are Shortened through Deformity. Am. J. Orthop. Surg. 1905, 2, 353. [Google Scholar] [CrossRef]
- Ombredanne, L. Allongement d’un fémur sur un membre trop court. Bull. Mem. Soc. Chir. 1913, 39, 1177. [Google Scholar]
- Putti, V.; Peltier, L.F. The operative lengthening of the femur. JAMA 1921, 77, 934–935. [Google Scholar] [CrossRef]
- Bier, A. Die Bedeutung des Blutergusses für die Heilung des Knochenbruches. Heilung von Pseudarthrosen und von verspäteter Callusbildung durch Bluteinspritzung Med Klinik. Urban&Schwarzenberg Berlin. Med. Klinik 1905, 6–7. [Google Scholar]
- Abbott, L.C.; Crego, C.H.; Adams, A.O. The operative lengthening of the lower extremity. Int. J. Orthod. Oral Surg. Radiogr. 1929, 15, 110–124. [Google Scholar] [CrossRef]
- Brandes, M. Über Störungen der Konsolidation nach orthopädischen Osteotomien langer Röhrenknochen. Verh. Deut. Orthop. Ges. 1928, 2, 775. [Google Scholar]
- Bosworth, D.M. Skeletal distraction of the tibia. Surg. Gynaecol. 1938, 66, 912–924. [Google Scholar]
- Wittmoser, R. Pressure osteosynthesis. Langenbeck’s Arch. Klin. Chir. Ver. Dtsch. Z. Chir. 1953, 276, 229–231. [Google Scholar]
- Bertrand, P. Technic of lengthening of the femur in extreme shortness. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1951, 37, 530–533. [Google Scholar]
- Wagner, H. Surgical leg prolongation. Chirurgei 1971, 42, 260–266. [Google Scholar]
- Ilizarov, G.A.; Lediaev, V.I.; Shitin, V.P. The course of compact bone reparative regeneration in distraction osteosynthesis under different conditions of bone fragment fixation (experimental study). Eksp. Khir. Anesteziol. 1969, 14, 3–12. [Google Scholar] [PubMed]
- Ilizarov, G.A. Transosseous Osteosynthesis: Theoretical and Clinical Aspects of the Regeneration and Growth of Tissue; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Ilizarov, G.A. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin. Orthop. Relat. Res. 1989, 238, 249–281. [Google Scholar] [CrossRef]
- Ilizarov, G.A. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin. Orthop. Relat. Res. 1989, 239, 263–285. [Google Scholar] [CrossRef]
- Raschke, M.J.; Mann, J.W.; Oedekoven, G.; Claudi, B.F. Segmental transport after unreamed intramedullary nailing. Preliminary report of a “Monorail” system. Clin. Orthop. Relat. Res. 1992, 282, 233–240. [Google Scholar]
- Kocaoglu, M.; Eralp, L.; Rashid, H.U.; Sen, C.; Bilsel, K. Reconstruction of segmental bone defects due to chronic osteomyelitis with use of an external fixator and an intramedullary nail. J. Bone Jt. Surg. 2006, 88, 2137–2145. [Google Scholar]
- Oh, C.W.; Song, H.R.; Roh, J.Y.; Oh, J.K.; Min, W.K.; Kyung, H.S.; Kim, J.W.; Kim, P.T.; Ihn, J.C. Bone transport over an intramedullary nail for reconstruction of long bone defects in tibia. Arch. Orthop. Trauma Surg. 2008, 128, 801–808. [Google Scholar] [CrossRef]
- Bas, A.; Daldal, F.; Eralp, L.; Kocaoglu, M.; Uludag, S.; Sari, S. Treatment of Tibial and Femoral Bone Defects With Bone Transport Over an Intramedullary Nail. J. Orthop. Trauma 2020, 34, e353–e359. [Google Scholar] [CrossRef]
- Kim, S.J.; Mandar, A.; Song, S.H.; Song, H.R. Pitfalls of lengthening over an intramedullary nail in tibia: A consecutive case series. Arch. Orthop. Trauma Surg. 2012, 132, 185–191. [Google Scholar] [CrossRef]
- Oh, C.W.; Apivatthakakul, T.; Oh, J.K.; Kim, J.W.; Lee, H.J.; Kyung, H.S.; Baek, S.G.; Jung, G.H. Bone transport with an external fixator and a locking plate for segmental tibial defects. Bone Jt. J. 2013, 95, 1667–1672. [Google Scholar] [CrossRef]
- Park, K.H.; Oh, C.W.; Kim, J.W.; Oh, J.K.; Yoon, Y.C.; Seo, I.; Ha, S.S.; Chung, S.H. Matched Comparison of Bone Transport Using External Fixator Over a Nail Versus External Fixator Over a Plate for Segmental Tibial Bone Defects. J. Orthop. Trauma 2021, 35, e397–e404. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthet. 2000, 45, 346–353. [Google Scholar] [PubMed]
- Cuthbert, R.J.; Churchman, S.M.; Tan, H.B.; McGonagle, D.; Jones, E.; Giannoudis, P.V. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone 2013, 57, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Karger, C.; Kishi, T.; Schneider, L.; Fitoussi, F.; Masquelet, A.C. Treatment of posttraumatic bone defects by the induced membrane technique. Orthop. Traumatol. Surg. Res. 2012, 98, 97–102. [Google Scholar] [CrossRef]
- Taylor, B.C.; Hancock, J.; Zitzke, R.; Castaneda, J. Treatment of Bone Loss with the Induced Membrane Technique: Techniques and Outcomes. J. Orthop. Trauma 2015, 29, 554–557. [Google Scholar] [CrossRef]
- Zappaterra, T.; Ghislandi, X.; Adam, A.; Huard, S.; Gindraux, F.; Gallinet, D.; Lepage, D.; Garbuio, P.; Tropet, Y.; Obert, L. Induced membrane technique for the reconstruction of bone defects in upper limb. A prospective single center study of nine cases. Chir. Main 2011, 30, 255–263. [Google Scholar] [CrossRef]
- Mauffrey, C.; Barlow, B.T.; Smith, W. Management of segmental bone defects. J. Am. Acad. Orthop. Surg. 2015, 23, 143–153. [Google Scholar]
- Giannoudis, P.V.; Faour, O.; Goff, T.; Kanakaris, N.; Dimitriou, R. Masquelet technique for the treatment of bone defects: Tips-tricks and future directions. Injury 2011, 42, 591–598. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am. 2010, 41, 27–37. [Google Scholar] [CrossRef]
- Donegan, D.J.; Scolaro, J.; Matuszewski, P.E.; Mehta, S. Staged bone grafting following placement of an antibiotic spacer block for the management of segmental long bone defects. Orthopedics 2011, 34, e730–e735. [Google Scholar] [CrossRef]
- Taylor, B.C.; French, B.G.; Fowler, T.T.; Russell, J.; Poka, A. Induced membrane technique for reconstruction to manage bone loss. J. Am. Acad. Orthop. Surg. 2012, 20, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J.; Pittman, J.L. Biological rationale for the intramedullary canal as a source of autograft material. Orthop. Clin. N. Am. 2010, 41, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Schmidmaier, G.; Herrmann, S.; Green, J.; Weber, T.; Scharfenberger, A.; Haas, N.P.; Wildemann, B. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone 2006, 39, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.M.; Liu, F.; Pilapil, C.; Betz, O.B.; Vrahas, M.S.; Harris, M.B.; Evans, C.H. Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J. Orthop. Res. 2009, 27, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Pelissier, P.; Masquelet, A.C.; Bareille, R.; Pelissier, S.M.; Amedee, J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J. Orthop. Res. 2004, 22, 73–79. [Google Scholar] [CrossRef]
- Sagi, H.C.; Young, M.L.; Gerstenfeld, L.; Einhorn, T.A.; Tornetta, P. Qualitative and quantitative differences between bone graft obtained from the medullary canal (with a Reamer/Irrigator/Aspirator) and the iliac crest of the same patient. J. Bone Jt. Surg. Am. 2012, 94, 2128–2135. [Google Scholar] [CrossRef]
- Aho, O.M.; Lehenkari, P.; Ristiniemi, J.; Lehtonen, S.; Risteli, J.; Leskela, H.V. The mechanism of action of induced membranes in bone repair. J. Bone Jt. Surg. Am. 2013, 95, 597–604. [Google Scholar] [CrossRef]
- Nau, C.; Seebach, C.; Trumm, A.; Schaible, A.; Kontradowitz, K.; Meier, S.; Buechner, H.; Marzi, I.; Henrich, D. Alteration of Masquelet’s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury 2016, 47, 325–334. [Google Scholar] [CrossRef]
- Liu, H.; Hu, G.; Shang, P.; Shen, Y.; Nie, P.; Peng, L.; Xu, H. Histological characteristics of induced membranes in subcutaneous, intramuscular sites and bone defect. Orthop. Traumatol. Surg. Res. 2013, 99, 959–964. [Google Scholar] [CrossRef]
- Viateau, V.; Guillemin, G.; Calando, Y.; Logeart, D.; Oudina, K.; Sedel, L.; Hannouche, D.; Bousson, V.; Petite, H. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: An ovine model. Vet. Surg. 2006, 35, 445–452. [Google Scholar] [CrossRef]
- Wang, X.; Luo, F.; Huang, K.; Xie, Z. Induced membrane technique for the treatment of bone defects due to post-traumatic osteomyelitis. Bone Jt. Res. 2016, 5, 101–105. [Google Scholar] [CrossRef]
- Apard, T.; Bigorre, N.; Cronier, P.; Duteille, F.; Bizot, P.; Massin, P. Two-stage reconstruction of post-traumatic segmental tibia bone loss with nailing. Orthop. Traumatol. Surg. Res. 2010, 96, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A.O.; Gehrmann, S.; Glombitza, M.; Kaufmann, R.A.; Bostelmann, R.; Flohe, S.; Windolf, J. Reconstruction of septic diaphyseal bone defects with the induced membrane technique. Injury 2015, 46 (Suppl. 4), S121–S124. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Ueng, S.W.; Wang, C.J.; Lee, S.S.; Chen, C.Y.; Shin, C.H. Antibiotic-impregnated autogenic cancellous bone grafting is an effective and safe method for the management of small infected tibial defects: A comparison study. J. Trauma 2000, 48, 246–255. [Google Scholar] [CrossRef]
- Tarchala, M.; Engel, V.; Barralet, J.; Harvey, E.J. A pilot study: Alternative biomaterials in critical sized bone defect treatment. Injury 2018, 49, 523–531. [Google Scholar] [CrossRef]
- Cavadas, P.C.; Landin, L.; Ibanez, J.; Nthumba, P. Reconstruction of major traumatic segmental bone defects of the tibia with vascularized bone transfers. Plast. Reconstr. Surg. 2010, 125, 215–223. [Google Scholar] [CrossRef]
- Yazar, S.; Lin, C.H.; Wei, F.C. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast. Reconstr. Surg. 2004, 114, 1457–1466. [Google Scholar] [CrossRef]
- Taylor, G.I.; Miller, G.D.; Ham, F.J. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast. Reconstr. Surg. 1975, 55, 533–544. [Google Scholar] [CrossRef]
- Attia, S.; Diefenbach, J.; Schmermund, D.; Böttger, S.; Pons-Kühnemann, J.; Scheibelhut, C.; Heiss, C.; Howaldt, H.P. Donor-Site Morbidity after Fibula Transplantation in Head and Neck Tumor Patients: A Split-Leg Retrospective Study with Focus on Leg Stability and Quality of Life. Cancers 2020, 12, 2217. [Google Scholar] [CrossRef]
- Taylor, G.I. The current status of free vascularized bone grafts. Clin. Plast. Surg. 1983, 10, 185–209. [Google Scholar] [CrossRef]
- Donati, D.; Di Liddo, M.; Zavatta, M.; Manfrini, M.; Bacci, G.; Picci, P.; Capanna, R.; Mercuri, M. Massive bone allograft reconstruction in high-grade osteosarcoma. Clin. Orthop. Relat. Res. 2000, 377, 186–194. [Google Scholar] [CrossRef]
- Banic, A.; Hertel, R. Double vascularized fibulas for reconstruction of large tibial defects. J. Reconstr. Microsurg. 1993, 9, 421–428. [Google Scholar] [CrossRef]
- Taylor, G.I.; Watson, N. One-stage repair of compound leg defects with free, revascularized flaps of groin skin and iliac bone. Plast. Reconstr. Surg. 1978, 61, 494–506. [Google Scholar] [CrossRef]
- Sekiguchi, J.; Kobayashi, S.; Ohmori, K. Use of the osteocutaneous free scapular flap on the lower extremities. Plast. Reconstr. Surg. 1993, 91, 103–112. [Google Scholar] [CrossRef]
- Cole, J.D.; Justin, D.; Kasparis, T.; DeVlught, D.; Knobloch, C. The intramedullary skeletal kinetic distractor (ISKD): First clinical results of a new intramedullary nail for lengthening of the femur and tibia. Injury 2001, 32 (Suppl. 4), SD129–SD139. [Google Scholar] [CrossRef]
- Laubscher, M.; Mitchell, C.; Timms, A.; Goodier, D.; Calder, P. Outcomes following femoral lengthening: An initial comparison of the Precice intramedullary lengthening nail and the LRS external fixator monorail system. Bone Jt. J. 2016, 98, 1382–1388. [Google Scholar] [CrossRef]
- Liodakis, E.; Kenawey, M.; Krettek, C.; Ettinger, M.; Jagodzinski, M.; Hankemeier, S. Segmental transports for posttraumatic lower extremity bone defects: Are femoral bone transports safer than tibial? Arch. Orthop. Trauma Surg. 2011, 131, 229–234. [Google Scholar] [CrossRef]
- Baumgart, R.; Betz, A.; Schweiberer, L. A fully implantable motorized intramedullary nail for limb lengthening and bone transport. Clin. Orthop. Relat. Res. 1997, 343, 135–143. [Google Scholar] [CrossRef]
- Gardner, M.P.; Beason, A.M. Plate-Assisted Bone Segment Transport Versus Precice Bone Transport Nail. J. Orthop. Trauma 2021, 35 (Suppl. 4), S19–S24. [Google Scholar] [CrossRef]
- Kahler, O.U. Plate-assisted segmental bone transport with a lengthening nail and a plate: A new technique for treatment of tibial and femoral bone defects. Unfallchirurg 2018, 121, 874–883. [Google Scholar]
- Barinaga, G.; Beason, A.M.; Gardner, M.P. Novel Surgical Approach to Segmental Bone Transport Using a Magnetic Intramedullary Limb Lengthening System. J. Am. Acad. Orthop. Surg. 2018, 26, e477–e482. [Google Scholar] [CrossRef]
- Krettek, C. MagicTube: New possibilities for completely internal bone segmental transport and optional lengthening: New additional module for motorized lengthening nails for treatment of large bone defects. Unfallchirurg 2018, 121, 884–892. [Google Scholar] [CrossRef]
- Krettek, C.; El Naga, A. All Internal Segmental Bone Transport and Optional Lengthening with a Newly Developed Universal Cylinder-Kombi-Tube Module for Motorized Nails-Description of a Surgical Technique. J. Orthop. Trauma 2017, 31 (Suppl. 5), S39–S41. [Google Scholar] [CrossRef] [PubMed]
- Kirane, Y.M.; Fragomen, A.T.; Rozbruch, S.R. Precision of the PRECICE internal bone lengthening nail. Clin. Orthop. Relat. Res. 2014, 472, 3869–3878. [Google Scholar] [CrossRef] [PubMed]
- Paley, D. PRECICE intramedullary limb lengthening system. Expert Rev. Med. Devices 2015, 12, 231–249. [Google Scholar] [CrossRef]
- Schiedel, F.M.; Vogt, B.; Tretow, H.L.; Schuhknecht, B.; Gosheger, G.; Horter, M.J.; Rödl, R. How precise is the PRECICE compared to the ISKD in intramedullary limb lengthening? Reliability and safety in 26 procedures. Acta Orthop. 2014, 85, 293–298. [Google Scholar] [CrossRef] [PubMed]


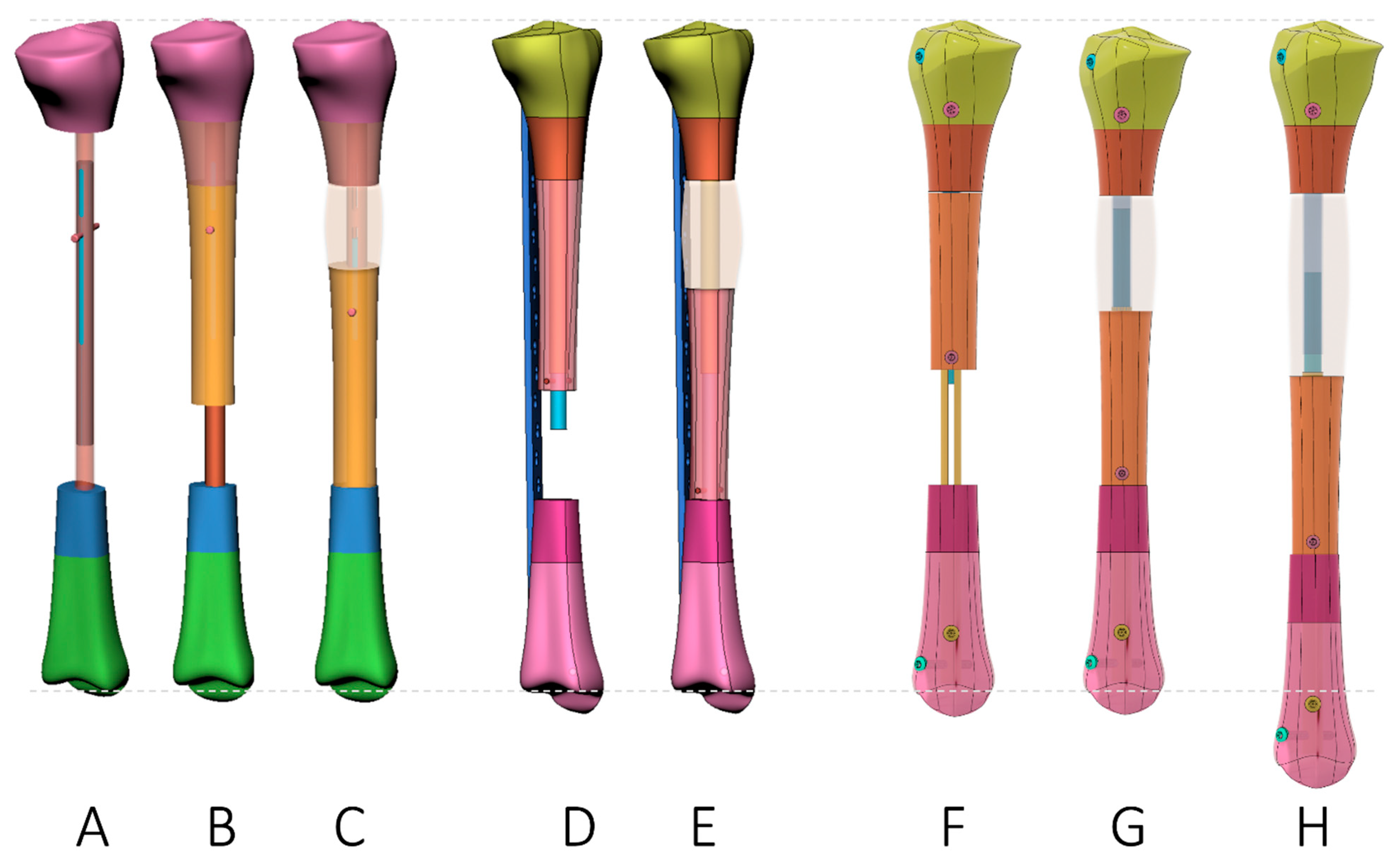
| Plate-Assisted Bone Segment Transport (PABST) [60,61] | Segment Transport Nails(STN) [62,63,64,65] | Nail-in-Nail System(MagicTube) [66,67] | |
|---|---|---|---|
| Concept |
|
|
|
| Advantage |
|
|
|
| Disadvantage |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosslenbroich, S.B.; Oh, C.-W.; Kern, T.; Mukhopadhaya, J.; Raschke, M.J.; Kneser, U.; Krettek, C. Current Management of Diaphyseal Long Bone Defects—A Multidisciplinary and International Perspective. J. Clin. Med. 2023, 12, 6283. https://doi.org/10.3390/jcm12196283
Rosslenbroich SB, Oh C-W, Kern T, Mukhopadhaya J, Raschke MJ, Kneser U, Krettek C. Current Management of Diaphyseal Long Bone Defects—A Multidisciplinary and International Perspective. Journal of Clinical Medicine. 2023; 12(19):6283. https://doi.org/10.3390/jcm12196283
Chicago/Turabian StyleRosslenbroich, Steffen Bernd, Chang-Wug Oh, Thomas Kern, John Mukhopadhaya, Michael Johannes Raschke, Ulrich Kneser, and Christian Krettek. 2023. "Current Management of Diaphyseal Long Bone Defects—A Multidisciplinary and International Perspective" Journal of Clinical Medicine 12, no. 19: 6283. https://doi.org/10.3390/jcm12196283
APA StyleRosslenbroich, S. B., Oh, C.-W., Kern, T., Mukhopadhaya, J., Raschke, M. J., Kneser, U., & Krettek, C. (2023). Current Management of Diaphyseal Long Bone Defects—A Multidisciplinary and International Perspective. Journal of Clinical Medicine, 12(19), 6283. https://doi.org/10.3390/jcm12196283









