Pedunculated Focal Nodular Hyperplasia: When in Doubt, Should We Cut It Out?
Abstract
1. Introduction
2. Current Landscape of Pedunculated Lesions in FNH
3. Epidemiology
4. Macroscopic and Microscopic Findings
5. Pathogenesis, Molecular Features and Associated Diseases
6. Clinical Manifestations and Diagnostic Workup
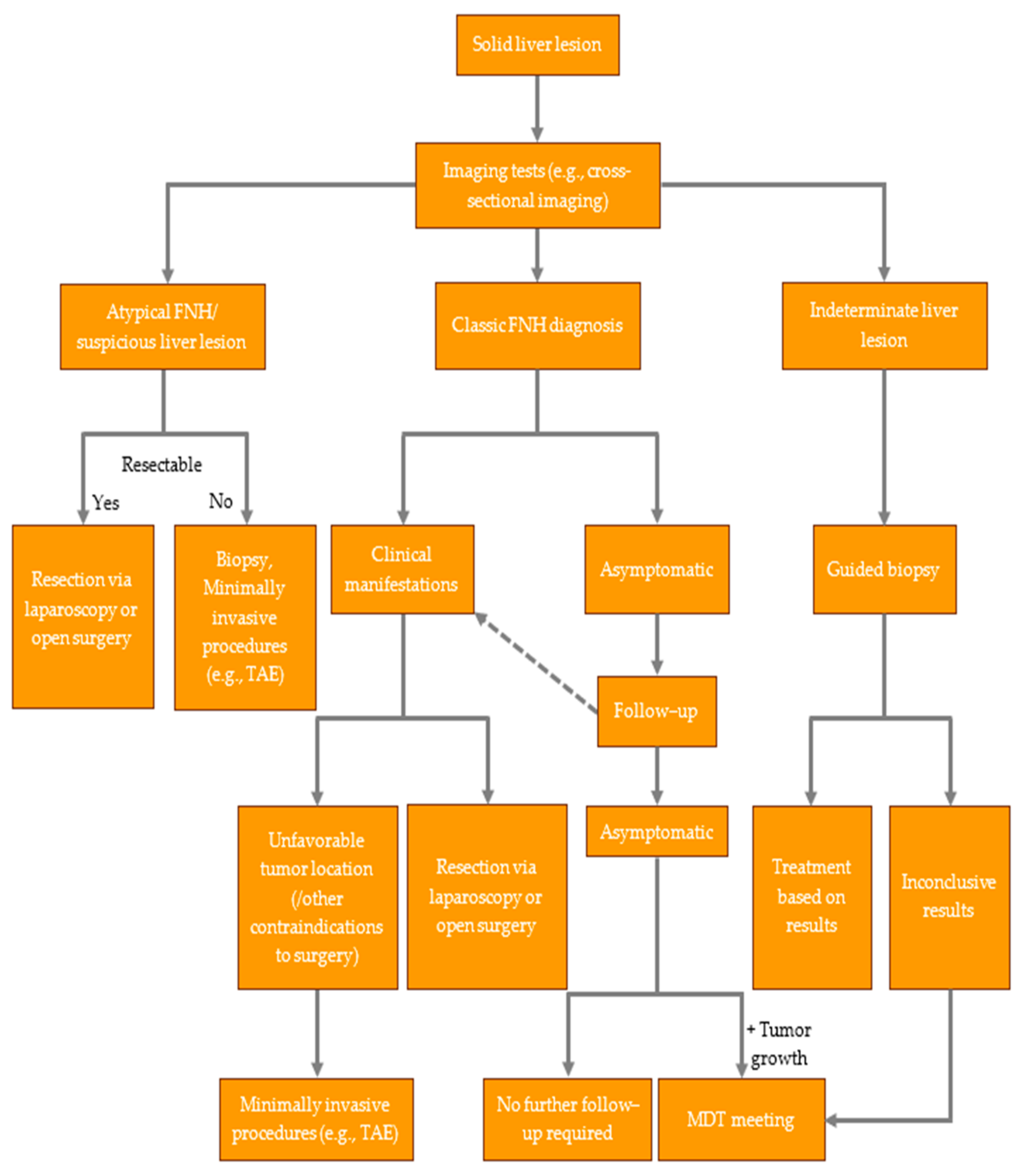
7. Management of FNH
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FNH | Focal nodular hyperplasia |
| N/A | Not available |
| TAE | Transarterial embolization |
| SD | Standard deviation |
| IVC | Inferior vena cava |
| GB | Gallbladder |
| HSCT | Hematopoietic Stem Cell Transplantation |
| HUMARA | Human androgen receptor assay |
| mRNA | Messenger RNA |
| ANGPT1 | Angiopoietin-1 |
| ANGPT2 | Angiopoietin-2 |
| AFP | Alpha-fetoprotein |
| US | Ultrasonography |
| CEUS | Contrast-enhanced ultrasound |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| Tc-99m | Technetium-99m |
| EGIST | Extra-gastrointestinal stromal tumor |
| PVA | Polyvinyl alcohol |
| MDT | Multidisciplinary team |
References
- Craig, J.R.; Peters, R.L.; Edmondson, H.A. Tumors of the Liver and Intrahepatic Bile Ducts. In Atlas of Tumor Pathology; Armed Forces Institute of Pathology: Washington, DC, USA, 1989. [Google Scholar]
- Wanless, I.R.; Mawdsley, C.; Adams, R. On the Pathogenesis of Focal Nodular Hyperplasia of the Liver. Hepatology 1985, 5, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Nahm, C.B.; Ng, K.; Lockie, P. Focal Nodular Hyperplasia—A Review of Myths and Truths. J. Gastrointest. Surg. 2011, 15, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, B.M.; Ronot, M.; Salvaggio, G. Imaging of Hepatic Focal Nodular Hyperplasia: Pictorial Review and Diagnostic Strategy. Semin Ultrasound CT MR 2016, 37, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Kondo, F. Benign Nodular Hepatocellular Lesions Caused by Abnormal Hepatic Circulation: Etiological Analysis and Introduction of a New Concept. J. Gastroenterol. Hepatol. 2001, 16, 1319–1328. [Google Scholar] [CrossRef]
- Morris-Stiff, G.; White, A.D.; Gomez, D.; Cameron, I.C.; Farid, S.; Toogood, G.J.; Lodge, J.P.A.; Prasad, K.R. Nodular Regenerative Hyperplasia (NRH) Complicating Oxaliplatin Chemotherapy in Patients Undergoing Resection of Colorectal Liver Metastases. Eur. J. Surg. Oncol. 2014, 40, 1016–1020. [Google Scholar] [CrossRef]
- Rubbia-Brandt, L.; Audard, V.; Sartoretti, P.; Roth, A.D.; Brezault, C.; Le Charpentier, M.; Dousset, B.; Morel, P.; Soubrane, O.; Chaussade, S.; et al. Severe Hepatic Sinusoidal Obstruction Associated with Oxaliplatin-Based Chemotherapy in Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2004, 15, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Sudour, H.; Mainard, L.; Baumann, C.; Clement, L.; Salmon, A.; Bordigoni, P. Focal Nodular Hyperplasia of the Liver Following Hematopoietic SCT. Bone Marrow Transplant. 2009, 43, 127–132. [Google Scholar] [CrossRef]
- Bouyn, C.I.-D.; Leclere, J.; Raimondo, G.; Le Pointe, H.D.; Couanet, D.; Valteau-Couanet, D.; Hartmann, O. Hepatic Focal Nodular Hyperplasia in Children Previously Treated for a Solid Tumor. Incidence, Risk Factors, and Outcome: Incidence, Risk Factors, and Outcome. Cancer 2003, 97, 3107–3113. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Fléjou, J.F.; Terris, B.; Belghiti, J.; Degott, C. Focal Nodular Hyperplasia of the Liver: A Comprehensive Pathologic Study of 305 Lesions and Recognition of New Histologic Forms. Am. J. Surg. Pathol. 1999, 23, 1441–1454. [Google Scholar] [CrossRef]
- Colombo, M. Malignant Neoplasm of the Liver. In Schiff’s Diseases of the Liver; Schiff, E.R., Sorrell, M.F., Maddrey, W.C., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2002; pp. 1377–1403. [Google Scholar]
- Perrakis, A.; Vassos, N.; Grützmann, R.; Croner, R.S. What Is Changing in Indications and Treatment of Focal Nodular Hyperplasia of the Liver. Is There Any Place for Surgery? Ann. Hepatol. 2017, 16, 333–341. [Google Scholar] [CrossRef]
- Eris, C.; Yildiz, M.K.; Odabasi, M.; Akbulut, S.; Abuoglu, H.; Ozkan, E. Gastric Outlet Obstruction Caused by Focal Nodular Hyperplasia of the Liver: A Case Report and Literature Review. Int. J. Surg. Case Rep. 2013, 4, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qin, L.-X.; Ji, Y.; Sun, H.-C.; Ye, Q.-H.; Wang, L.; Pan, Q.; Fan, J.; Tang, Z.-Y. Atypical Hepatic Focal Nodular Hyperplasia Presenting as Acute Abdomen and Misdiagnosed as Hepatocellular Carcinoma. Hepatol. Res. 2007, 37, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Bader, T.R.; Braga, L.; Semelka, R.C. Exophytic Benign Tumors of the Liver: Appearance on MRI. Magn. Reson. Imaging 2001, 19, 623–628. [Google Scholar] [CrossRef]
- Byrnes, V.; Cárdenas, A.; Afdhal, N.; Hanto, D. Symptomatic Focal Nodular Hyperplasia during Pregnancy: A Case Report. Ann. Hepatol. 2004, 3, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Leone, N.; Saettone, S.; De Paolis, P.; Carucci, P.; Brunello, F.; De Angelis, C.; Menozzi, G.; Rizzetto, M. Ectopic Livers and Related Pathology: Report of Three Cases of Benign Lesions. Dig. Dis. Sci. 2005, 50, 1818–1822. [Google Scholar] [CrossRef]
- Wasif, N.; Sasu, S.; Conway, W.C.; Bilchik, A. Focal Nodular Hyperplasia: Report of an Unusual Case and Review of the Literature. Am. Surg. 2008, 74, 1100–1103. [Google Scholar] [CrossRef]
- Khan, M.R.; Saleem, T.; Haq, T.U.; Aftab, K. Atypical Focal Nodular Hyperplasia of the Liver. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Terada, T. Projected Focal Nodular Hyperplasia of the Liver with Pronounced Atypical Ductular Reaction Resembling Ductal Plate and Expressing KIT. Hepatol. Res. 2012, 42, 721–726. [Google Scholar] [CrossRef]
- Dreizin, D.; Infante, J.; Tirada, N.; Raman, S.P.; Madrazo, B. Focal Nodular Hyperplasia within Accessory Liver: Imaging Findings at Computed Tomography and Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2014, 38, 424–426. [Google Scholar] [CrossRef]
- Badea, R.; Meszaros, M.; Al Hajjar, N.; Rusu, I.; Chiorean, L. Benign Nodular Hyperplasia of the Liver-Pedunculated Form: Diagnostic Contributions of Ultrasonography and Consideration of Exophytic Liver Tumors. J. Med. Ultrason. 2015, 42, 97–102. [Google Scholar] [CrossRef]
- Reddy, K.; Hooper, K.; Frost, A.; Hebert-Magee, S.; Bell, W.; Porterfield, J.R.; Ramesh, J. Pedunculated Focal Nodular Hyperplasia Masquerading as Perigastric Mass Identified by EUS-FNA. Gastrointest. Endosc. 2015, 81, 238–239, discussion 239–240. [Google Scholar] [CrossRef] [PubMed]
- Zeina, A.-R.; Glick, Y. Pedunculated Hepatic Focal Nodular Hyperplasia. Ann. Hepatol. 2016, 15, 929–931. [Google Scholar] [PubMed]
- Tsukui, M.; Morimoto, N.; Murayama, K. A Case of Pedunculated Focal Nodular Hyperplasia Treated by Laparoscopic Hepatectomy. J. Dig. Dis. Hepatol. 2017, 2, 1–5. [Google Scholar]
- Martiniuc, A.; Dumitraşcu, T. Pedunculated Focal Nodular Hyperplasia of the Liver. Surg. Gastroenterol. Oncol. 2018, 23, 79. [Google Scholar] [CrossRef][Green Version]
- Su, N.; Chen, C.; Dai, Q.; Wang, L.; Yang, M.; Xia, Y.; Jiang, Y.; Lv, K. Focal Nodular Hyperplasia on an Accessory Liver Lobe: A Case Report and Literature Review. Medicine 2020, 99, e21357. [Google Scholar] [CrossRef]
- Navarini, D.; De Britto, F.M.; Da Silva, L.A. Acute Abdomen Caused by a Large Pedunculated Pediculated Focal Nodular Hyperplasia of the Liver. Dig. Liver Dis. 2020, 16, 1513–1514. [Google Scholar] [CrossRef]
- Ben Ismail, I.; Zenaidi, H.; Jouini, R.; Rebii, S.; Zoghlami, A. Pedunculated Hepatic Focal Nodular Hyperplasia: A Case Report and Review of the Literature. Clin. Case Rep. 2021, 9, e04202. [Google Scholar] [CrossRef]
- Nandy, K.; Patkar, S.; Goel, M. Massive Pedunculated Focal Nodular Hyperplasia: A Diagnostic Dilemma. J. Gastrointest. Surg. 2023, 27, 1746–1748. [Google Scholar] [CrossRef]
- Akaguma, A.; Ishii, T.; Uchida, Y.; Chigusa, Y.; Ueda, Y.; Mandai, M.; Mogami, H. Laparoscopic Resection for Pedunculated Focal Nodular Hyperplasia of the Liver during Pregnancy. Oxf. Med. Case Rep. 2023, 2023, omad054. [Google Scholar] [CrossRef]
- Sawhney, S.; Jain, R.; Safaya, R.; Berry, M. Pedunculated Focal Nodular Hyperplasia. Pediatr. Radiol. 1992, 22, 231–232. [Google Scholar] [CrossRef]
- Ruiz Hierro, C.; Vázquez Rueda, F.; Vargas Cruz, V.; Lasso Betancor, C.E.; Ayala Montoro, J. Focal Nodular Hyperplasia on Accessory Lobe of the Liver: Preoperative Diagnosis and Management. J. Pediatr. Surg. 2013, 48, 251–254. [Google Scholar] [CrossRef]
- Lee, J.; Molitor, M.; Alashari, M.; Winters, W.; Skarda, D.E. Exophytic Focal Nodular Hyperplasia Torsion: A Rare Cause of Sudden-Onset Epigastric Pediatric Abdominal Pain. J. Pediatr. Surg. Case Rep. 2014, 2, 407–409. [Google Scholar] [CrossRef][Green Version]
- Koolwal, J.; Birkemeier, K.L.; Zreik, R.T.; Mattix, K.D. Pedunculated Focal Nodular Hyperplasia in a Healthy Toddler. Proc. (Bayl. Univ. Med. Cent.) 2018, 31, 97–99. [Google Scholar] [CrossRef]
- Edmondson, H.A. Tumors of the Liver and Intrahepatic Bile Ducts; Armed Forces Institute of Pathology: Washington, DC, USA, 1958. [Google Scholar]
- Vilgrain, V. Focal Nodular Hyperplasia. Eur. J. Radiol. 2006, 58, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Ma, I.T.; Rojas, Y.; Masand, P.M.; Castro, E.C.; Himes, R.W.; Kim, E.S.; Goss, J.A.; Nuchtern, J.G.; Finegold, M.J.; Thompson, P.A.; et al. Focal Nodular Hyperplasia in Children: An Institutional Experience with Review of the Literature. J. Pediatr. Surg. 2015, 50, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Chiorean, L.; Cui, X.-W.; Tannapfel, A.; Franke, D.; Stenzel, M.; Kosiak, W.; Schreiber-Dietrich, D.; Jüngert, J.; Chang, J.-M.; Dietrich, C.F. Benign Liver Tumors in Pediatric Patients—Review with Emphasis on Imaging Features. World J. Gastroenterol. 2015, 21, 8541–8561. [Google Scholar] [CrossRef]
- Lautz, T.; Tantemsapya, N.; Dzakovic, A.; Superina, R. Focal Nodular Hyperplasia in Children: Clinical Features and Current Management Practice. J. Pediatr. Surg. 2010, 45, 1797–1803. [Google Scholar] [CrossRef]
- Ortega, G.; Price, M.; Choo, S.; Goldstein, S.D.; Stewart, D.; Abdullah, F. Multidisciplinary Management of Focal Nodular Hyperplasia in Children: Experience with 10 Cases: Experience with 10 Cases. JAMA Surg. 2013, 148, 1068–1070. [Google Scholar] [CrossRef]
- Meyers, R.L. Tumors of the Liver in Children. Surg. Oncol. 2007, 16, 195–203. [Google Scholar] [CrossRef]
- Venturi, A.; Piscaglia, F.; Vidili, G.; Flori, S.; Righini, R.; Golfieri, R.; Bolondi, L. Diagnosis and Management of Hepatic Focal Nodular Hyperplasia. J. Ultrasound 2007, 10, 116–127. [Google Scholar] [CrossRef]
- Caseiro-Alves, F.; Zins, M.; Mahfouz, A.-E.; Rahmouni, A.; Vilgrain, V.; Menu, Y.; Mathieu, D. Calcification in Focal Nodular Hyperplasia: A New Problem for Differentiation from Fibrolamellar Hepatocellular Carcinoma. Radiology 1996, 198, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, L.; Morana, G.; Kirchin, M.A.; Schneider, G. Accurate Differentiation of Focal Nodular Hyperplasia from Hepatic Adenoma at Gadobenate Dimeglumine-Enhanced MR Imaging: Prospective Study. Radiology 2005, 236, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Boulahdour, H.; Cherqui, D.; Charlotte, F.; Rahmouni, A.; Dhumeaux, D.; Zafrani, E.S.; Meignan, M. The Hot Spot Hepatobiliary Scan in Focal Nodular Hyperplasia. J. Nucl. Med. 1993, 34, 2105–2110. [Google Scholar]
- Hussain, S.M.; Terkivatan, T.; Zondervan, P.E.; Lanjouw, E.; de Rave, S.; IJzermans, J.N.M.; de Man, R.A. Focal Nodular Hyperplasia: Findings at State-of-the-Art MR Imaging, US, CT, and Pathologic Analysis. Radiographics 2004, 24, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; van den Bos, I.C.; Dwarkasing, R.S.; Kuiper, J.-W.; den Hollander, J. Hepatocellular Adenoma: Findings at State-of-the-Art Magnetic Resonance Imaging, Ultrasound, Computed Tomography and Pathologic Analysis. Eur. Radiol. 2006, 16, 1873–1886. [Google Scholar] [CrossRef]
- Van Aalten, S.M.; Verheij, J.; Terkivatan, T.; Dwarkasing, R.S.; de Man, R.A.; Ijzermans, J.N.M. Validation of a Liver Adenoma Classification System in a Tertiary Referral Centre: Implications for Clinical Practice. J. Hepatol. 2011, 55, 120–125. [Google Scholar] [CrossRef]
- Roncalli, M.; Sciarra, A.; Tommaso, L.D. Benign Hepatocellular Nodules of Healthy Liver: Focal Nodular Hyperplasia and Hepatocellular Adenoma. Clin. Mol. Hepatol. 2016, 22, 199–211. [Google Scholar] [CrossRef]
- Xue, D.-Q.; Yang, L. Development of Focal Nodular Hyperplasia after Cyclophosphamide-Based Chemotherapy in a Patient with Breast Cancer. Case Rep. Hepatol. 2018, 2018, 5409316. [Google Scholar] [CrossRef]
- Joyner, B.L., Jr.; Levin, T.L.; Goyal, R.K.; Newman, B. Focal Nodular Hyperplasia of the Liver: A Sequela of Tumor Therapy. Pediatr. Radiol. 2005, 35, 1234–1239. [Google Scholar] [CrossRef]
- Mathieu, D.; Zafrani, E.S.; Anglade, M.C.; Dhumeaux, D. Association of Focal Nodular Hyperplasia and Hepatic Hemangioma. Gastroenterology 1989, 97, 154–157. [Google Scholar] [CrossRef]
- Buscarini, E.; Danesino, C.; Plauchu, H.; de Fazio, C.; Olivieri, C.; Brambilla, G.; Menozzi, F.; Reduzzi, L.; Blotta, P.; Gazzaniga, P.; et al. High Prevalence of Hepatic Focal Nodular Hyperplasia in Subjects with Hereditary Hemorrhagic Telangiectasia. Ultrasound Med. Biol. 2004, 30, 1089–1097. [Google Scholar] [CrossRef]
- Guariso, G.; Fiorio, S.; Altavilla, G.; Gamba, P.G.; Toffolutti, T.; Chiesura-Corona, M.; Tedeschi, U.; Zancan, L. Congenital Absence of the Portal Vein Associated with Focal Nodular Hyperplasia of the Liver and Cystic Dysplasia of the Kidney. Eur. J. Pediatr. 1998, 157, 287–290. [Google Scholar] [CrossRef]
- Kinjo, T.; Aoki, H.; Sunagawa, H.; Kinjo, S.; Muto, Y. Congenital Absence of the Portal Vein Associated with Focal Nodular Hyperplasia of the Liver and Congenital Choledochal Cyst: A Case Report. J. Pediatr. Surg. 2001, 36, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Sempoux, C.; Paradis, V.; Komuta, M.; Wee, A.; Calderaro, J.; Balabaud, C.; Quaglia, A.; Bioulac-Sage, P. Hepatocellular Nodules Expressing Markers of Hepatocellular Adenomas in Budd-Chiari Syndrome and Other Rare Hepatic Vascular Disorders. J. Hepatol. 2015, 63, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Wanless, I.R.; Albrecht, S.; Bilbao, J.; Frei, J.V.; Heathcote, E.J.; Roberts, E.A.; Chiasson, D. Multiple Focal Nodular Hyperplasia of the Liver Associated with Vascular Malformations of Various Organs and Neoplasia of the Brain: A New Syndrome. Mod. Pathol. 1989, 2, 456–462. [Google Scholar] [PubMed]
- Libbrecht, L.; Cassiman, D.; Verslype, C.; Maleux, G.; Van Hees, D.; Pirenne, J.; Nevens, F.; Roskams, T. Clinicopathological Features of Focal Nodular Hyperplasia-like Nodules in 130 Cirrhotic Explant Livers. Am. J. Gastroenterol. 2006, 101, 2341–2346. [Google Scholar] [CrossRef]
- De Pasquale, M.D.; Monti, L.; D’Andrea, M.L.; De Ioris, M.A.; Castellano, A. Focal Nodular Hyperplasia and Hepatic Regenerating Nodules in Pediatric Oncology Patients: How Much Invasive Approach Is Necessary? Ann. Hepatol. 2013, 12, 308–314. [Google Scholar] [CrossRef]
- Towbin, A.J.; Luo, G.G.; Yin, H.; Mo, J.Q. Focal Nodular Hyperplasia in Children, Adolescents, and Young Adults. Pediatr. Radiol. 2011, 41, 341–349. [Google Scholar] [CrossRef]
- Scalori, A.; Tavani, A.; Gallus, S.; La Vecchia, C.; Colombo, M. Oral Contraceptives and the Risk of Focal Nodular Hyperplasia of the Liver: A Case-Control Study. Am. J. Obstet. Gynecol. 2002, 186, 195–197. [Google Scholar] [CrossRef]
- Klatskin, G. Hepatic Tumors: Possible Relationship to Use of Oral Contraceptives. Gastroenterology 1977, 73, 386–394. [Google Scholar] [CrossRef]
- Fukahori, S.; Kawano, T.; Obase, Y.; Umeyama, Y.; Sugasaki, N.; Kinoshita, A.; Fukushima, C.; Yamakawa, M.; Omagari, K.; Mukae, H. Fluctuation of Hepatic Focal Nodular Hyperplasia Size with Oral Contraceptives Use. Am. J. Case Rep. 2019, 20, 1124–1127. [Google Scholar] [CrossRef]
- Ashhab, A.A.; Abu-Sulb, A.; Yang, J.D.; Noureddin, M.; Sundaram, V.; Kuo, A.; Ayoub, W.S. Estrogen-Driven Growth of Focal Nodular Hyperplasia: Truth or Myth? ACG Case Rep. J. 2021, 8, e00531. [Google Scholar] [CrossRef]
- Chandrasegaram, M.D.; Shah, A.; Chen, J.W.; Ruszkiewicz, A.; Astill, D.S.; England, G.; Raju, R.S.; Neo, E.L.; Dolan, P.M.; Tan, C.P.; et al. Oestrogen Hormone Receptors in Focal Nodular Hyperplasia. HPB 2015, 17, 502–507. [Google Scholar] [CrossRef]
- Mathieu, D.; Kobeiter, H.; Maison, P.; Rahmouni, A.; Cherqui, D.; Zafrani, E.S.; Dhumeaux, D. Oral Contraceptive Use and Focal Nodular Hyperplasia of the Liver. Gastroenterology 2000, 118, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-H.; Fan, J.; Wu, Z.-Q.; Ma, Z.-C.; Zhou, X.-D.; Zhou, J.; Qiu, S.-J.; Qin, L.-X.; Ye, Q.-H.; Sun, H.-C.; et al. Focal Nodular Hyperplasia of the Liver in 86 Patients. Hepatobiliary Pancreat. Dis. Int. 2007, 6, 52–57. [Google Scholar] [PubMed]
- Kim, M.J.; Han, S.Y.; Baek, Y.H.; Lee, S.W.; Kwon, H.J. A Case of Focal Nodular Hyperplasia with Growth Progression during Pregnancy. Clin. Mol. Hepatol. 2014, 20, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Figueiredo, S.; Kefleyesus, A.; Sempoux, C.; Uldry, E.; Halkic, N. Focal Nodular Hyperplasia Associated with a Giant Hepatocellular Adenoma: A Case Report and Review of Literature. World J. Hepatol. 2021, 13, 1450–1458. [Google Scholar] [CrossRef]
- Langrehr, J.M.M.D.; Pfitzmann, R.; Hermann, M.; Radke, C.; Neuhaus, P.; Pech, M.; Denecke, T.; Felix, R.; Hänninen, E.L. Hepatocellular Carcinoma in Association with Hepatic Focal Nodular Hyperplasia. Acta Radiol. 2006, 47, 340–344. [Google Scholar] [CrossRef]
- Ercan, C.; Coto-Llerena, M.; Gallon, J.; Fourie, L.; Marinucci, M.; Hess, G.F.; Vosbeck, J.; Taha-Mehlitz, S.; Boldanova, T.; Meier, M.-A.; et al. Genomic Analysis of Focal Nodular Hyperplasia with Associated Hepatocellular Carcinoma Unveils Its Malignant Potential: A Case Report. Commun. Med. 2022, 2, 11. [Google Scholar] [CrossRef]
- Haubert, L.; Yearsley, M.; Bloomston, M. Hepatocellular Carcinoma Arising within Focal Nodular Hyperplasia. Am. Surg. 2010, 76, 335–336. [Google Scholar] [CrossRef]
- Coopersmith, C.M.; Lowell, J.A.; Hassan, A.; Howard, T.K. Hepatocellular Carcinoma in a Patient with Focal Nodular Hyperplasia. HPB 2002, 4, 135–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bioulac-Sage, P.; Rebouissou, S.; Sa Cunha, A.; Jeannot, E.; Lepreux, S.; Blanc, J.-F.; Blanché, H.; le Bail, B.; Saric, J.; Laurent-Puig, P.; et al. Clinical, Morphologic, and Molecular Features Defining so-Called Telangiectatic Focal Nodular Hyperplasias of the Liver. Gastroenterology 2005, 128, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Paradis, V.; Benzekri, A.; Dargère, D.; Bièche, I.; Laurendeau, I.; Vilgrain, V.; Belghiti, J.; Vidaud, M.; Degott, C.; Bedossa, P. Telangiectatic Focal Nodular Hyperplasia: A Variant of Hepatocellular Adenoma. Gastroenterology 2004, 126, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Paradis, V.; Laurent, A.; Flejou, J.F.; Vidaud, M.; Bedossa, P. Evidence for the Polyclonal Nature of Focal Nodular Hyperplasia of the Liver by the Study of X-Chromosome Inactivation. Hepatology 1997, 26, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Rebouissou, S.; Bioulac-Sage, P.; Zucman-Rossi, J. Molecular Pathogenesis of Focal Nodular Hyperplasia and Hepatocellular Adenoma. J. Hepatol. 2008, 48, 163–170. [Google Scholar] [CrossRef]
- Bioulac-Sage, P. Angiopoietines play a physiopathological role in focal nodular hyperplasia. Gastroenterol. Clin. Biol. 2004, 28, 200–201. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Jeng, Y.-M.; Yeh, S.-H.; Chen, P.-J. P53 Gene and Wnt Signaling in Benign Neoplasms: β-Catenin Mutations in Hepatic Adenoma but Not in Focal Nodular Hyperplasia. Hepatology 2002, 36, 927–935. [Google Scholar] [CrossRef]
- Gaffey, M.J.; Iezzoni, J.C.; Weiss, L.M. Clonal Analysis of Focal Nodular Hyperplasia of the Liver. Am. J. Pathol. 1996, 148, 1089–1096. [Google Scholar]
- Gong, L.; Li, Y.-H.; Su, Q.; Li, G.; Zhang, W.-D.; Zhang, W. Use of X-Chromosome Inactivation Pattern and Laser Microdissection to Determine the Clonal Origin of Focal Nodular Hyperplasia of the Liver. Pathology 2009, 41, 348–355. [Google Scholar] [CrossRef]
- Cai, Y.-R.; Gong, L.; Teng, X.-Y.; Zhang, H.-T.; Wang, C.-F.; Wei, G.-L.; Guo, L.; Ding, F.; Liu, Z.-H.; Pan, Q.-J.; et al. Clonality and Allelotype Analyses of Focal Nodular Hyperplasia Compared with Hepatocellular Adenoma and Carcinoma. World J. Gastroenterol. 2009, 15, 4695–4708. [Google Scholar] [CrossRef]
- Zheng, S.; Cummings, O.W.; Saxena, R.; Zhang, S.; Wang, M.; Williamson, S.R.; Cheng, M.; Lopez-Beltran, A.; Montironi, R.; Hodges, K.B.; et al. Clonality and TP53 Mutation Analysis of Focal Nodular Hyperplasia of the Liver. Am. J. Clin. Pathol. 2010, 134, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Ringe, B.; Klempnauer, J.; Lamesch, P.; Gratz, K.F.; Prokop, M.; Maschek, H.; Tusch, G.; Pichlmayr, R. Benign Liver Tumors: Differential Diagnosis and Indications for Surgery. World J. Surg. 1997, 21, 983–990, discussion 990-1. [Google Scholar] [CrossRef] [PubMed]
- Cherqui, D.; Rahmouni, A.; Charlotte, F.; Boulahdour, H.; Métreau, J.M.; Meignan, M.; Fagniez, P.L.; Zafrani, E.S.; Mathieu, D.; Dhumeaux, D. Management of Focal Nodular Hyperplasia and Hepatocellular Adenoma in Young Women: A Series of 41 Patients with Clinical, Radiological, and Pathological Correlations. Hepatology 1995, 22, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Takemura, S.; Tanaka, S.; Hamano, G.; Ito, T.; Aota, T.; Koda, M.; Ohsawa, M.; Kubo, S. Ruptured Focal Nodular Hyperplasia Observed during Follow-up: A Case Report. Surg. Case Rep. 2017, 3, 44. [Google Scholar] [CrossRef]
- Shehri, A. Focal Nodular Hyperplasia in Children Presenting as Acute Cholecystitis. Int. J. Health Sci. 2010, 4, 194–197. [Google Scholar]
- Bente, A.; Ouedraogo, M.; Kasbawala, K.N.; Glasgow, K.M. Compression of Cystic Duct Caused by Focal Nodular Hyperplasia. J. Surg. Case Rep. 2019, 2019, rjz254. [Google Scholar] [CrossRef]
- Filippone, A.; Basilico, R.; Fabio, D. Focal Liver Lesions: Detection, Characterization, Ablation; Lencioni, R., Cioni, D., Bartolozzi, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Malhi, H.; Grant, E.G.; Duddalwar, V. Contrast-Enhanced Ultrasound of the Liver and Kidney. Radiol. Clin. N. Am. 2014, 52, 1177–1190. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, B.K.; Shim, C.S. Focal Nodular Hyperplasia: With a Focus on Contrast Enhanced Ultrasound. Korean J. Hepatol. 2010, 16, 414. [Google Scholar] [CrossRef]
- Ungermann, L.; Eliáš, P.; Žižka, J.; Ryška, P.; Klzo, L. Focal Nodular Hyperplasia: Spoke-Wheel Arterial Pattern and Other Signs on Dynamic Contrast-Enhanced Ultrasonography. Eur. J. Radiol. 2007, 63, 290–294. [Google Scholar] [CrossRef]
- Bartolotta, T.V.; Taibbi, A.; Matranga, D.; Malizia, G.; Lagalla, R.; Midiri, M. Hepatic Focal Nodular Hyperplasia: Contrast-Enhanced Ultrasound Findings with Emphasis on Lesion Size, Depth and Liver Echogenicity. Eur. Radiol. 2010, 20, 2248–2256. [Google Scholar] [CrossRef]
- Fang, C.; Anupindi, S.A.; Back, S.J.; Franke, D.; Green, T.G.; Harkanyi, Z.; Jüngert, J.; Kwon, J.K.; Paltiel, H.J.; Squires, J.H.; et al. Contrast-Enhanced Ultrasound of Benign and Malignant Liver Lesions in Children. Pediatr. Radiol. 2021, 51, 2181–2197. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.-D.; Lu, M.-D.; Liu, G.-J.; Shen, S.-L.; Xu, Z.-F.; Xie, X.-Y.; Wang, Y.; Zhou, L.-Y. Contrast-Enhanced Ultrasound Features of Histologically Proven Focal Nodular Hyperplasia: Diagnostic Performance Compared with Contrast-Enhanced CT. Eur. Radiol. 2013, 23, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Bertin, C.; Egels, S.; Wagner, M.; Huynh-Charlier, I.; Vilgrain, V.; Lucidarme, O. Contrast-Enhanced Ultrasound of Focal Nodular Hyperplasia: A Matter of Size. Eur. Radiol. 2014, 24, 2561–2571. [Google Scholar] [CrossRef]
- Myers, L.; Ahn, J. Focal Nodular Hyperplasia and Hepatic Adenoma. Clin. Liver Dis. 2020, 24, 389–403. [Google Scholar] [CrossRef]
- Uggowitzer, M.; Kugler, C.; Gröll, R.; Mischinger, H.J.; Stacher, R.; Fickert, P.; Weiglein, A. Sonographic Evaluation of Focal Nodular Hyperplasias (FNH) of the Liver with a Transpulmonary Galactose-Based Contrast Agent (Levovist). Br. J. Radiol. 1998, 71, 1026–1032. [Google Scholar] [CrossRef]
- Shamsi, K.; De Schepper, A.; Degryse, H.; Deckers, F. Focal Nodular Hyperplasia of the Liver: Radiologic Findings. Abdom. Imaging 1993, 18, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.K.; Johnson, C.D.; Bender, C.E.; Welch, T.J. CT of Focal Nodular Hyperplasia of the Liver. AJR Am. J. Roentgenol. 2000, 174, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Brancatelli, G.; Federle, M.P.; Grazioli, L.; Blachar, A.; Peterson, M.S.; Thaete, L. Focal Nodular Hyperplasia: CT Findings with Emphasis on Multiphasic Helical CT in 78 Patients. Radiology 2001, 219, 61–68. [Google Scholar] [CrossRef]
- Kim, T.; Hori, M.; Onishi, H. Liver Masses with Central or Eccentric Scar. Semin. Ultrasound CT MR 2009, 30, 418–425. [Google Scholar] [CrossRef]
- Attal, P.; Vilgrain, V.; Brancatelli, G.; Paradis, V.; Terris, B.; Belghiti, J.; Taouli, B.; Menu, Y. Telangiectatic Focal Nodular Hyperplasia: US, CT, and MR Imaging Findings with Histopathologic Correlation in 13 Cases. Radiology 2003, 228, 465–472. [Google Scholar] [CrossRef]
- Kehagias, D.; Moulopoulos, L.; Antoniou, A.; Hatziioannou, A.; Smyrniotis, V.; Trakadas, S.; Lahanis, S.; Vlahos, L. Focal Nodular Hyperplasia: Imaging Findings. Eur. Radiol. 2001, 11, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Bieze, M.; van den Esschert, J.W.; Nio, C.Y.; Verheij, J.; Reitsma, J.B.; Terpstra, V.; van Gulik, T.M.; Phoa, S.S.K.S. Diagnostic Accuracy of MRI in Differentiating Hepatocellular Adenoma from Focal Nodular Hyperplasia: Prospective Study of the Additional Value of Gadoxetate Disodium. AJR Am. J. Roentgenol. 2012, 199, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.J.; Brown, J.J.; Narra, V.R. Magnetic Resonance Imaging of Focal Liver Lesions: Approach to Imaging Diagnosis. Hepatology 2011, 54, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.A.; Lichtenstein, G.R. Hepatic Scintigraphy in the Evaluation of Solitary Solid Liver Masses. J. Nucl. Med. 1993, 34, 697–705. [Google Scholar] [PubMed]
- Hanaoka, J.; Shimada, M.; Utsunomiya, T. Huge Focal Nodular Hyperplasia Difficult to Distinguish from Well-Differentiated Hepatocellular Carcinoma. Hepatol. Res. 2012, 42, 727–731. [Google Scholar] [CrossRef]
- Navarro, A.P.; Gomez, D.; Lamb, C.M. Focal Nodular Hyperplasia: A Review of Current Indications for and Outcome of Hepatic Resection. HPB 2014, 16, 503–511. [Google Scholar] [CrossRef]
- Sergi, C. Hepatocellular Carcinoma, Fibrolamellar Variant: Diagnostic Pathologic Criteria and Molecular Pathology Update. A Primer. Diagnostics 2015, 6, 3. [Google Scholar] [CrossRef]
- Dahnert, W.F. Liver, Bile Ducts, Pancreas and Spleen. In Radiology Review Manual; Lippincot Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 679–768. [Google Scholar]
- Zhang, X.; Zhou, Z. Hepatic Hemangioma Masquerading as a Tumor Originating from the Stomach. Oncol. Lett. 2015, 9, 1406–1408. [Google Scholar] [CrossRef]
- Portinari, M.; Liboni, A.; Feo, C.V. Strangulated Adenoma of the Liver: A Unique Cause of Acute Abdomen. Ann. Ital. Chir. 2014, 85, S2239253X14022439. [Google Scholar]
- Akatsu, T.; Sakamoto, M.; Shimazu, M. Pedunculated Angiomyolipoma of the Liver with a Predominant Pelioid Pattern. Virchows Arch. 2004, 444, 467–469. [Google Scholar] [CrossRef]
- Koh, C.C.; Sheu, J.C. Hepatic Lymphangioma-a Case Report. Pediatr. Surg. Int. 2000, 16, 515–516. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, Y.K.; Cho, B.H.; Moon, W.S. Pedunculated Hepatic Mass. Liver Int. 2011, 31, 541. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.N.; Lee, W.C.; Jeng, L.B. Pedunculated Hepatocellular Carcinoma: Clinicopathologic Study of 18 Surgically Resected Cases. World J. Surg. 2002, 26, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.J.; Lee, H.K.; Yu, H.C. Exophytic Combined Hepatocellular Carcinoma and Cholangiocarcinoma. Clin. Mol. Hepatol. 2012, 18, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Hayashi, K.; Mike, M. An Exophytic Hepatic Metastasis of Mucinous Colon Cancer. Jpn. J. Clin. Oncol. 2015, 45, 605–606. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Wang, J.-H.; Lu, S.-N.; Hung, C.-H.; Wei, Y.-C.; Hu, T.-H.; Chen, C.-H.; Yen, Y.-H.; Lee, C.-M.; Eng, H.-L. Natural Course of Hepatic Focal Nodular Hyperplasia: A Long-Term Follow-up Study with Sonography. J. Clin. Ultrasound 2009, 37, 132–137. [Google Scholar] [CrossRef]
- Virgilio, E.; Cavallini, M. Managing Focal Nodular Hyperplasia of the Liver: Surgery or Minimally-Invasive Approaches? A Review of the Preferable Treatment Options. Anticancer Res. 2018, 38, 33–36. [Google Scholar]
- Hedayati, P.; VanSonnenberg, E.; Shamos, R.; Gillespie, T.; McMullen, W. Treatment of Symptomatic Focal Nodular Hyperplasia with Percutaneous Radiofrequency Ablation. J. Vasc. Interv. Radiol. 2010, 21, 582–585. [Google Scholar] [CrossRef]
- Cheng, Z.; Liang, P.; Yu, X.; Han, Z.; Liu, F.; Yu, J.; Li, X. Percutaneous Microwave Ablation for Benign Focal Liver Lesions: Initial Clinical Results. Oncol. Lett. 2017, 13, 429–434. [Google Scholar] [CrossRef]
- Yao, Z.; Zeng, Q.; Yu, X.; Lin, S.; Jiang, S.; Ma, D.; Li, K. Case Report: Ultrasound-Guided Percutaneous Microwave Ablation of Focal Nodular Hyperplasia in a 9-Year-Old Girl. Front. Pediatr. 2021, 9, 710779. [Google Scholar]
- Zhang, D.-L.; Chen, S.; Lin, Y.-C.; Ye, W.; Li, K.; Wu, S.-S. Ultrasound-Guided Thermal Ablation versus Laparoscopic Surgery for Focal Nodular Hyperplasia of the Liver: A Retrospective Controlled Study. Front. Oncol. 2022, 12, 932889. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chang, J.; Zhang, D.; Lu, Q.; Wu, S.; Li, K. Ultrasound-Guided Percutaneous Thermal Ablation of Hepatic Focal Nodular Hyperplasia—A Multicenter Retrospective Study. Front. Bioeng. Biotechnol. 2022, 9, 826926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, M.; Duan, F.; Yuan, K.; Yan, J.; Chang, Z. Early- and Intermediate-Term Outcome of Transarterial Embolization for Symptomatic Hepatic Focal Nodular Hyperplasia. J. Interv. Med. 2018, 1, 86–91. [Google Scholar]
- Bozkaya, H.; Cinar, C.; Besir, F.H.; Parıldar, M.; Oran, I. Minimally Invasive Treatment of Giant Haemangiomas of the Liver: Embolisation with Bleomycin. Cardiovasc. Radiol. 2014, 37, 101–107. [Google Scholar] [CrossRef]
- Bröker, M.E.E.; Klompenhouwer, A.J.; Gaspersz, M.P.; Alleleyn, A.M.E.; Dwarkasing, R.S.; Pieters, I.C.; de Man, R.A.; IJzermans, J.N.M. Growth of Focal Nodular Hyperplasia Is Not a Reason for Surgical Intervention, but Patients Should Be Referred to a Tertiary Referral Centre. World J. Surg. 2018, 42, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Monden, K.; Iwasaki, T.; Hioki, M.; Sadamori, H.; Takakura, N. Large Focal Nodular Hyperplasia of the Liver Treated with Transcatheter Arterial Embolization and Laparoscopic Resection: A Case Report. Asian J. Endosc. Surg. 2023, 16, 579–583. [Google Scholar] [CrossRef]
- Ott, R.; Hohenberger, W. Focal nodular hyperplasia and liver cell adenoma: Operation or observation? Zentralbl. Chir. 1998, 123, 145–153. [Google Scholar]
- Hau, H.M.; Atanasov, G.; Tautenhahn, H.-M.; Ascherl, R.; Wiltberger, G.; Schoenberg, M.B.; Morgül, M.H.; Uhlmann, D.; Moche, M.; Fuchs, J.; et al. The Value of Liver Resection for Focal Nodular Hyperplasia: Resection Yes or No? Eur. J. Med. Res. 2015, 20, 86. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Li, H.; Liu, F.; Li, B.; Wei, Y. Surgical Outcomes of Laparoscopic versus Open Liver Resection for Hepatocellular Carcinoma for Various Resection Extent. Medicine 2017, 96, e6460. [Google Scholar] [CrossRef]
- Sakata, M.; Syoji, T.; Nishiyama, R.; Taniguchi, M.; Yamazaki, M.; Higashi, Y.; Suzuki, K.; Kawamura, T.; Yonekawa, H.; Maruo, H. Laparoscopic Partial Hepatectomy of Focal Nodular Hyperplasia. Case Rep. Gastroenterol. 2012, 6, 720–725. [Google Scholar] [CrossRef]
- Zarfati, A.; Chambers, G.; Pio, L.; Guerin, F.; Fouquet, V.; Franchi-Abella, S.; Branchereau, S. Management of Focal Nodular Hyperplasia of the Liver: Experience of 50 Pediatric Patients in a Tertiary Center. J. Pediatr. Surg. 2020, 55, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Ahn, J.; Reddy, R.K. ACG Clinical Guideline: The Diagnosis and Management of Focal Liver Lesions. Am. J. Gastroenterol. 2014, 109, 1328–1347. [Google Scholar] [CrossRef] [PubMed]
- EASL Clinical Practice Guidelines on the Management of Benign Liver Tumours. J. Hepatol. 2016, 65, 386–398. [CrossRef] [PubMed]
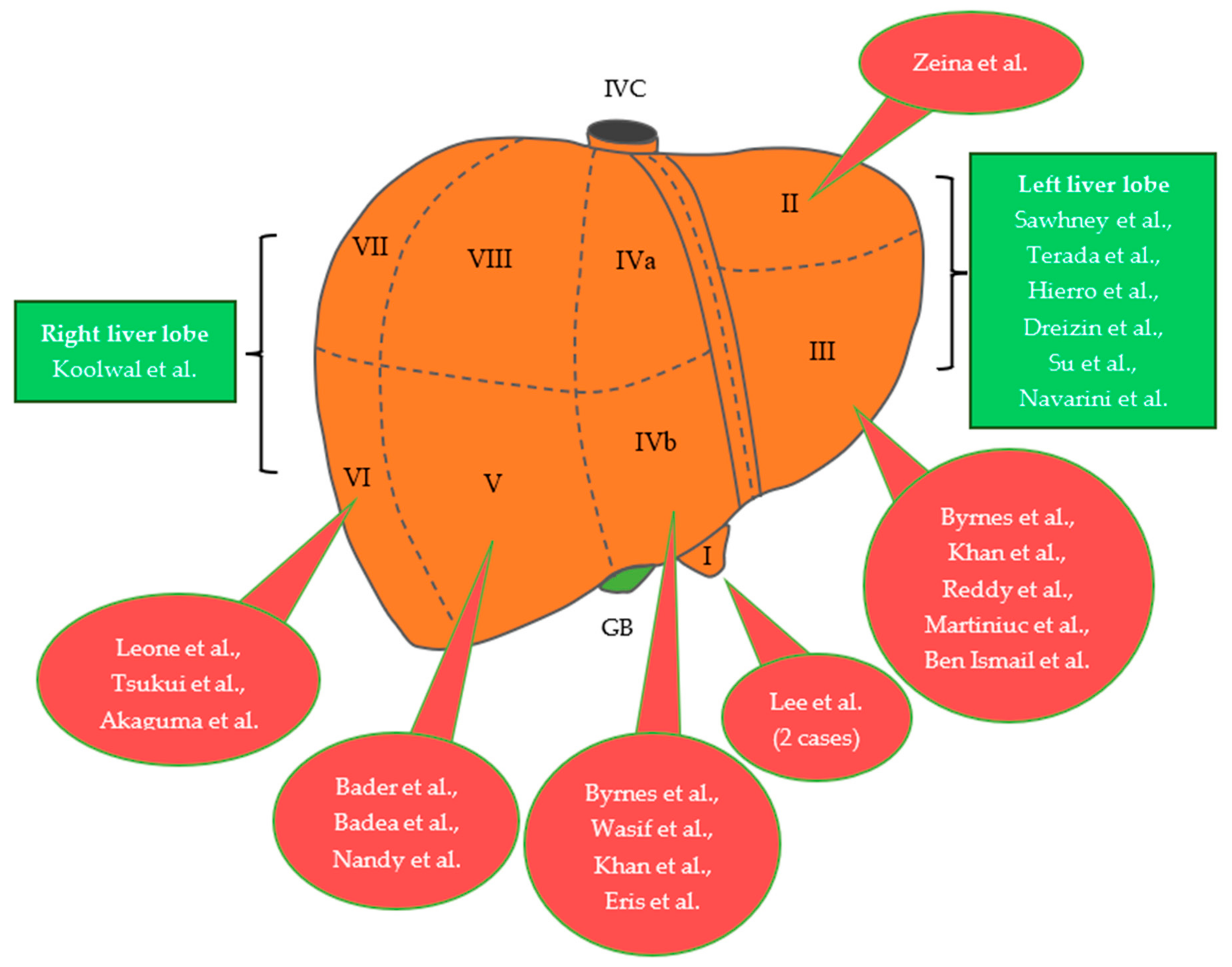
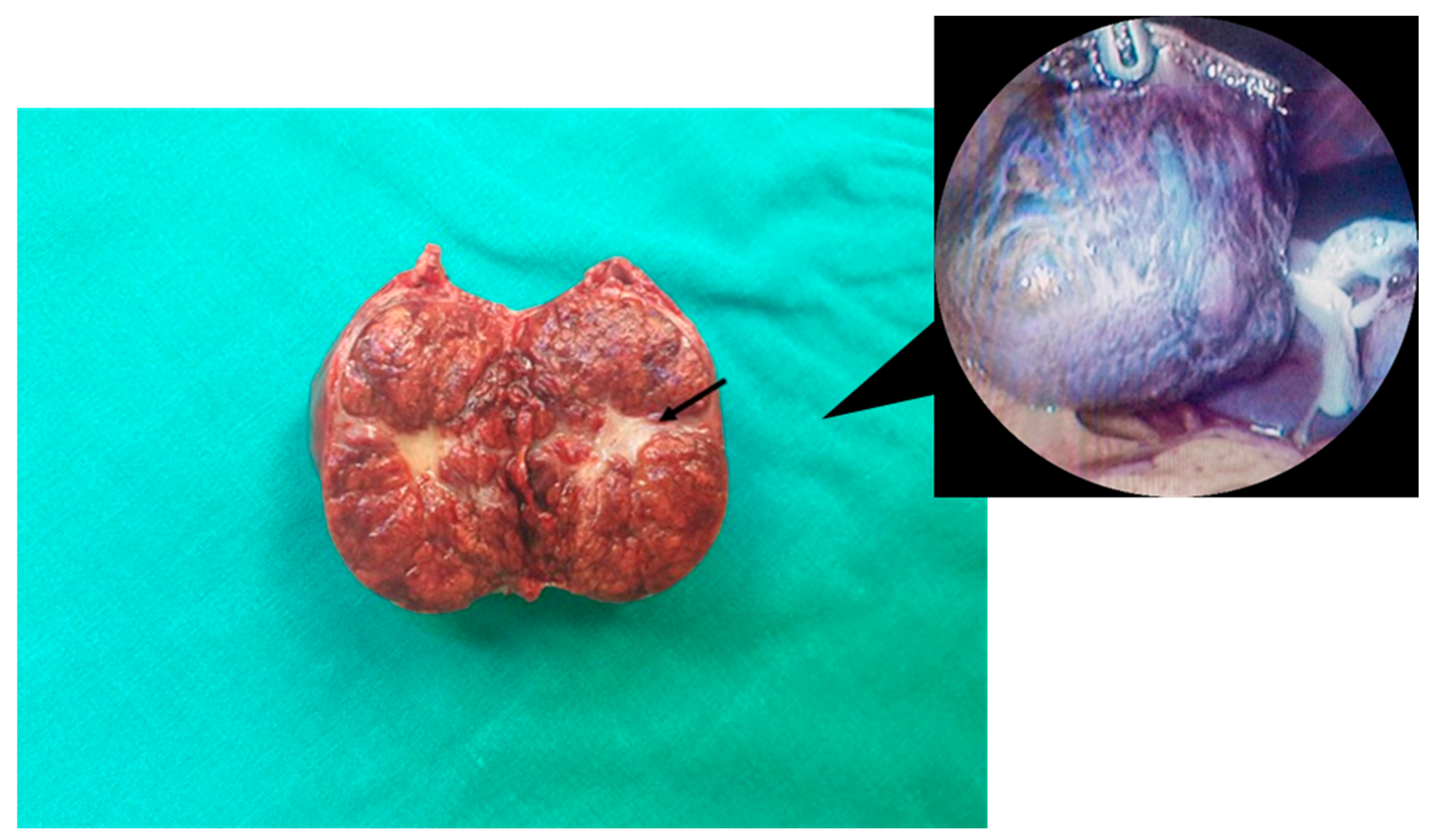
| Author, Year | Age | Gender | Size of Lesion | Symptoms | Procedure | Location |
|---|---|---|---|---|---|---|
| Bader et al., 2001 [15] | N/A | N/A | 4.5 cm in diameter | + | Surgery | V |
| Byrnes et al., 2004 [16] | 30 | Female | 16 × 18 cm, 3.5 × 3 cm | + | Open surgery | IVb, III |
| Leone et al., 2005 [17] | 61 | Female | 6 cm in diameter | − | Follow-up | VI |
| Wasif et al., 2008 [18] | 48 | Female | 3.2 × 2.4 × 2.4 cm | + | Laparoscopic Resection | IVb |
| Khan et al., 2011 [19] | 29 | Female | 10 × 10 × 10 cm, Large pedunculated mass | + | Embolization & Surgery | IVb, III |
| Terada et al., 2012 [20] | 26 | Female | 4 × 5 × 4 cm | − | Laparoscopic Resection | Left liver lobe |
| Eris et al., 2013 [13] | 23 | Female | 7.8 × 5.5 × 5.6 cm | + | Open surgery | IVb |
| Dreizin et al., 2014 [21] | 45 | Male | Pedunculated mass | + | Laparoscopic Resection | Left liver lobe |
| Badea et al., 2015 [22] | 29 | Female | 8 × 5 cm | + | Laparoscopic Resection | V |
| Reddy et al., 2015 [23] | 35 | Female | 2.89 cm × 2.7 cm | + | Laparoscopic Resection | III |
| Zeina et al., 2016 [24] | 25 | Female | 4.8 cm in diameter | + | N/A | II |
| Tsukui et al., 2017 [25] | 39 | Male | 3.9 × 3 × 3.7 cm | − | Laparoscopic Resection | VI |
| Martiniuc et al., 2018 [26] | 40 | Female | 4.2 × 5 cm | − | Open surgery (Mini- laparotomy) | III |
| Su et al., 2020 [27] | 33 | Female | 8.3 × 5.9 × 5.6 cm | − | Open surgery | Left liver lobe |
| Navarini et al., 2020 [28] | 26 | Female | 13 × 7 cm | + | Open surgery | Left liver lobe |
| Ben Ismail et al., 2021 [29] | 38 | Female | 4.5 × 3.5 × 3 cm | − | Open surgery | III |
| Nandy et al., 2023 [30] | 40 | Female | 8.2 × 14.6 × 16 cm | + | TAE & Surgery | V |
| Akaguma et al., 2023 [31] | 35 | Female | 7 × 7 × 5 cm | +/− | Laparoscopic Resection | VI |
| Author, Year | Age | Gender | Size of Lesion | Symptoms | Procedure | Location |
|---|---|---|---|---|---|---|
| Sawhney et al., 1992 [32] | 12 | Female | Pedunculated mass | − | Surgery | Left liver lobe |
| Hierro et al., 2013 [33] | 13 | Female | 10 × 9 × 8 cm | + | Open surgery | Left liver lobe |
| Lee et al., 2014 [34] | 7 | Male | 4.8 × 3 × 3.6 cm | + | Open surgery | I |
| 13 | Female | 3.1 × 4.1 × 5.5 cm | + | Open surgery | I | |
| Koolwal et al., 2018 [35] | 3 | Female | 5.9 cm in diameter | + | Surgery | Right liver lobe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsalikidis, C.; Mitsala, A.; Pappas-Gogos, G.; Romanidis, K.; Tsaroucha, A.K.; Pitiakoudis, M. Pedunculated Focal Nodular Hyperplasia: When in Doubt, Should We Cut It Out? J. Clin. Med. 2023, 12, 6034. https://doi.org/10.3390/jcm12186034
Tsalikidis C, Mitsala A, Pappas-Gogos G, Romanidis K, Tsaroucha AK, Pitiakoudis M. Pedunculated Focal Nodular Hyperplasia: When in Doubt, Should We Cut It Out? Journal of Clinical Medicine. 2023; 12(18):6034. https://doi.org/10.3390/jcm12186034
Chicago/Turabian StyleTsalikidis, Christos, Athanasia Mitsala, George Pappas-Gogos, Konstantinos Romanidis, Alexandra K. Tsaroucha, and Michail Pitiakoudis. 2023. "Pedunculated Focal Nodular Hyperplasia: When in Doubt, Should We Cut It Out?" Journal of Clinical Medicine 12, no. 18: 6034. https://doi.org/10.3390/jcm12186034
APA StyleTsalikidis, C., Mitsala, A., Pappas-Gogos, G., Romanidis, K., Tsaroucha, A. K., & Pitiakoudis, M. (2023). Pedunculated Focal Nodular Hyperplasia: When in Doubt, Should We Cut It Out? Journal of Clinical Medicine, 12(18), 6034. https://doi.org/10.3390/jcm12186034






