Jaw Bone Invasion of Oral Squamous Cell Carcinoma Is Associated with Osteoclast Count and Expression of Its Regulating Proteins in Patients and Organoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Patients and Clinical Data
2.2. Immunohistochemistry
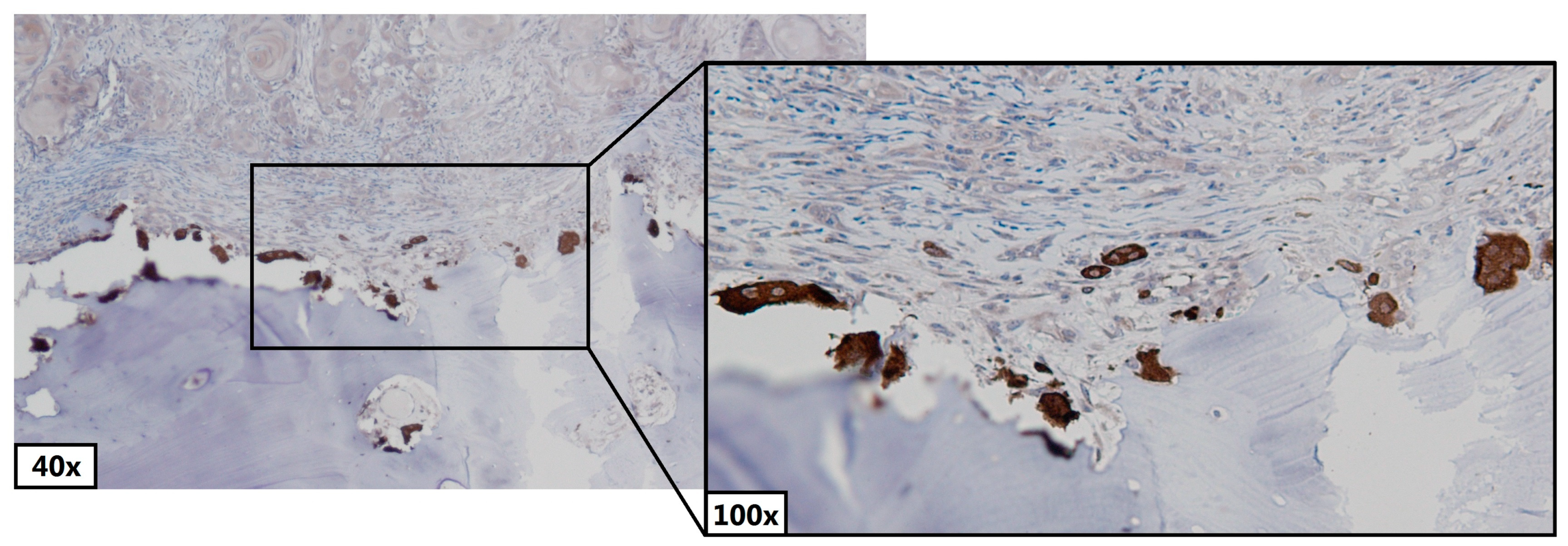
2.3. Counting Osteoclasts and Pre-Osteoclasts
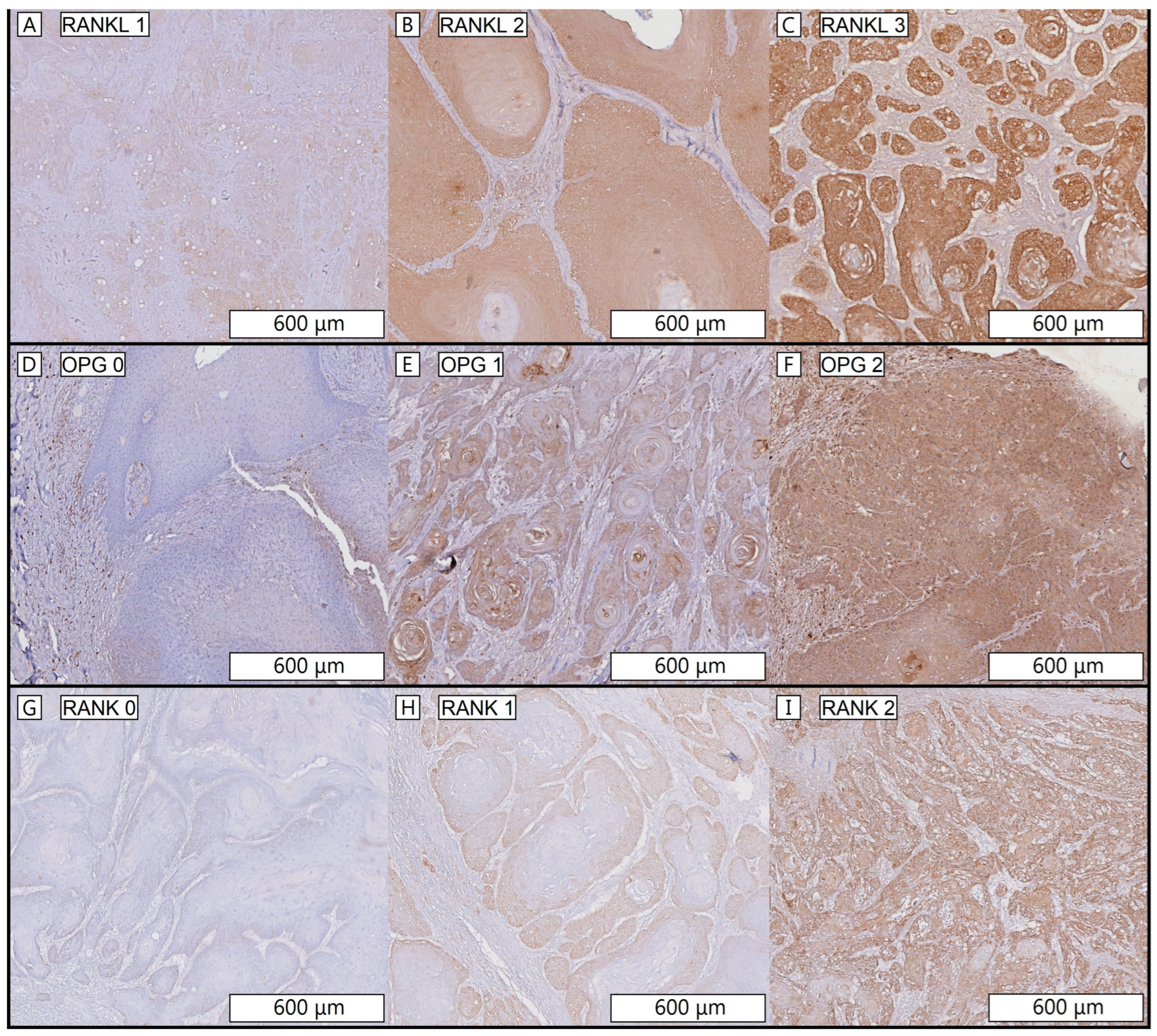
2.4. Scoring RANKL, RANK and OPG Intensity Staining
2.5. Organoid Culture and RNA Isolation
2.6. cDNA Synthesis and Quantitative PCR
2.7. Statistics
3. Results
3.1. Patients
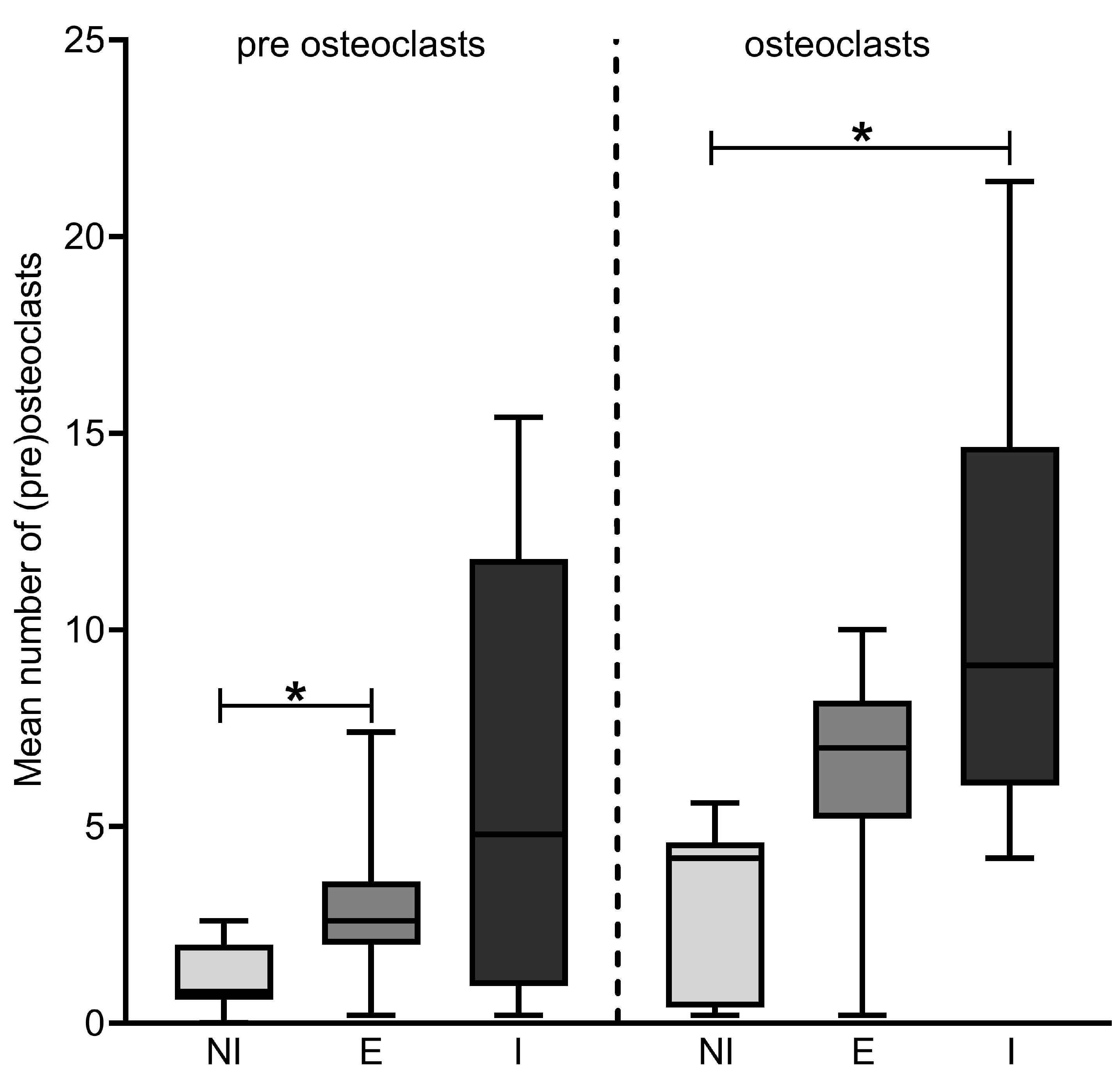
3.2. Number of Osteoclasts
3.3. RANKL, OPG and RANK Stainings
3.4. Expression in Tumor versus Normal Mucosa
3.5. Expression within the Tumor
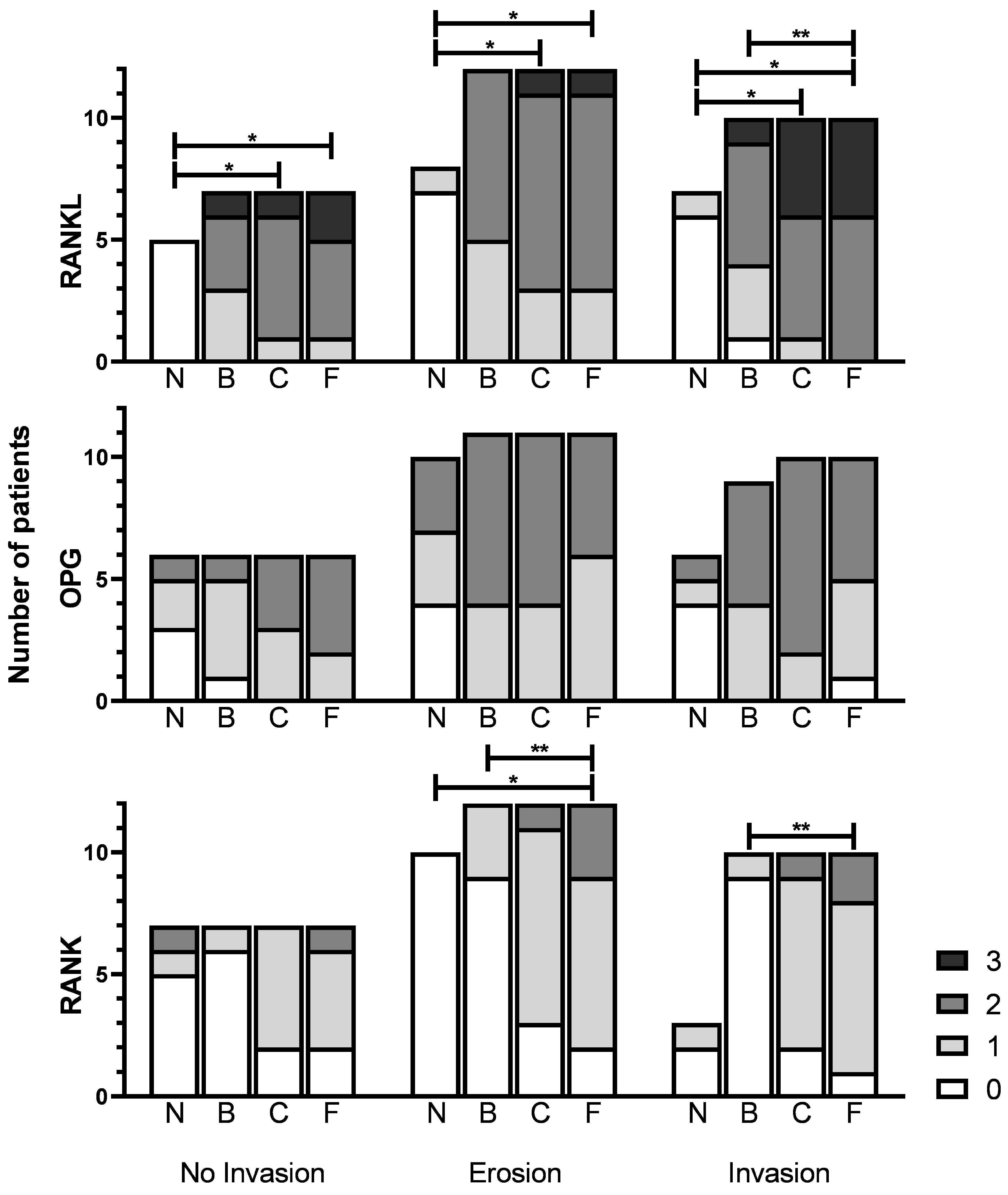
3.6. Expression between Invasion Categories
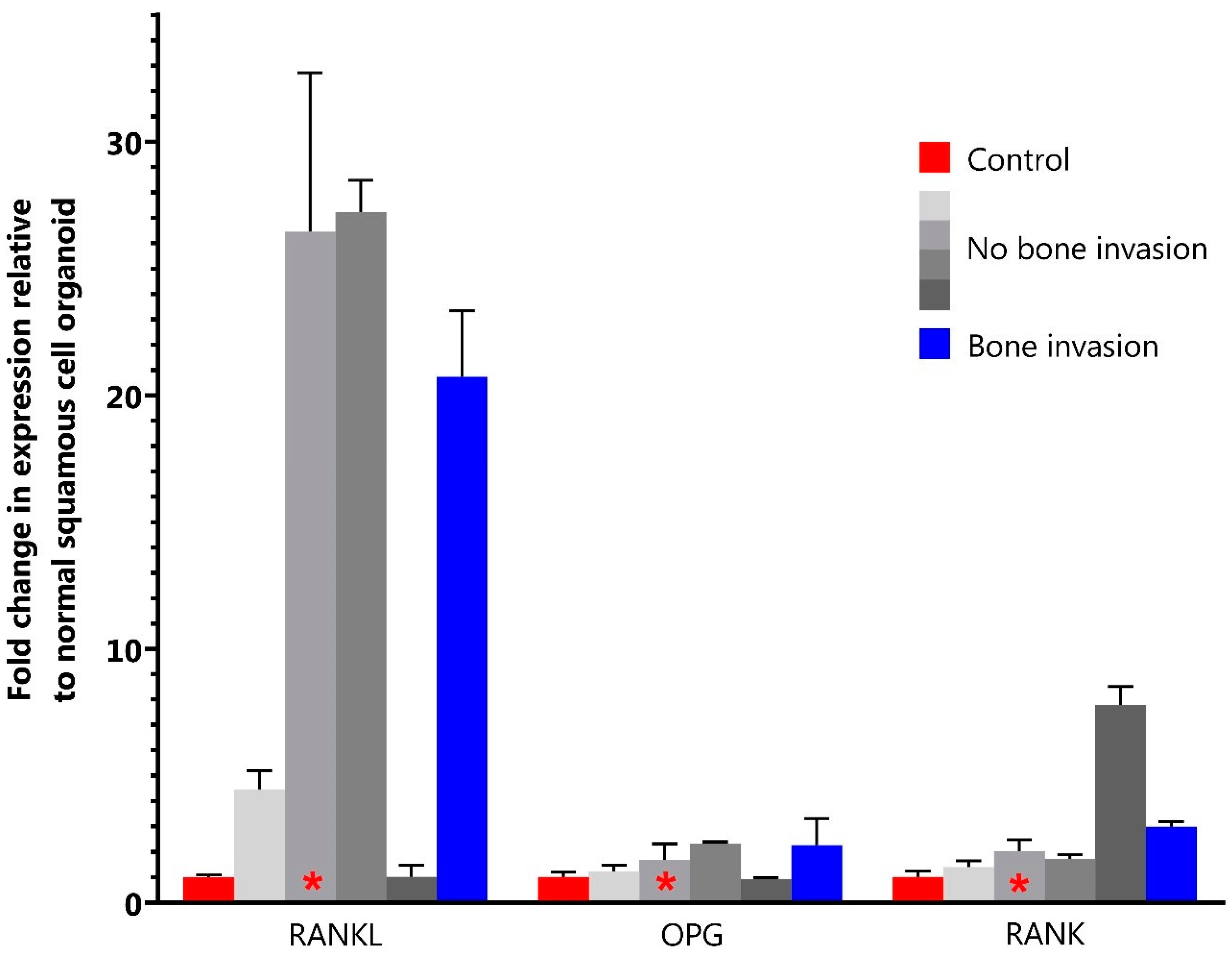
3.7. Organoid Staining and qPCR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Speight, P.M.; Farthing, P.M. The pathology of oral cancer. Br. Dent. J. 2018, 225, 841–847. [Google Scholar] [CrossRef]
- Brown, J.S.; Lowe, D.; Kalavrezos, N.; D’Souza, J.; Magennis, P.; Woolgar, J. Patterns of invasion and routes of tumor entry into the mandible by oral squamous cell carcinoma. Head Neck 2002, 24, 370–383. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Vaassen, L.A.A.; Speel, E.-J.M.; Kessler, P.A.W.H. Bone invasion by oral squamous cell carcinoma: Molecular alterations leading to osteoclastogenesis e a review of literature. J. Craniomaxillofac. Surg. 2017, 45, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Van Cann, E.M.; Dom, M.; Koole, R.; Merkx, M.A.W.; Stoelinga, P.J.W. Health related quality of life after mandibular resection for oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2005, 41, 687–693. [Google Scholar] [CrossRef]
- Van Gemert, J.; Holtslag, I.; Van Der Bilt, A.; Merkx, M.; Koole, R.; Van Cann, E. Health-related quality of life after segmental resection of the lateral mandible: Free fibula flap versus plate reconstruction. J. Cranio-Maxillofacial Surg. 2015, 43, 658–662. [Google Scholar] [CrossRef]
- Jimi, E.; Shin, M.; Furuta, H.; Tada, Y.; Kusukawa, J. The RANKL/RANK system as a therapeutic target for bone invasion by oral squamous cell carcinoma (Review). Int. J. Oncol. 2013, 42, 803–809. [Google Scholar] [CrossRef]
- Jimi, E.; Furuta, H.; Matsuo, K.; Tominaga, K.; Takahashi, T.; Nakanishi, O. The cellular and molecular mechanisms of bone invasion by oral squamous cell carcinoma. Oral Dis. 2011, 17, 462–468. [Google Scholar] [CrossRef]
- Nagai, M.; Kyakumoto, S.; Sato, N. Cancer cells responsible for humoral hypercalcemia express mRNA encoding a secreted form of ODF/TRANCE that induces osteoclast formation. Biochem. Biophys. Res. Commun. 2000, 269, 532–536. [Google Scholar] [CrossRef]
- Farrugia, A.N.; Atkins, G.J.; To, L.B.; Pan, B.; Horvath, N.; Kostakis, P.; Findlay, D.M.; Bardy, P.; Zannettino, A.C.W. Receptor activator of nuclear factor-κB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res. 2003, 63, 5438–5445. [Google Scholar] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Sezer, O.; Heider, U.; Zavrski, I.; Kühne, C.A.; Hofbauer, L.C. RANK ligand and osteoprotegerin in myeloma bone disease. Blood 2003, 101, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Schiavon, G.; Vincenzi, B.; Gaeta, L.; Pantano, F.; Russo, A.; Ortega, C.; Porta, C.; Galluzzo, S.; Armento, G.; et al. Receptor activator of NF-kB (rank) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS ONE 2011, 6, e19234. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V.; et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.; Ye, L.; Sanders, A.J.; Mason, M.D.; Jiang, W.G. Expression profile of receptor activator of nuclear-κb (rank), rank ligand (rankl) and osteoprotegerin (OPG) in breast cancer. Anticancer Res. 2013, 33, 199–206. [Google Scholar]
- Sasaki, A.; Ishikawa, K.; Haraguchi, N.; Inoue, H.; Ishio, T.; Shibata, K.; Ohta, M.; Kitano, S.; Mori, M. Receptor activator of nuclear factor-κB ligand (RANKL) expression in hepatocellular carcinoma with bone metastasis. Ann. Surg. Oncol. 2007, 14, 1191–1199. [Google Scholar] [CrossRef]
- Peng, X.; Guo, W.; Ren, T.; Lou, Z.; Lu, X.; Zhang, S.; Lu, Q.; Sun, Y. Differential Expression of the RANKL/RANK/OPG System Is Associated with Bone Metastasis in Human Non-Small Cell Lung Cancer. PLoS ONE 2013, 8, e58361. [Google Scholar] [CrossRef]
- Roux, S.; Meignin, V.; Quillard, J.; Meduri, G.; Guiochon-Mantel, A.; Fermand, J.P.; Milgrom, E.; Mariette, X. RANK (receptor activator of nuclear factor-κB) and RANKL expression in multiple myeloma. Br. J. Haematol. 2002, 117, 86–92. [Google Scholar] [CrossRef]
- Chen, G.; Sircar, K.; Aprikian, A.; Potti, A.; Goltzman, D.; Rabbani, S.A. Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer 2006, 107, 289–298. [Google Scholar] [CrossRef]
- Santini, D.; Perrone, G.; Roato, I.; Godio, L.; Pantano, F.; Grasso, D.; Russo, A.; Vincenzi, B.; Fratto, M.E.; Sabbatini, R.; et al. Expression pattern of receptor activator of NFκB (RANK) in a series of primary solid tumors and related bone metastases. J. Cell. Physiol. 2011, 226, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Millen, R.; De Kort, W.W.B.; Koomen, M.; van Son, G.J.F.; Gobits, R.; Penning de Vries, B.; Begthel, H.; Zandvliet, M.; Doornaert, P.; Raaijmakers, C.P.J.; et al. Patient-derived head and neck cancer organoids allow treatment stratification and serve as a tool for biomarker validation and identification. Med 2023, 4, 290–310.e12. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Browne, R.M. Factors influencing the patterns of invasion of the mandible by oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 1995, 24, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.L.; Tsao, S.W.; Burman, J.F.; Pittam, M.R.; Clifford, P.; Shaw, H.J. Patterns and mechanisms of bone invasion by squamous carcinomas of the head and neck. Am. J. Surg. 1983, 146, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Clohisy, D.R.; Palkert, D.; Ramnaraine, M.L.R.; Pekurovsky, I.; Oursler, M.J. Human breast cancer induces osteoclast activation and increases the number of osteoclasts at sites of tumor osteolysis. J. Orthop. Res. 1996, 14, 396–402. [Google Scholar] [CrossRef]
- Maurizi, A.; Rucci, N. The Osteoclast in Bone Metastasis: Player and Target. Cancers 2018, 10, 218. [Google Scholar] [CrossRef]
- Chuang, F.H.; Hsue, S.S.; Wu, C.W.; Chen, Y.K. Immunohistochemical expression of RANKL, RANK, and OPG in human oral squamous cell carcinoma. J. Oral Pathol. Med. 2009, 38, 753–758. [Google Scholar] [CrossRef]
- Cui, N.; Nomura, T.; Takano, N.; Wang, E.; Zhang, W.; Onda, T.; Shibahara, T. Osteoclast-related cytokines from biopsy specimens predict mandibular invasion by oral squamous cell carcinoma. Exp. Ther. Med. 2010, 1, 755–760. [Google Scholar] [CrossRef]
- Elmusrati, A.A.; Pilborough, A.E.; Khurram, S.A.; Lambert, D.W. Cancer-associated fibroblasts promote bone invasion in oral squamous cell carcinoma. Br. J. Cancer 2017, 117, 867–875. [Google Scholar] [CrossRef]
- Gibo, T.; Yamada, S.I.; Kawamoto, M.; Uehara, T.; Kurita, H. Immunohistochemical Investigation of Predictive Biomarkers for Mandibular Bone Invasion in Oral Squamous Cell Carcinoma. Pathol. Oncol. Res. 2020, 26, 2381–2389. [Google Scholar] [CrossRef]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; De Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Saad, F.; Coleman, R.; Shore, N.; Fizazi, K.; Tombal, B.; Miller, K.; Sieber, P.; Karsh, L.; Damião, R.; et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial. Lancet 2012, 379, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Good, C.R.; O’Keefe, R.J.; Edward Puzas, J.; Schwarz, E.M.; Rosier, R.N. Immunohistochemical study of receptor activator of nuclear factor kappa-B ligand (RANK-L) in human osteolytic bone tumors. J. Surg. Oncol. 2002, 79, 174–179. [Google Scholar] [CrossRef]
| Antibody | Manufacturer | Ordering Number | Antigen Retrieval | Primary Antibody Dilution | Secondary Antibody |
|---|---|---|---|---|---|
| Cathepsin K | Abcam, Cambridge, UK | ab19027 | pH6 Citrate | 1:500 | Goat anti-rabbit HRP |
| RANKL | Abcam, Cambridge, UK | ab13918 | pH6 citrate | 1:100 | Goat anti-rabbit HRP |
| RANK | Abcam, Cambridge, UK | ab9957 | pH9 EDTA | 1:100 | Goat anti-mouse HRP |
| OPG | Abcam, Cambridge, UK | ab183910 | pH9 EDTA | 1:200 | Goat anti-rabbit HRP |
| No Invasion (n = 7) | Erosion (n = 12) | Invasion (n = 10) | |
|---|---|---|---|
| Age (Median (range) | 67 (43–88) | 72 (60–79) | 63 (40–79) |
| Gender (M:F) | 1:6 | 8:4 | 5:5 |
| Tumor site | |||
| - Lower gum | 4 | 9 | 6 |
| - Upper gum | 2 | 3 | 3 |
| - Cheek | 1 | ||
| - Mandible * | 1 | ||
| Growth Pattern | |||
| - Cohesive | 5 | 5 | 4 |
| - Non-cohesive | 2 | 7 | 6 |
| Peri-neural invasion | |||
| - No | 7 | 3 | 4 |
| - Yes | 0 | 9 | 6 |
| Angioinvasion | |||
| - No | 7 | 12 | 9 |
| - Yes | 0 | 0 | 1 |
| No Invasion (n = 7) | Erosion (n = 12) | Invasion (n = 10) | ||||||||||
| RANKL Score | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Normal mucosa * | 5* | 0 | 0 | 0 | 7 | 1 | 0 | 0 | 6 | 1 | 0 | 0 |
| Back of tumor | 0 | 3 | 3 | 1 | 0 | 5 | 7 | 0 | 1 | 3 | 5 | 1 |
| Center of tumor | 0 | 1 | 5 | 1 | 0 | 3 | 8 | 1 | 0 | 1 | 5 | 4 |
| Front of tumor | 0 | 1 | 4 | 2 | 0 | 3 | 8 | 1 | 0 | 0 | 6 | 4 |
| No Invasion (n = 6) | Erosion (n = 11) | Invasion (n = 10) | ||||||||||
| OPG Score | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | |||
| Normal mucosa ** | 3 | 2 | 1 | 4 | 3 | 3 | 4 | 1 | 1 | |||
| Back of tumor *** | 1 | 4 | 2 | 0 | 4 | 7 | 0 | 4 | 5 | |||
| Center of tumor | 0 | 3 | 3 | 0 | 4 | 7 | 0 | 2 | 8 | |||
| Front of tumor | 0 | 2 | 4 | 0 | 6 | 5 | 1 | 4 | 5 | |||
| No Invasion (n = 7) | Erosion (n = 12) | Invasion (n = 10) | ||||||||||
| RANK Score | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | |||
| Normal mucosa **** | 5 | 1 | 1 | 10 | 0 | 0 | 2 | 1 | 0 | |||
| Back of tumor | 6 | 1 | 0 | 9 | 3 | 0 | 9 | 1 | 0 | |||
| Center of tumor | 2 | 5 | 0 | 3 | 8 | 1 | 2 | 7 | 1 | |||
| Front of tumor | 2 | 4 | 1 | 2 | 7 | 3 | 1 | 7 | 2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Kort, W.W.B.; Haakma, W.E.; van Es, R.J.J.; Gawlitta, D.; Driehuis, E.; Gansevoort, M.; Willems, S.M. Jaw Bone Invasion of Oral Squamous Cell Carcinoma Is Associated with Osteoclast Count and Expression of Its Regulating Proteins in Patients and Organoids. J. Clin. Med. 2023, 12, 6035. https://doi.org/10.3390/jcm12186035
de Kort WWB, Haakma WE, van Es RJJ, Gawlitta D, Driehuis E, Gansevoort M, Willems SM. Jaw Bone Invasion of Oral Squamous Cell Carcinoma Is Associated with Osteoclast Count and Expression of Its Regulating Proteins in Patients and Organoids. Journal of Clinical Medicine. 2023; 12(18):6035. https://doi.org/10.3390/jcm12186035
Chicago/Turabian Stylede Kort, Willem W. B., Wisse E. Haakma, Robert J. J. van Es, Debby Gawlitta, Else Driehuis, Merel Gansevoort, and Stefan M. Willems. 2023. "Jaw Bone Invasion of Oral Squamous Cell Carcinoma Is Associated with Osteoclast Count and Expression of Its Regulating Proteins in Patients and Organoids" Journal of Clinical Medicine 12, no. 18: 6035. https://doi.org/10.3390/jcm12186035
APA Stylede Kort, W. W. B., Haakma, W. E., van Es, R. J. J., Gawlitta, D., Driehuis, E., Gansevoort, M., & Willems, S. M. (2023). Jaw Bone Invasion of Oral Squamous Cell Carcinoma Is Associated with Osteoclast Count and Expression of Its Regulating Proteins in Patients and Organoids. Journal of Clinical Medicine, 12(18), 6035. https://doi.org/10.3390/jcm12186035






