Lifestyles, Left Atrial Structure and Function, and Cognitive Decline in Adults with Metabolic Syndrome
Abstract
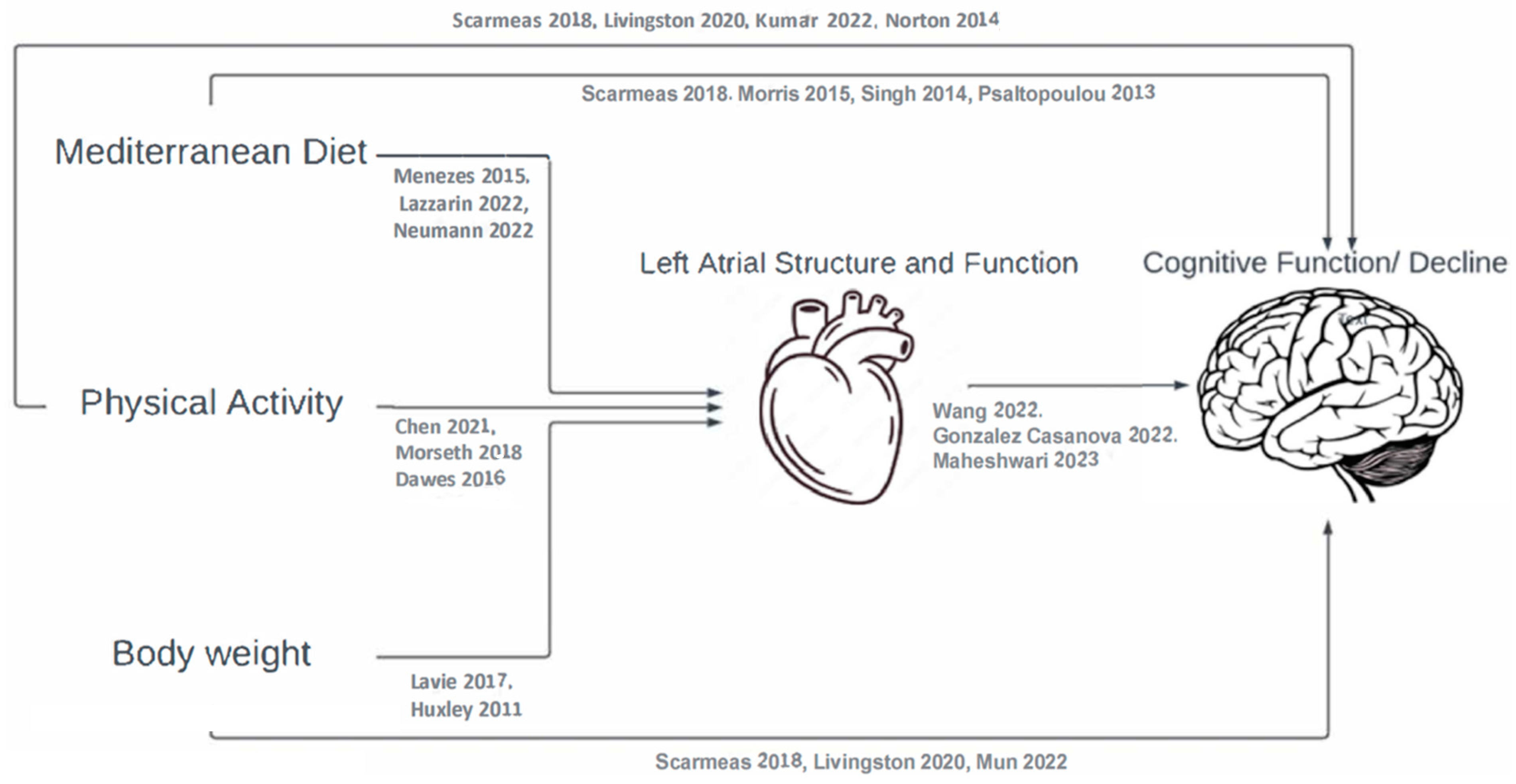
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Data Sources and Measurements
2.3. Quantitative Variables and Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N.J.A.o.n. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Mun, Y.S.; Park, H.K.; Kim, J.; Yeom, J.; Kim, G.H.; Chun, M.Y.; Lee, H.A.; Yoon, S.J.; Park, K.W.; Kim, E.-J.; et al. Association Between Body Mass Index and Cognitive Function in Mild Cognitive Impairment Regardless of APOE ε4 Status. Dement. Neurocognitive Disord. 2022, 21, 30–41. [Google Scholar] [CrossRef]
- Kumar, M.; Srivastava, S.; Muhammad, T. Relationship between physical activity and cognitive functioning among older Indian adults. Sci. Rep. 2022, 12, 2725. [Google Scholar] [CrossRef]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Menezes, A.R.; Lavie, C.J.; De Schutter, A.; Milani, R.V.; O’Keefe, J.; DiNicolantonio, J.J.; Morin, D.P.; Abi-Samra, F.M. Lifestyle Modification in the Prevention and Treatment of Atrial Fibrillation. Prog. Cardiovasc. Dis. 2015, 58, 117–125. [Google Scholar] [CrossRef]
- Lazzarin, T.; Garcia, L.R.; Martins, D.; Queiroz, D.A.R.; Tonon, C.R.; Balin, P.D.S.; Polegato, B.F.; Paiva, S.A.R.; Azevedo, P.S.; Minicucci, M.; et al. Role of Nutrients and Foods in Attenuation of Cardiac Remodeling through Oxidative Stress Pathways. Antioxidants 2022, 11, 2064. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.A.; Jagemann, B.; Makarova, N.; Börschel, C.S.; Aarabi, G.; Gutmann, F.; Schnabel, R.B.; Zyriax, B.-C. Mediterranean Diet and Atrial Fibrillation: Lessons Learned from the AFHRI Case–Control Study. Nutrients 2022, 14, 3615. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Voskoboinik, A.; Gerche, A.L.; Marwick, T.H.; McMullen, J.R. Prevention of Pathological Atrial Remodeling and Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 2846–2864. [Google Scholar] [CrossRef] [PubMed]
- Morseth, B.; Løchen, M.-L.; Ariansen, I.; Myrstad, M.; Thelle, D.S. The ambiguity of physical activity, exercise and atrial fibrillation. Eur. J. Prev. Cardiol. 2018, 25, 624–636. [Google Scholar] [CrossRef]
- Dawes, T.J.W.; Corden, B.; Cotter, S.; de Marvao, A.; Walsh, R.; Ware, J.S.; Cook, S.A.; O’Regan, D.P. Moderate Physical Activity in Healthy Adults Is Associated with Cardiac Remodeling. Circ. Cardiovasc. Imaging 2016, 9, e004712. [Google Scholar] [CrossRef]
- Lavie Carl, J.; Pandey, A.; Lau Dennis, H.; Alpert Martin, A.; Sanders, P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis. J. Am. Coll. Cardiol. 2017, 70, 2022–2035. [Google Scholar] [CrossRef]
- Huxley, R.R.; Lopez, F.L.; Folsom, A.R.; Agarwal, S.K.; Loehr, L.R.; Soliman, E.Z.; Maclehose, R.; Konety, S.; Alonso, A. Absolute and Attributable Risks of Atrial Fibrillation in Relation to Optimal and Borderline Risk Factors. Circulation 2011, 123, 1501–1508. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, M.J.; Inciardi, R.M.; Norby, F.L.; Johansen, M.C.; Parikh, R.; Van’t Hof, J.R.; Alonso, A.; Soliman, E.Z.; Mosley, T.H.; et al. Association of Echocardiographic Measures of Left Atrial Function and Size with Incident Dementia. JAMA 2022, 327, 1138–1148. [Google Scholar] [CrossRef]
- Gonzalez Casanova, I.; Alonso-Gomez, A.M.; Romaguera, D.; Toledo, E.; Fortuny, E.; Lopez, L.; Ramallal, R.; Salas-Salvado, J.; Tojal-Sierra, L.; Castaner, O.; et al. Association of Left Atrial Structure and Function with Cognitive Function in Adults with Metabolic Syndrome. Am. J. Cardiol. 2022, 183, 122–128. [Google Scholar] [CrossRef]
- Maheshwari, A.; Norby, F.L.; Inciardi, R.M.; Wang, W.; Zhang, M.J.; Soliman, E.Z.; Alonso, A.; Johansen, M.C.; Gottesman, R.F.; Solomon, S.D.; et al. Left Atrial Mechanical Dysfunction and the Risk for Ischemic Stroke in People Without Prevalent Atrial Fibrillation or Stroke: A Prospective Cohort Study. Ann. Intern. Med. 2023, 176, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Buil-Cosiales, P.; Corella, D.; Bulló, M.; Fitó, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; López-Miranda, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2019, 48, 387o–388o. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program with Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2018, 42, 777–788. [Google Scholar] [PubMed]
- Abed, H.S.; Wittert, G.A.; Leong, D.P.; Shirazi, M.G.; Bahrami, B.; Middeldorp, M.E.; Lorimer, M.F.; Lau, D.H.; Antic, N.A.; Brooks, A.G.; et al. Effect of Weight Reduction and Cardiometabolic Risk Factor Management on Symptom Burden and Severity in Patients with Atrial Fibrillation: A Randomized Clinical Trial. JAMA 2013, 310, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventos, R.M.; et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef]
- Fitzmaurice, G.M.; Laird, N.M.; Ware, J.H. Applied Longitudinal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Llinàs-Reglà, J.; Vilalta-Franch, J.; López-Pousa, S.; Calvó-Perxas, L.; Torrents Rodas, D.; Garre-Olmo, J. The Trail Making Test. Assessment 2017, 24, 183–196. [Google Scholar] [CrossRef]
- López, L.; Rossello, X.; Romaguera, D.; Alonso-Gómez, Á.M.; Toledo, E.; Fortuny, E.; Noris, M.; Mas-Lladó, C.; Fiol, M.; Ramallal, R.; et al. The Palma Echo Platform: Rationale and Design of an Echocardiography Core Lab. Front. Cardiovasc. Med. 2022, 9, 909347. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Machino-Ohtsuka, T.; Seo, Y.; Tada, H.; Ishizu, T.; Machino, T.; Yamasaki, H.; Igarashi, M.; Xu, D.; Sekiguchi, Y.; Aonuma, K. Left atrial stiffness relates to left ventricular diastolic dysfunction and recurrence after pulmonary vein isolation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011, 22, 999–1006. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Molina, L.; Sarmiento, M.; Peñafiel, J.; Donaire, D.; Garcia-Aymerich, J.; Gomez, M.; Ble, M.; Ruiz, S.; Frances, A.; Schröder, H.; et al. Validation of the Regicor Short Physical Activity Questionnaire for the Adult Population. PLoS ONE 2017, 12, e0168148. [Google Scholar] [CrossRef] [PubMed]
- Valeri, L.; Vanderweele, T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychol. Methods 2013, 18, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Féart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, B.; Trifan, G.; Isasi, C.R.; Lipton, R.B.; Sotres-Alvarez, D.; Cai, J.; Tarraf, W.; Stickel, A.; Mattei, J.; Talavera, G.A.; et al. Association of Mediterranean Diet with Cognitive Decline Among Diverse Hispanic or Latino Adults from the Hispanic Community Health Study/Study of Latinos. JAMA Netw. Open. 2022, 5, e2221982. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef]
| % or Mean (SD) | Total (476) |
|---|---|
| Site | |
| Mallorca | 27.1 |
| Navarra | 20.8 |
| Vitoria | 52.1 |
| Age (years) | 65.2 (4.9) |
| Married | 77.9 |
| People living in the household | 1.3 (1.0) |
| Schooling (years) | 12.0 (5.2) |
| Employed | 17.7 |
| Health status | |
| Body Mass Index (kg/m2) | 32.5 (3.3) |
| Diabetes (self-reported) | 22.7 |
| Hypertension (self-reported) | 83.6 |
| Depression (self-reported) | 17.9 |
| Arrythmia (self-reported) | 5.5 |
| Health Behaviors | |
| Current Smoker | 9.7 |
| Former Smoker | 51.3 |
| Mediterranean Diet Score (17-item screener) | 7.5 (2.9) |
| Moderate to vigorous physical activity (MET-min/day) | 269 (318) |
| General Echocardiographic Measures of Left Atrial Substrate | |
| Volume Index (mL/m2) | 23.3 (7.5) |
| Peak Systolic Longitudinal Strain (%) | 27.3 (6.8) |
| Conduit Strain (%) | −11.9 (4.4) |
| Contractile Strain (%) | −15.4 (4.9) |
| Stiffness Index (U) | 0.4 (0.2) |
| Trail Making Test A (seconds) | |
| Baseline | 48.1 (21.2) |
| 2-year difference | 1.9 (20.9) |
| Mediterranean Score | MVPA MET—Min/day | Body Mass Index | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect Estimate (95% CI) | Direct | Indirect | Total | Direct | Indirect | Total | Direct | Indirect | Total |
| Volume Index (mL/m2) | 0.01 (−0.10, 0.13) | −0.01 (−0.03, 0.00) | 0.00 (−0.11, 0.11) | −0.05 (−0.16, 0.05) | −0.02 (−0.03, 0.00) | −0.07 (−0.17, 0.03) | 0.01 (−0.08, 0.09) | 0.00 (−0.01, 0.01) | 0.00 (−0.09, 0.09) |
| Peak Systolic Longitudinal Strain (%) | −0.02 (−0.13, 0.08) | 0.01 (−0.01, 0.02) | −0.01 (−0.12, 0.09) | −0.05 (−0.15, 0.05) | 0.00 (−0.01, 0.01) | −0.05 (−0.15, 0.05) | 0.00 (−0.08, 0.09) | −0.01 (−0.02, 0.00) | 0.01 (−0.09, 0.08) |
| Conduit Strain (%) | 0.01 (−0.12, 0.09) | 0.01 (−0.01, 0.01) | −0.01 (−0.12, 0.10) | −0.05 (−0.15, 0.05) | 0.00 (−0.01, 0.01) | −0.05 (−0.15, 0.05) | 0.00 (−0.08, 0.08) | 0.00 (−0.01, 0.00) | 0.00 (−0.09, 0.08) |
| Contractile Strain (%) | −0.01 (−0.12, 0.10) | 0.00 (−0.01, 0.01) | −0.01 (−0.11, 0.10) | −0.05 (−0.15, 0.05) | 0.00 (−0.01, 0.01) | −0.05 (−0.15, 0.05) | 0.00 (−0.08, 0.09) | −0.01 (−0.02, 0.00) | −0.01 (−0.09, 0.08) |
| Stiffness Index (U) | −0.02 (−0.12, 0.09) | 0.00 (−0.02, 0.02) | −0.02 (−0.12, 0.09) | −0.06 (−0.15, 0.04) | 0.01 (−0.01, 0.02) | −0.05 (0.15, 0.05) | 0.00 (−0.08, 0.09) | −0.02 (−0.03, 0.00) | −0.01 (−0.09, 0.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Casanova, I.; Alonso-Gómez, Á.M.; Romaguera, D.; Toledo, E.; Li, L.; Fortuny, E.; López, L.; Ramallal, R.; Salas-Salvadó, J.; Tojal-Sierra, L.; et al. Lifestyles, Left Atrial Structure and Function, and Cognitive Decline in Adults with Metabolic Syndrome. J. Clin. Med. 2023, 12, 6066. https://doi.org/10.3390/jcm12186066
Gonzalez Casanova I, Alonso-Gómez ÁM, Romaguera D, Toledo E, Li L, Fortuny E, López L, Ramallal R, Salas-Salvadó J, Tojal-Sierra L, et al. Lifestyles, Left Atrial Structure and Function, and Cognitive Decline in Adults with Metabolic Syndrome. Journal of Clinical Medicine. 2023; 12(18):6066. https://doi.org/10.3390/jcm12186066
Chicago/Turabian StyleGonzalez Casanova, Ines, Ángel M. Alonso-Gómez, Dora Romaguera, Estefanía Toledo, Linzi Li, Elena Fortuny, Luis López, Raúl Ramallal, Jordi Salas-Salvadó, Lucas Tojal-Sierra, and et al. 2023. "Lifestyles, Left Atrial Structure and Function, and Cognitive Decline in Adults with Metabolic Syndrome" Journal of Clinical Medicine 12, no. 18: 6066. https://doi.org/10.3390/jcm12186066
APA StyleGonzalez Casanova, I., Alonso-Gómez, Á. M., Romaguera, D., Toledo, E., Li, L., Fortuny, E., López, L., Ramallal, R., Salas-Salvadó, J., Tojal-Sierra, L., Castañer, O., & Alonso, A. (2023). Lifestyles, Left Atrial Structure and Function, and Cognitive Decline in Adults with Metabolic Syndrome. Journal of Clinical Medicine, 12(18), 6066. https://doi.org/10.3390/jcm12186066








