1. Introduction
Functional digestive diseases are widespread and represent an important public health issue. Some of the most common associated symptoms include irritable bowel syndrome and functional dyspestia [
1,
2,
3], the occurrences of which are often concurrent [
4,
5,
6,
7,
8]. Moreover, the uncertainty regarding their pathogenesis has impeded effective treatment options [
1,
2,
3]. Although several drugs have been proposed, their effectiveness is not very high, which prompts the search for new drugs for these purposes [
1,
2,
3].
Functional digestive diseases are often accompanied by low levels of inflammation in the intestinal wall with its infiltration by lymphocytes, eosinophils, mast cells, and other cells [
9,
10,
11,
12,
13]. This inflammation is considered a response to the increased permeability of the intestinal barrier, which is a common feature of these diseases [
14,
15,
16,
17,
18,
19], and can disrupt the mechanisms of gastrointestinal sensitivity and motility, contributing to the development of functional digestive diseases [
9,
10,
11,
12,
13]. Increased permeability can result in enterocytic disorders, manifested, in part, by the increased formation of fatty acid-binding proteins (FABPs) [
20,
21,
22]. Moreover, the formation of intestinal mucus glycoproteins, including mucin-2 (MUC-2), can increase as a compensatory reaction under these conditions [
23,
24].
The composition and function of the gut microbiota also contribute to the pathogenesis of functional digestive diseases. For instance, the short-chain fatty acids (SCFAs) produced by certain microorganisms are used by enterocytes as energy sources and regulatory molecules [
25,
26,
27]. Probiotics, which affect the composition and function of the gut microbiota, are currently used in the treatment of irritable bowel syndrome and other digestive system disorders [
28,
29]. The use of drugs that strengthen the intestinal barrier seems very promising in these diseases because they may decrease intestinal minimal inflammation, and this may contribute to the reduction in hypersensitivity and motor disorders of the digestive tract. One such drug is rebamipide [
30,
31,
32].
Although rebamipide is effective in the treatment of numerous digestive diseases [
33], its efficacy in treating overlap with the diarrheal variant of irritable bowel syndrome and functional dyspepsia (D-IBSoFD) has not been investigated. Therefore, the primary aim of this study was to evaluate the effect of rebamipide on symptom severity, intestinal barrier status, and intestinal microbiota composition and function in patients with D-IBSoFD. In particular, this randomized, controlled, single-blind trial assessed how rebamipide impacts intestinal permeability by detecting changes in the abundance of serum zonulin, a primary marker of increased intestinal permeability [
34]. Moreover, its effect on inflammation within the intestinal wall was also assessed via the enumeration of eosinophils and intraepithelial lymphocytes (IEL). Damage to enterocytes was also assessed based on changes in the abundance of FABPs, and the extent of compensatory MUC-2 hyperproduction within the mucosal tissues was determined. Finally, we evaluated the effect of rebamipide on the gut microbiota composition and metabolic function. The observed effects were compared with trimebutine—a standard drug used to treat this disease [
35,
36]. This drug has excellent safety profile and, acting on the intestinal opiate system, can normalize the motor activity and visceral sensitivity of the gastrointestinal tract [
37]. An additional comparison group treated with combinatorial trimebutine and rebamipide was included to evaluate the advantages and disadvantages of this complex regimen compared to either drug alone.
2. Materials and Methods
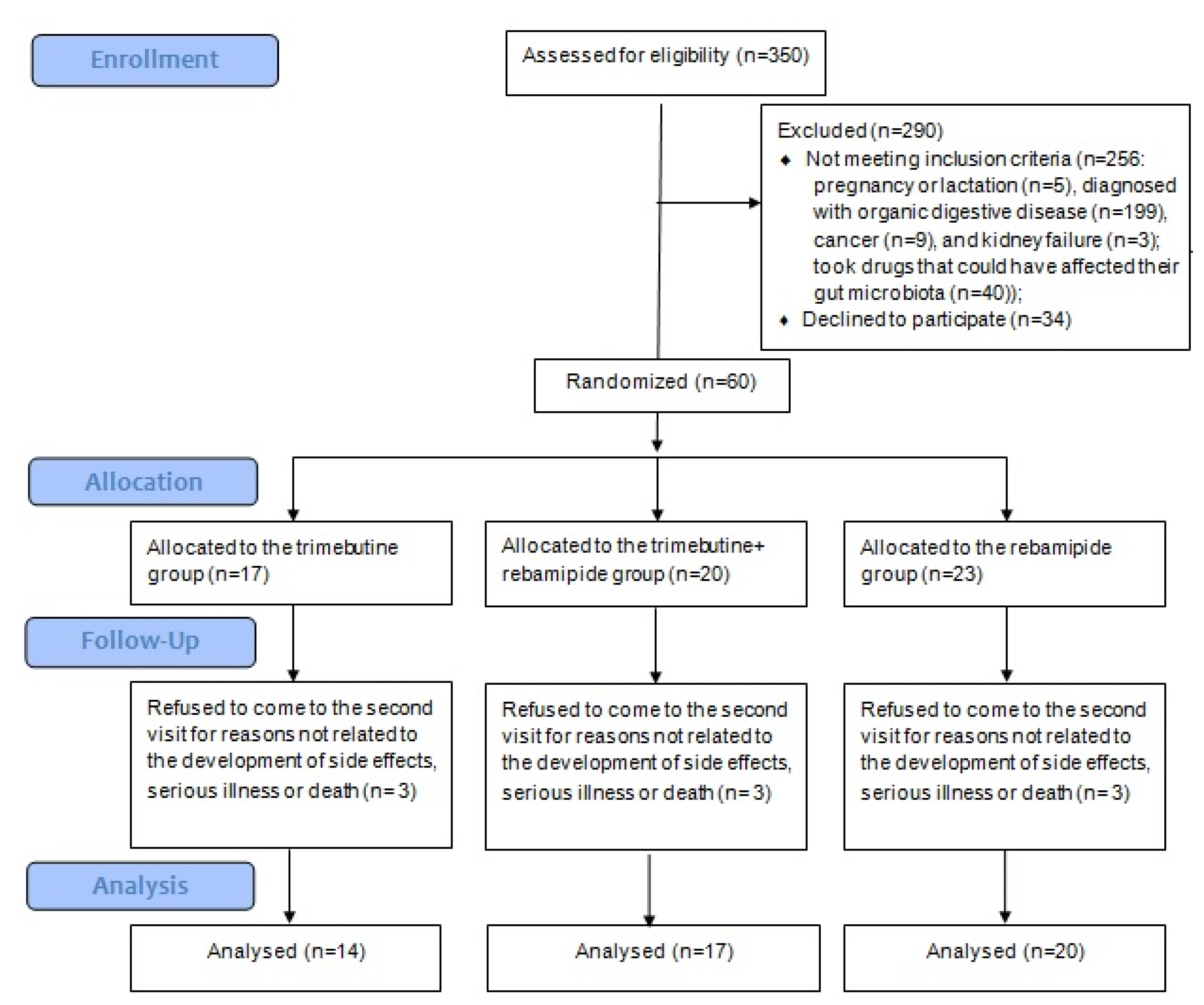
This randomized, controlled, single-blind trial was conducted in accordance with the Declaration of Helsinki and approved by the Local Ethics Committee of Sechenov University (No. 06-21 dated 7 April 2021). All participants signed an informed consent form to participate in this study. There were no data to calculate the sample size. Clinical trial registration: NCT05379036.
2.1. Patients
All patients admitted to the chronic bowel disease department of Sechenov University with diarrhea, abdominal pain, or abdominal discomfort were screened for inclusion in the study. The inclusion criteria included diagnosis of D-IBSoFD in accordance with international and national guidelines [
1,
38,
39] and age 18–59 years. The exclusion criteria included (i) diagnosis of an organic digestive system disease (including consequences of abdominal surgery,
Helicobacter pylori infection, peptic ulcer, inflammatory bowel disease, intestinal infections, and celiac disease), (ii) pregnancy or breastfeeding, (iii) use of drugs affecting the intestinal microbiota (e.g., probiotics, prebiotics, antibiotics, and prokinetics) within 6 weeks of beginning the study, and (iv) refusal to participate in the study.
Patients, who prematurely discontinued the studied drugs, took additional drugs that could affect the gut microbiota composition or digestive system function during the follow-up period, and those who refused to visit the clinic at the end of the study to perform the required examinations, were also excluded.
2.2. Interventions
All included patients were randomized by random number method into three groups. The main group (REB group) received 100 mg of rebamipide (RebagitTM) three times a day and placebo three times a day for 2 months. The comparison group (TRI group) received 200 mg trimebutine three times a day and placebo three times a day for 2 months. An additional comparison group (T + R group) received 100 mg rebamipide (RebagitTM) three times a day and 200 mg trimebutine three times a day for 2 months. The indicated doses of these drugs are standard when they are used for the treatment of digestive diseases. There was no placebo-only group, as the ethics committee deemed it unethical to leave patients with untreated pain.
2.3. Outcomes
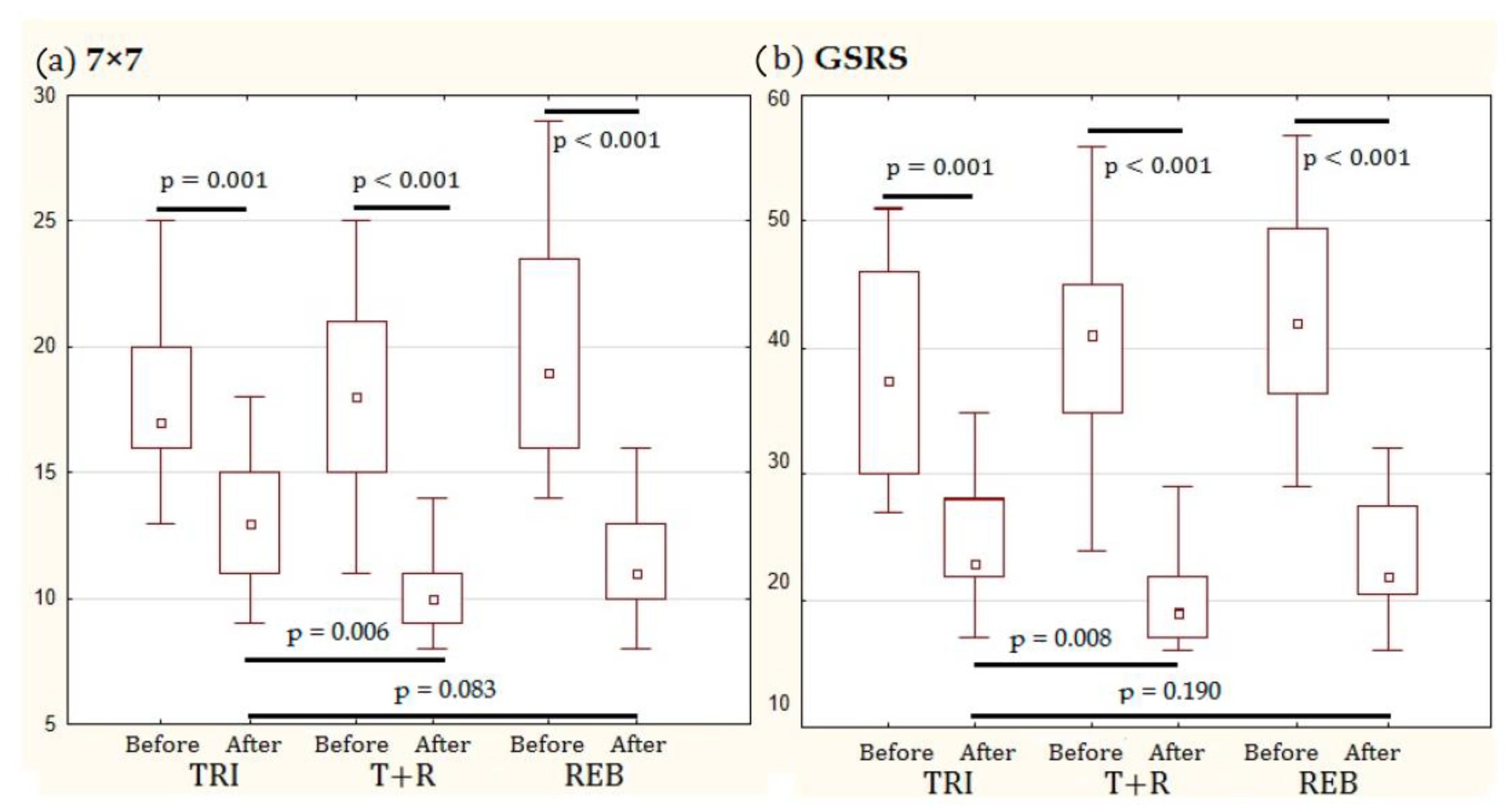
The primary outcomes were (i) changes in general health, determined using the SF-36 questionnaire [
40]; (ii) changes in the severity of major digestive symptoms, assessed using the 7 × 7 [
41] and Gastrointestinal Symptom Rating Scale (GSRS) [
42] questionnaires; and (iii) changes in the severity of inflammation in the duodenum and sigmoid colon, assessed by the level of lymphocytic and eosinophilic infiltration in the intestinal mucosa. The secondary outcomes included (i) changes in the amount of MUC-2 in the duodenal and sigmoid mucus, (ii) changes in the abundance of FABPs in the epithelium of the duodenum and sigmoid colon, (iii) changes in the serum zonulin level, (iv) changes in gut microbiome taxa, and (v) changes in the fecal SCFA levels.
2.4. Study Protocol
At the beginning of the study (the first visit), all patients filled out the SF-36, 7 × 7, and GSRS questionnaires. The next morning, blood was collected to determine the serum zonulin level (ELISA; Immundiagnostik AG; Bensheim, Germany); feces were collected and frozen to determine the gut microbiome composition using 16S rRNA gene sequencing; and the spectrum SCFAs were determined using standard chromatographic methods. Additionally, gastroduodenoscopy with biopsy of the postbulbar region of the duodenum was performed. The next day, colonoscopy with biopsy of the sigmoid colon was performed. The biopsy samples of the duodenum and sigmoid colon mucosa were evaluated to semi-quantitatively determine the IEL (Group 1: 0–5 IEL per 100 enterocytes, Group 2: 6–10 IEL per 100 enterocytes, Group 3: 11–15 IEL per 100 enterocytes, Group 4: 16–25 IEL per 100 enterocytes, and Group 5: >25 IEL per 100 enterocytes) and eosinophil (average number of five fields at a magnification of 400×) content (
Figure 1). FABP and MUC-2 levels were also analyzed in the duodenal and sigmoid biopsy samples, as described below. Subsequently, patients began taking their assigned drugs for 2 months. On completion of the 2 month period, patients were invited to the clinic for repeated examinations (the second visit). Patient compliance was assessed during their interview at the second visit. The differences in all indicators between the first and second visits were then calculated.
2.5. Gut Microbiome Analysis
A stool sample was obtained from each patient and placed in a sterile disposable container the morning after admission and immediately frozen at −80 °C [
43].
Just before the library preparation, the frozen samples were placed in a container with ice to thaw for 30 min. A 10 μg sample was taken with a spatula and placed in test tubes for homogenization. Sample tubes were incubated for 10 min at 65 °C, and then for 10 min more at 95 °C. Subsequently, the samples were homogenized using a MagNA Lyser automatic homogenizer (Roche) according to the manufacturer’s instructions, following which they were centrifuged at 14,000 rpm for 10 min. The resulting supernatant (400 µL) was used for further isolation of nucleic acids. Total DNA was isolated using reagents of the MagNA Pure Compact Nucleic Acid Isolation Kit I (Roche) in a MagNA Pure LC automated nucleic acid extraction system. The isolated DNA was stored at −20 °C. A NanoDrop 1000 (Thermo Fisher Scientific, Waltham, WA, USA) was used for DNA qualitative and quantitative evaluation. The 16S libraries were prepared according to the 16S Metagenomic Sequencing Library Preparation protocol (Illumina, San Diego, CA, USA) recommended by Illumina for the MiSeq sequencer. The following primers were used for the amplification of V3-V4 16S rDNA variable regions: TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-CCTACGGGNGGCWGCAG and GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-GACTACHVGGGTATCTAATCC [
44]. The part of the sequence before the dash refers to Illumina adapters. These primers are aimed at the amplification of bacterial (more than 90% taxon coverage) but not archaeal (less than 5%) rRNA genes. The average amplicon length was about 450 bp with minimal variation. Applied Biosystems 2720 Thermal Cycler amplifier (Thermo Fisher Scientific, USA) was used. The amplification program was as follows: 95 °C—3 min; 30 cycles: 95 °C—30 s, 55 °C—30 s, 72 °C—30 s; 72 °C—5 min; and 4 °C, finally. PCR products were purified using Agencourt AMPure XP beads (Beckman Coulter, Carlsbad, CA, USA) according to the manufacturer’s protocol.
Then, the second round of amplification was performed for double indexing of samples using a specific combination of index sequences from the Nextera XT Index kit (Illumina, USA). The amplification program was similar except that the number of cycles was 8. PCR products were also purified using Agencourt AMPure XP beads. The concentration of the resulting 16S libraries was determined using the Qubit® 2.0 fluorimeter (Invitrogen, San Diego, CA, USA) and QuantiT™ dsDNA High-Sensitivity Assay Kit.
The purified amplicons were mixed equimolarly according to the obtained concentrations. The quality of the prepared libraries was performed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) using an Agilent DNA 1000 Kit Bioanalyzer (Agilent Technologies, USA).
Sequencing was performed with the MiSeq sequencer (Illumina) in the paired-end mode (2 × 250 bp) using the MiSeq Reagent Kit v3. An average of 152 thousand reads per sample was obtained (from 34 to 380 thousand reads).
After sequencing, forward and reverse Illumina reads were pre-trimmed with Trimmomatic 0.38 and merged with MeFiT 1.0 tool [
45] into a single amplicon sequence (because of the small length of the overlapping regions with high quality). Then, the merged reads were processed with DADA2 1.22 package (Bioconductor project) [
46]. Taxonomic annotation of inferred RSVs was performed using naive RDP classifier algorithm (built-in default DADA2 annotation engine) based on the Silva 138.1 database [
47]. Taxon assignment confidence threshold was set to 80%. The sufficiency of sequencing depth (i.e., the read count) was ensured with rarefaction curves analysis (at the RSV, genera, and family levels). For most of the samples, there was a plateau when the actual number of reads in a sample was reached. Intergroup comparisons were performed using the ALDEx2 1.26 package [
48].
2.6. FABP Analysis
To assess the FABP level in enterocytes, biopsied mucosal fragments were placed in 200 mcL of lysis solution comprising 9 M urea, 5% mercaptoethanol, 2% triton X-100, and 2% ampholine with pH 3.5–10. The specimen was ground in the homogenizer and centrifuging at 800× g for 5 min. The supernatant fraction containing the solubilized protein extract was fractionated by two-dimensional electrophoresis. The first fractionation stage included separation of biopsy specimen proteins according to their isoelectric points. During the second stage, a polyacrylamide gel column was placed in Laemmli’s buffer after isoelectric focusing to displace Triton X-100 from its bond with proteins and replace it with sodium dodecyl sulphate, thus ensuring separation of protein oligomers into subunits in a 5–20% polyacrylamide gel gradient. To quantitatively evaluate the protein contents, two-dimensional electrophoregrams were constructed via scanning with an Epson Expression 1680 scanner and processed using the ImageMaster 2DPlatinum ver.7 software package (GE Healthcare, Opfikon, Switzerland). To calculate the quantitative ratio of FABP), computer-aided densimetry was performed on the biopsied mucosal specimens. A minimum of three evenly mapped electropherograms were used for protein quantification. The optical density scatter was less than ±1.5%. The percentage of isoforms was normalized to the percentage of the β-hemoglobin fraction.
2.7. MUC-2 Analysis
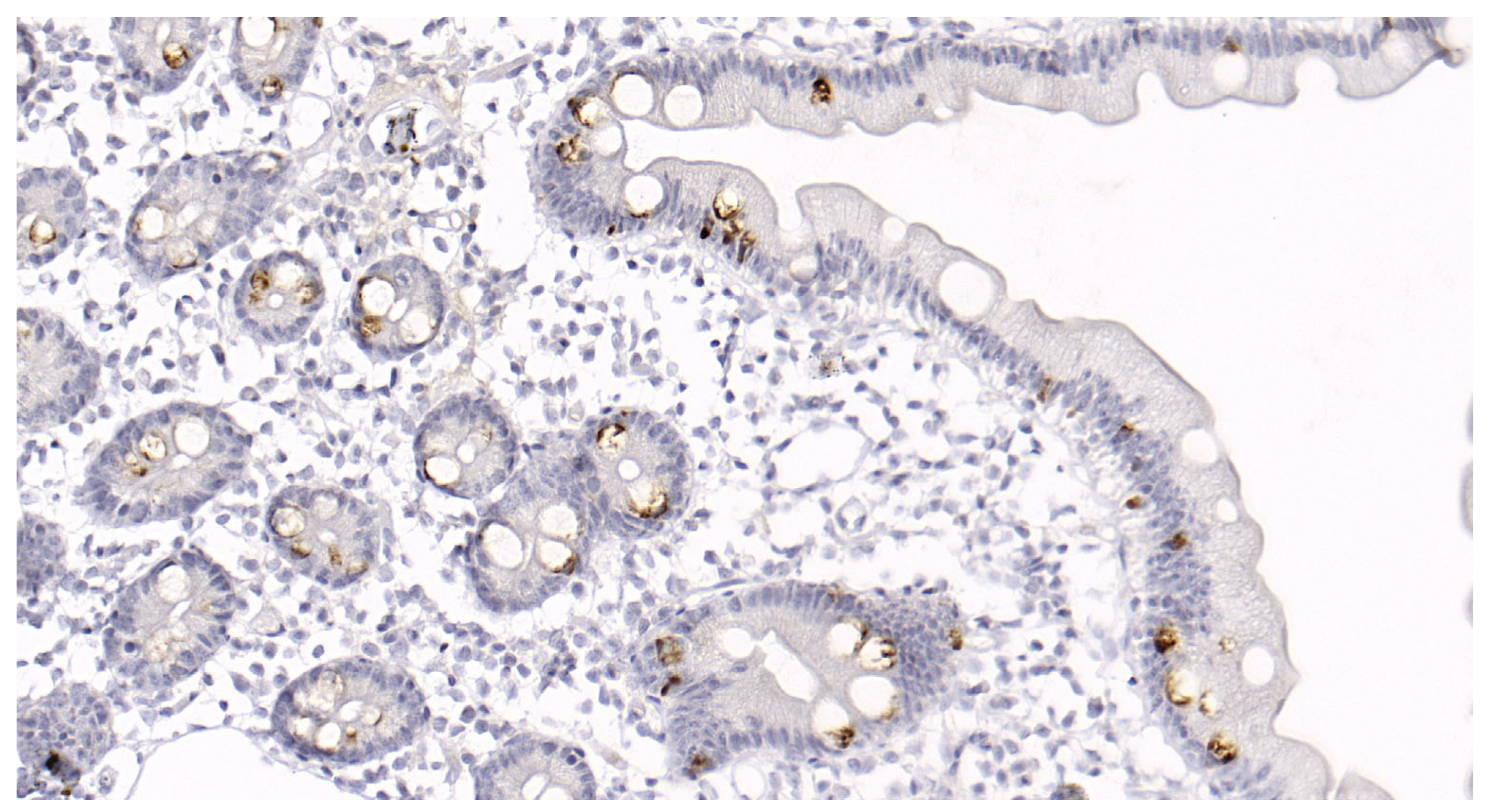
MUC-2 glycoprotein content was examined in the biopsied mucosal specimens fixed in formalin and paraffinized. The slices were deparaffinized in xylol and rehydrated using an automatic immunostaining device (BenchMark XT, Ventana Medical Systems Inc., Tucson, AZ, USA). Preliminary treatment was performed using CC1 (a prediluted solution for cell conditioning) for 60 min. The tissue sections were incubated with mouse polyclonal antibodies against human MUC-2 (Cell Marque, Rocklin, CA, USA; MRQ-18) at 37 °C for 20 min. A DAB Ventana
® I-view detection kit was then used according to the manufacturer’s instructions. The staining percentage was divided into five groups, 0: no staining; 1: <10% epithelial cell staining; 2: 10–25% epithelial cell staining; 3: 25–50% epithelial cell staining; 4: >50% epithelial cell staining (
Figure 2).
2.8. Statistical Analysis
Statistical data processing was performed using STATISTICA 10 (StatSoft Inc., Tulsa, OK, USA). Data are presented as median (interquartile range). Comparisons of several groups were performed using the Kruskal–Wallis method. Comparisons between two groups were performed using the Mann–Whitney U test. Categorical variables were compared using Fisher’s two-tailed exact test. Wilcoxon test was used to assess the significance of changes in the values of indicators between the first and second visits. Correlations were analyzed using the Spearman’s rank correlation coefficient. Differences were considered significant at p < 0.05.
4. Discussion
One of the primary outcomes of the current study was the change in general health, assessed using the SF-36 questionnaire. All three treatment regimens elicited beneficial effects on all tested parameters of general health. However, rebamipide was superior to trimebutine in improving mental health. Interestingly, the combined use of trimebutine and rebamipide had a worse effect on several general health parameters than rebamipide alone.
Regarding the effect of the tested regimens on digestive symptoms, four groups of symptoms were identified. The severity of the first group of symptoms (i.e., acid reflux, pain in the stomach area, fullness in the stomach after a meal, feeling of bloating, hunger pain, nausea, total abdominal bloating, rumbling, abdominal pain decreased after bowel movement, increased stool frequency, and urgent need to have a bowel movement) decreased equally following treatment with all regimens. Thus, similar efficacy was achieved by rebamipide and trimebutine monotherapies with no added benefit provided by the combination regimen. Moreover, a significant improvement in the severity of the second group of symptoms (i.e., heartburn, burping, passing gas or flatus, and sensation of incomplete bowel emptying) occurred only in patients administered rebamipide, with no significant difference observed between those who were administered trimebutine + rebamipide and trimebutine alone. The severity of the third group of symptoms (i.e., feeling of burning in the stomach area and early satiety) did not change significantly during the study for any of the tested regimens. However, this may be because these symptoms were mild on enrolment in the study before beginning treatment. Moreover, stool consistency improved significantly in all regimens; however, it improved the most with the rebamipide regimen, with no significant additional improvement observed in patients receiving the combination treatment. The overall improvement in digestive symptoms was the greatest in those who received rebamipide, with no significant difference between patients who additionally received trimebutine and those who did not receive it.
Assessment of changes in the severity of intestinal inflammation was among primary outcomes. Significant decreases in all tested parameters were observed in only those groups treated with rebamipide, with no significant benefit noted following the additional administration of trimebutine. In fact, the eosinophil count in the duodenal mucosa was the only marker of intestinal inflammation that was significantly decreased in the group administered trimebutine alone. However, this might be due to the natural course of the disease. To test this hypothesis, an additional study with a placebo group is required. Additionally, the association between a decrease in lymphocytic infiltration and reduced severity of various symptoms may indicate a pathogenetic relationship, which requires further investigation.
To the best of our knowledge, this is the first study to evaluate the dynamics of various FABPs in the enterocytes of patients with D-IBSoFD under various treatment regimens. Although significant differences were not observed in the abundance of FABP5 among the groups, a significant decrease was detected in the level of FABP2—a biomarker of intestinal epithelium damage [
49]—in the groups treated with rebamipide, regardless of whether they also received trimebutine or not. Moreover, while the level of FABP1 was significantly reduced in the sigmoid colon of all three groups, it was only reduced in the duodenum of the REB and TRI groups, but not in the duodenum of T + R group. The reason for this is unclear.
Although there was no significant difference in the effects of regimens with and without rebamipide for many markers of compromised intestinal barrier, in most cases there were trends that improvements were greater in regimens with rebamipide than in regimen without it. More studies with a larger number of included patients are needed that are likely to turn these trends into significant differences.
The level of serum zonulin was significantly decreased only in the REB group. However, why similar effects were not observed in the T + R group is unclear. An unknown interaction might have occurred between these drugs.
In the trimebutine group, the abundance of Lachnospiraceae and Lactobacillaceae, which are considered useful (as they form a lot of SCFA and do not have pathogenicity factors), decreased during the study. In the rebamipide group, there was an increase in the abundance of taxa of the Clostridia class that considered beneficial, and a decrease in the abundance of beneficial bacteria of the Lactobacillaceae family. In the group of patients taking both drugs, an increase in the content of beneficial bacteria Lachnoclostridium, Blautia and Dorea, as well as a decrease in the abundance of endotoxin-producing Proteobacteria, was found. In all groups, there were also other changes in the composition of the gut microbiota, the significance of which remains to be seen. None of the regimens significantly impacted total SCFA production by the gut microbiota. Therefore, the exact role of changes in the composition of the gut microbiota under the influence of these drugs in this disease should be established in the following studies.
Trimebutine, acting as a comparator drug, has shown its effectiveness in the treatment of irritable bowel syndrome (IBS) previously. A meta-analysis has shown that it improves the general condition in this disease [
50]. Another meta-analysis confirmed its positive effect on abdominal pain reduction in IBS [
51]. The third meta-analysis showed that trimebutine was as effective in treating functional dyspepsia as metoclopramide, domperidone and itopride and superior to placebo [
35]. Thus, our data on the high efficacy of trimebutine in the treatment of IBS and functional dyspepsia are consistent with the results obtained earlier.
To the best of our knowledge, this is the first study to comprehensively examine the effect of a drug capable of restoring the intestinal barrier in patients with D-IBSoFD in a randomized controlled trial. However, we did not include a placebo-only group, as the ethics committee deemed it unethical to leave patients with untreated pain, which is a limitation of the study. Hence, further placebo-controlled studies are required to verify our results.