Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios
Abstract
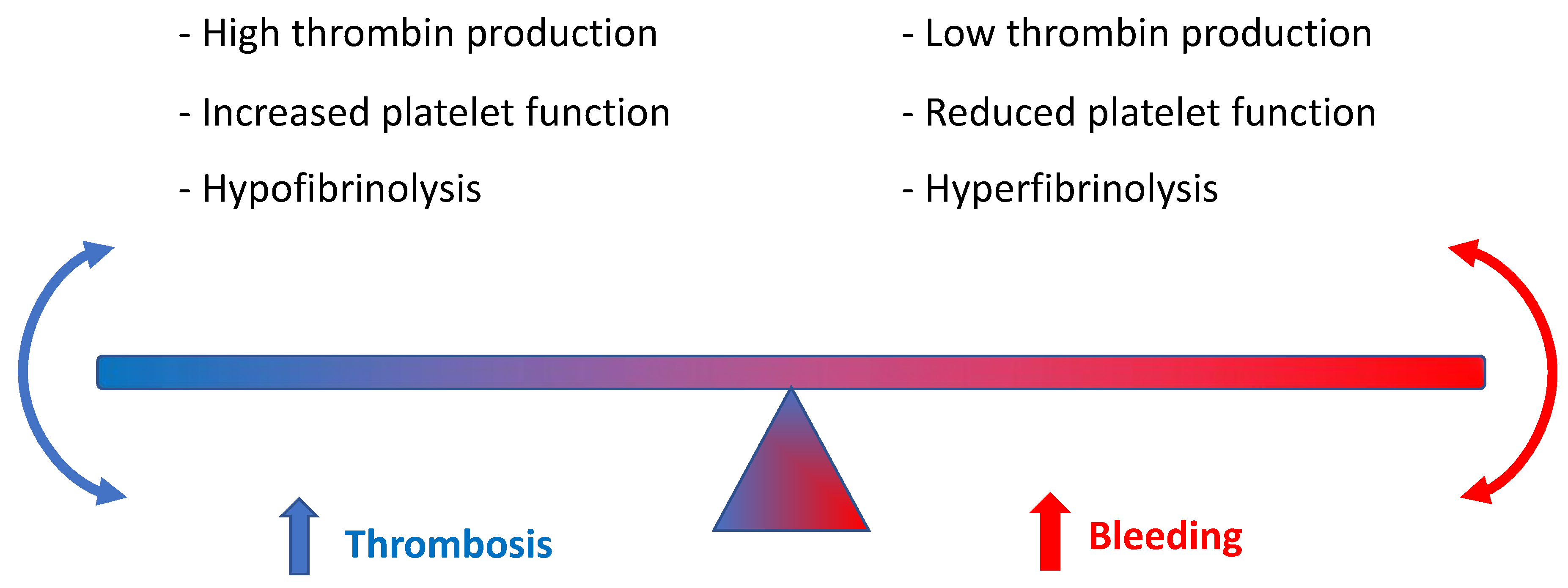
1. Introduction
2. Methods
3. Drug–Drug Interactions (DDIs) of DOACs and Polypharmacy
4. How to Switch between Different Anticoagulants
5. DOACs in Patients with Advanced Chronic Liver Disease (CLD)
6. DOACs Management in the Preoperative and Postoperative Setting
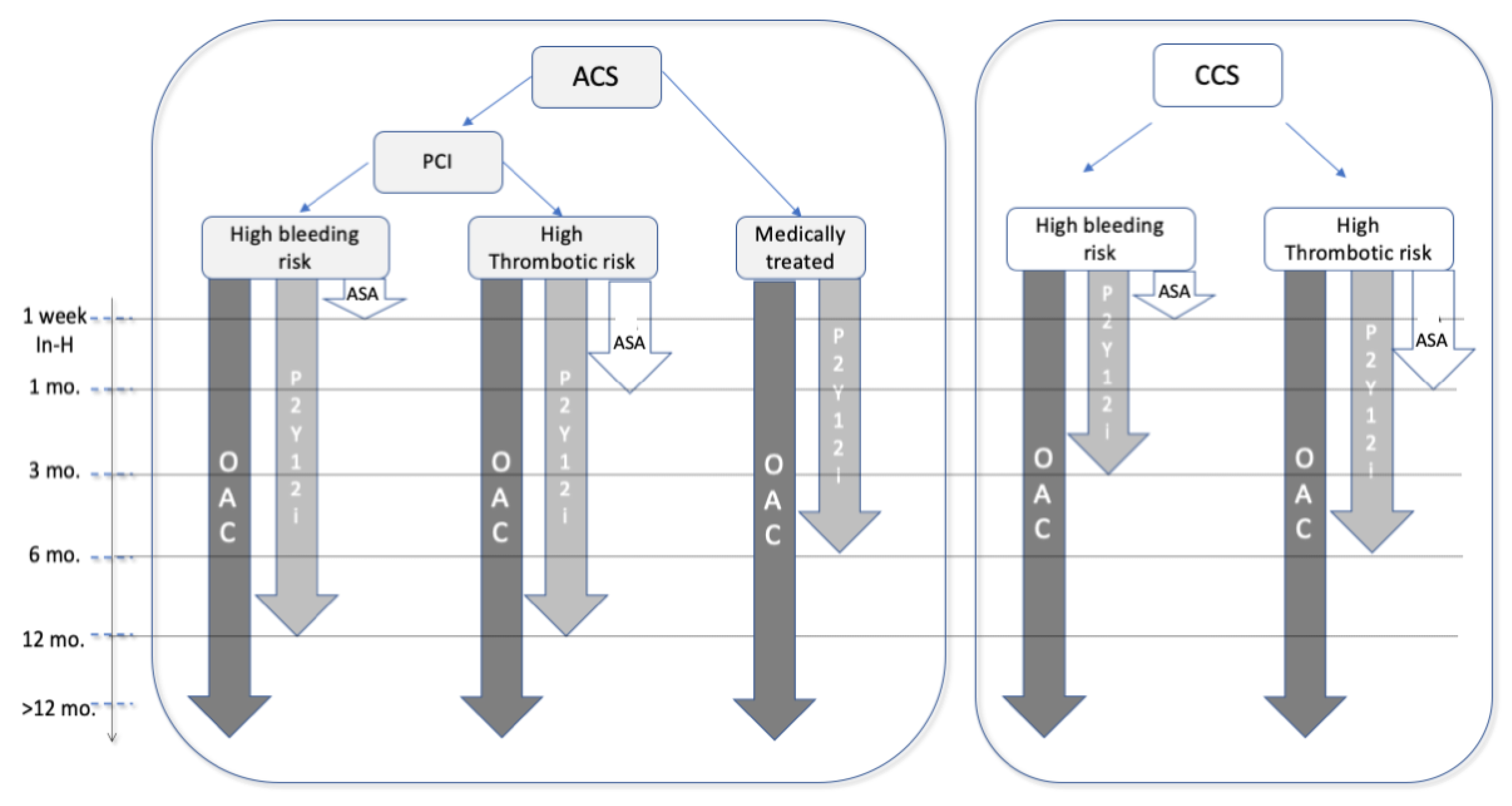
Anticoagulant and Antiplatelet Combined Therapy
7. Elective and Urgent PCI
8. Pacemaker and Implantable Cardioverter-Defibrillator (ICD) Implantation
9. Catheter Ablation of Atrial Fibrillation (CAAF)
10. AF Patients Undergoing Non-Cardiac Surgery
11. DOACs in Patients with Atrial Fibrillation and Malignancy
12. DOACs in the Elderly Population
13. DOACSs and Frailty
14. DOACs in Under and Overweight Patients
15. Adherence to Oral Anticoagulant Intake
16. Differences between VKAs and DOACs
17. Differences in Adherence between Single DOACs
18. Patient Characteristics Associated with Adherence and Persistence to OACs
19. Age
20. Gender
21. Socioeconomic Status and Education
22. Comorbidities
23. Previous Thrombosis and Bleeding
24. Psychological Factors
25. Polypharmacy
26. Frequency of Administration
27. Factors Related to the Health System
28. Consequences of Non-Adherence and Non-Persistence
29. How to Improve OAC Adherence and Persistence in Patients
30. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aronis, K.N.; Hylek, E.M. Evidence gaps in the era of Non–Vitamin K oral anticoagulants. J. Am. Heart Assoc. 2018, 7, e007338. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.K.; Bar, B. Direct oral anticoagulant agents: Pharmacologic profile, indications, coagulation monitoring, and reversal agents. J. Stroke Cerebrovasc. Dis. 2018, 27, 2049–2058. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Kapoor, A.; Ellis, A.; Shaffer, N.; Gurwitz, J.; Chandramohan, A.; Saulino, J.; Ishak, A.; Okubanjo, T.; Michota, F.; Hylek, E. Comparative effectiveness of venous thromboembolism prophylaxis options for the patient undergoing total hip and knee replacement: A network meta-analysis. J. Thromb. Haemost. 2017, 15, 284–294. [Google Scholar] [CrossRef]
- Salmasi, S.; Loewen, P.S.; Tandun, R.; Andrade, J.G.; De Vera, M.A. Adherence to oral anticoagulants among patients with atrial fibrillation: A systematic review and meta-analysis of observational studies. BMJ Open 2020, 10, e034778. [Google Scholar] [CrossRef]
- Mar, P.L.; Gopinathannair, R.; Gengler, B.E.; Chung, M.K.; Perez, A.; Dukes, J.; Ezekowitz, M.D.; Lakkireddy, D.; Lip, G.Y.H.; Miletello, M.; et al. Drug Interactions Affecting Oral Anticoagulant Use. Circ. Arrhythm. Electrophysiol. 2022, 15, e007956. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Stecker, E.; Warden, B.A. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J. Am. Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef]
- Wiggins, B.S.; Dixon, D.L.; Neyens, R.R.; Page, R.L., 2nd; Gluckman, T.J. Select Drug-Drug Interactions With Direct Oral Anticoagulants: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 1341–1350. [Google Scholar] [CrossRef]
- Burnett, A.E.; Mahan, C.E.; Vazquez, S.R.; Oertel, L.B.; Garcia, D.A.; Ansell, J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J. Thromb. Thrombolysis 2016, 41, 206–232. [Google Scholar] [CrossRef]
- Vazquez, S.R. Drug-drug interactions in an era of multiple anticoagulants: A focus on clinically relevant drug interactions. Blood 2018, 132, 2230–2239. [Google Scholar] [CrossRef]
- Mueck, W.; Kubitza, D.; Becka, M. Co-administration of rivaroxaban with drugs that share its elimination pathways: Pharmacokinetic effects in healthy subjects. Br. J. Clin. Pharmacol. 2013, 76, 455–466. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; Camm, A.J.; Kirchhof, P. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013, 15, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Nobili, A.; Marengoni, A.; Tettamanti, M.; Salerno, F.; Pasina, L.; Franchi, C.; Iorio, A.; Marcucci, M.; Corrao, S.; Licata, G.; et al. Association between clusters of diseases and polypharmacy in hospitalized elderly patients: Results from the REPOSI study. Eur. J. Intern. Med. 2011, 22, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Raparelli, V.; Olshansky, B.; Lip, G.Y. Polypharmacy and major adverse events in atrial fibrillation: Observations from the AFFIRM trial. Clin. Res. Cardiol. 2016, 105, 412–420. [Google Scholar] [CrossRef]
- Leiss, W.; Méan, M.; Limacher, A.; Righini, M.; Jaeger, K.; Beer, H.J.; Osterwalder, J.; Frauchiger, B.; Matter, C.M.; Kucher, N.; et al. Polypharmacy is associated with an increased risk of bleeding in elderly patients with venous thromboembolism. J. Gen. Intern. Med. 2015, 30, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Herink, M.C.; Zhuo, Y.F.; Williams, C.D.; DeLoughery, T.G. Clinical Management of Pharmacokinetic Drug Interactions with Direct Oral Anticoagulants (DOACs). Drugs 2019, 79, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Foerster, K.I.; Hermann, S.; Mikus, G.; Haefeli, W.E. Drug-Drug Interactions with Direct Oral Anticoagulants. Clin. Pharmacokinet. 2020, 59, 967–980. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Kanuri, S.H.; Kreutz, R.P. Pharmacogenomics of Novel Direct Oral Anticoagulants: Newly Identified Genes and Genetic Variants. J. Pers. Med. 2019, 9, 7. [Google Scholar] [CrossRef]
- Barrett, A.; Moore, M.; Ferrins, P.; Thornton, P.; Murphy, P.; Quinn, J. From a direct oral anticoagulant to warfarin: Reasons why patients switch. Ir. J. Med. Sci. 2018, 187, 719–721. [Google Scholar] [CrossRef]
- Steffel, J.; Heidbüchel, H. 2021 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: Comment-Authors’ reply. Europace 2021, 23, 1685–1686. [Google Scholar] [CrossRef]
- Keller, C.; Katz, R.; Cushman, M.; Fried, L.F.; Shlipak, M. Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: A cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Nephrol. 2008, 9, 9. [Google Scholar] [CrossRef]
- Iseki, K.; Kinjo, K.; Kimura, Y.; Osawa, A.; Fukiyama, K. Evidence for high risk of cerebral hemorrhage in chronic dialysis patients. Kidney Int. 1993, 44, 1086–1090. [Google Scholar] [CrossRef]
- Sood, P.; Kumar, G.; Nanchal, R.; Sakhuja, A.; Ahmad, S.; Ali, M.; Kumar, N.; Ross, E.A. Chronic kidney disease and end-stage renal disease predict higher risk of mortality in patients with primary upper gastrointestinal bleeding. Am. J. Nephrol. 2012, 35, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Giubilato, S.; Di Fusco, S.A.; Piccioni, L.; Rao, C.M.; Iorio, A.; Cipolletta, L.; D’Elia, E.; Gelsomino, S.; Rossini, R.; et al. Anticoagulation in Atrial Fibrillation Cardioversion: What Is Crucial to Take into Account. J. Clin. Med. 2021, 10, 3212. [Google Scholar] [CrossRef]
- Aursulesei, V.; Costache, I.I. Anticoagulation in chronic kidney disease: From guidelines to clinical practice. Clin. Cardiol. 2019, 42, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, F.; Cooper, C.; Petrescu, I.; George, J.; Mansuri, S. Safety of apixaban compared to warfarin in hemodialysis patients: Do antiplatelets make a difference? Eur. J. Haematol. 2021, 106, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, M.I.; Ardeshirrouhanifard, S.; Segal, J.B.; Streiff, M.B.; Deitelzweig, S.B.; Brotman, D.J. Safety and effectiveness of apixaban versus warfarin for acute venous thromboembolism in patients with end-stage kidney disease: A national cohort study. J. Hosp. Med. 2022, 17, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.; Sood, N.; Bunz, T.J.; Coleman, C.I. Rivaroxaban versus apixaban in non-valvular atrial fibrillation patients with end-stage renal disease or receiving dialysis. Eur. J. Haematol. 2020, 104, 328–335. [Google Scholar] [CrossRef]
- Kumar, S.; Lim, E.; Covic, A.; Verhamme, P.; Gale, C.P.; Camm, A.J.; Goldsmith, D. Anticoagulation in Concomitant Chronic Kidney Disease and Atrial Fibrillation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Chantrarat, T.; Hauythan, S. The change of renal functions after nonvitamin K oral anticoagulants in patients with atrial fibrillation. Int. J. Cardiol. Heart Vasc. 2021, 35, 100844. [Google Scholar] [CrossRef] [PubMed]
- Kujovich, J.L. Coagulopathy in liver disease: A balancing act. Hematology 2015, 2015, 243–249. [Google Scholar] [CrossRef]
- Amitrano, L.; Guardascione, M.A.; Brancaccio, V.; Balzano, A. Coagulation disorders in liver disease. Semin. Liver Dis. 2002, 22, 83–96. [Google Scholar] [CrossRef]
- Northup, P.G.; Caldwell, S.H. Coagulation in Liver Disease: A Guide for the Clinician. Clin. Gastroenterol. Hepatol. 2013, 11, 1064–1074. [Google Scholar] [CrossRef]
- Northup, P.G.; McMahon, M.M.; Ruhl, A.P.; Altschuler, S.E.; Volk-Bednarz, A.; Caldwell, S.H.; Berg, C.L. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am. J. Gastroenterol. 2006, 101, 1524–1528, quiz 1680. [Google Scholar] [CrossRef]
- Dabbagh, O.; Oza, A.; Prakash, S.; Sunna, R.; Saettele, T.M. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest 2010, 137, 1145–1149. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Chang, W.H.; Mueller, S.H.; Tan, Y.Y.; Lai, A.G. Antithrombotic therapy in patients with liver disease: Population-based insights on variations in prescribing trends, adherence, persistence and impact on stroke and bleeding. Lancet Reg. Health Eur. 2021, 10, 100222. [Google Scholar] [CrossRef]
- Graff, J.; Harder, S. Anticoagulant therapy with the oral direct factor Xa inhibitors rivaroxaban, apixaban and edoxaban and the thrombin inhibitor dabigatran etexilate in patients with hepatic impairment. Clin. Pharmacokinet. 2013, 52, 243–254. [Google Scholar] [CrossRef]
- Qamar, A.; Vaduganathan, M.; Greenberger, N.J.; Giugliano, R.P. Oral Anticoagulation in Patients With Liver Disease. J. Am. Coll. Cardiol. 2018, 71, 2162–2175. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.D.; Aronow, H.D.; Shobayo, F.; Hume, A.L.; Taveira, T.H.; Matson, K.L.; Zhang, Y.; Wen, X. Comparative Effectiveness and Safety of Direct Oral Anticoagulants and Warfarin in Patients With Atrial Fibrillation and Chronic Liver Disease: A Nationwide Cohort Study. Circulation 2023, 147, 782–794. [Google Scholar] [CrossRef]
- Božič-Mijovski, M.; Malmström, R.E.; Malovrh, P.; Antovic, J.P.; Vene, N.; Šinigoj, P.; Mavri, A. Diluted thrombin time reliably measures low to intermediate plasma dabigatran concentrations. Ann. Clin. Biochem. 2016, 53, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.; Nowak, G.; Bucha, E. Ecarin chromogenic assay--a new method for quantitative determination of direct thrombin inhibitors like hirudin. Pathophysiol. Haemost. Thromb. 2003, 33, 184–191. [Google Scholar] [CrossRef]
- Beyer, J.; Trujillo, T.; Fisher, S.; Ko, A.; Lind, S.E.; Kiser, T.H. Evaluation of a Heparin-Calibrated Antifactor Xa Assay for Measuring the Anticoagulant Effect of Oral Direct Xa Inhibitors. Clin. Appl. Thromb. Hemost. 2016, 22, 423–428. [Google Scholar] [CrossRef]
- Connolly, S.J.; Crowther, M.; Eikelboom, J.W.; Gibson, C.M.; Curnutte, J.T.; Lawrence, J.H.; Yue, P.; Bronson, M.D.; Lu, G.; Conley, P.B.; et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N. Engl. J. Med. 2019, 380, 1326–1335. [Google Scholar] [CrossRef]
- Gottlieb, M.; Khishfe, B. Idarucizumab for the Reversal of Dabigatran. Ann. Emerg. Med. 2017, 69, 554–558. [Google Scholar] [CrossRef]
- Lucà, F.; Giubilato, S.; Fusco, S.A.D.; Leone, A.; Poli, S.; Rao, C.M.; Iorio, A.; Gelsomino, S.; Gabrielli, D.; Colivicchi, F.; et al. The Combination of Oral Anticoagulant and Antiplatelet Therapies: Stay One Step Ahead. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with atrial fibrillation and coronary artery disease–double trouble. Adv. Med. Sci. 2018, 63, 30–35. [Google Scholar] [CrossRef]
- Capodanno, D.; Di Maio, M.; Greco, A.; Bhatt, D.L.; Gibson, C.M.; Goette, A.; Lopes, R.D.; Mehran, R.; Vranckx, P.; Angiolillo, D.J. Safety and Efficacy of Double Antithrombotic Therapy with Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e017212. [Google Scholar] [CrossRef]
- Capodanno, D.; Angiolillo, D.J. Management of antiplatelet and anticoagulant therapy in patients with atrial fibrillation in the setting of acute coronary syndromes or percutaneous coronary interventions. Circ. Cardiovasc. Interv. 2014, 7, 113–124. [Google Scholar] [CrossRef]
- Capodanno, D.; Huber, K.; Mehran, R.; Lip, G.Y.; Faxon, D.P.; Granger, C.B.; Vranckx, P.; Lopes, R.D.; Montalescot, G.; Cannon, C.P. Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 74, 83–99. [Google Scholar] [CrossRef]
- Capodanno, D.; Alfonso, F.; Levine, G.N.; Valgimigli, M.; Angiolillo, D.J. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J. Am. Coll. Cardiol. 2018, 72, 2915–2931. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Caretta, G.; Vagnarelli, F.; Marini, M.; Iorio, A.; Di Fusco, S.A.; Pozzi, A.; Gabrielli, D.; Colivicchi, F.; De Luca, L.; et al. Clinical characteristics, management and outcomes of patients with acute coronary syndrome and atrial fibrillation: Real-world data from two nationwide registries in Italy. J. Cardiovasc Med. (Hagerstown) 2020, 21, 99–105. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Rubboli, A.; Bolognese, L.; Gonzini, L.; Urbinati, S.; Murrone, A.; Scotto di Uccio, F.; Ferrari, F.; Lucà, F.; Caldarola, P.; et al. Antithrombotic management of patients with acute coronary syndrome and atrial fibrillation undergoing coronary stenting: A prospective, observational, nationwide study. BMJ Open 2020, 10, e041044. [Google Scholar] [CrossRef]
- Buccheri, S.; Capodanno, D.; James, S.; Angiolillo, D.J. Bleeding after antiplatelet therapy for the treatment of acute coronary syndromes: A review of the evidence and evolving paradigms. Expert. Opin. Drug Saf. 2019, 18, 1171–1189. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: A consensus document from the Academic Research Consortium for High Bleeding Risk. Circulation 2019, 140, 240–261. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Collet, J.P.; Haude, M.; Byrne, R.; Chung, E.H.; Fauchier, L.; Halvorsen, S.; Lau, D.; Lopez-Cabanillas, N.; Lettino, M.; et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: A joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2019, 21, 192–193. [Google Scholar] [CrossRef]
- Potpara, T.S.; Mujovic, N.; Proietti, M.; Dagres, N.; Hindricks, G.; Collet, J.P.; Valgimigli, M.; Heidbuchel, H.; Lip, G.Y.H. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: Meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials. Europace 2020, 22, 33–46. [Google Scholar] [CrossRef]
- Gargiulo, G.; Goette, A.; Tijssen, J.; Eckardt, L.; Lewalter, T.; Vranckx, P.; Valgimigli, M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: A systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur. Heart J. 2019, 40, 3757–3767. [Google Scholar] [CrossRef]
- Gibson, C.M.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; Van Eickels, M. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N. Engl. J. Med. 2016, 375, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N. Engl. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef]
- Vranckx, P.; Valgimigli, M.; Eckardt, L.; Tijssen, J.; Lewalter, T.; Gargiulo, G.; Batushkin, V.; Campo, G.; Lysak, Z.; Vakaliuk, I. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet 2019, 394, 1335–1343. [Google Scholar] [CrossRef]
- Dewilde, W.J.; Oirbans, T.; Verheugt, F.W.; Kelder, J.C.; De Smet, B.J.; Herrman, J.P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar] [CrossRef]
- Colleran, R.; Byrne, R.A.; Ndrepepa, G.; Alvarez-Covarrubias, H.A.; Mayer, K.; Kuna, C.; Rai, H.; Kastrati, A.; Cassese, S. Antithrombotic Therapy with or without Aspirin after Percutaneous Coronary Intervention or Acute Coronary Syndrome in Patients Taking Oral Anticoagulation: A Meta-Analysis and Network Analysis of Randomized Controlled Trials. Cardiovasc. Revasc Med. 2022, 36, 99–106. [Google Scholar] [CrossRef]
- Vranckx, P.; Verheugt, F.W.; de Maat, M.P.; Ulmans, V.A.; Regar, E.; Smits, P.; ten Berg, J.M.; Lindeboom, W.; Jones, R.L.; Friedman, J.; et al. A randomised study of dabigatran in elective percutaneous coronary intervention in stable coronary artery disease patients. EuroIntervention 2013, 8, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Bálint, A.; Kupó, P.; Tornyos, D.; El Alaoui El Abdallaoui, O.; Jánosi, A.; Komócsi, A. Oral anticoagulation and outcomes in patients with acute myocardial infarction: Insights from the Hungarian Myocardial Infarction Registry. Int. J. Clin. Pract. 2021, 75, e14179. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, S.A.; Lucà, F.; Gulizia, M.M.; Gabrielli, D.; Colivicchi, F. Emerging clinical setting of direct oral anticoagulants: Atherothrombotic events prevention. J. Cardiovasc Med. (Hagerstown) 2020, 21, 1–5. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Oldgren, J.; Yang, S.; Wallentin, L.; Ezekowitz, M.; Reilly, P.; Eikelboom, J.; Brueckmann, M.; Yusuf, S.; Connolly, S.J. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation 2012, 125, 669–676. [Google Scholar] [CrossRef]
- Kupó, P.; Szakács, Z.; Solymár, M.; Habon, T.; Czopf, L.; Hategan, L.; Csányi, B.; Borbás, J.; Tringer, A.; Varga, G.; et al. Direct Anticoagulants and Risk of Myocardial Infarction, a Multiple Treatment Network Meta-Analysis. Angiology 2020, 71, 27–37. [Google Scholar] [CrossRef]
- Essebag, V.; Verma, A.; Healey, J.S.; Krahn, A.D.; Kalfon, E.; Coutu, B.; Ayala-Paredes, F.; Tang, A.S.; Sapp, J.; Sturmer, M.; et al. Clinically Significant Pocket Hematoma Increases Long-Term Risk of Device Infection: BRUISE CONTROL INFECTION Study. J. Am. Coll. Cardiol. 2016, 67, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.H.; Healey, J.S.; Wells, G.A.; Ayala-Paredes, F.; Coutu, B.; Sumner, G.L.; Becker, G.; Verma, A.; Philippon, F.; Kalfon, E.; et al. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur. Heart J. 2018, 39, 3973–3979. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Martin, A.C.; Weizman, O.; Sellal, J.M.; Algalarrondo, V.; Amara, W.; Bouzeman, A.; Gandjbakhch, E.; Lellouche, N.; Louembe, J.; Menet, A.; et al. Impact of peri-procedural management of direct oral anticoagulants on pocket haematoma after cardiac electronic device implantation: The StimAOD multicentre prospective study. Europace 2023, 25, euad057. [Google Scholar] [CrossRef]
- Mendoza, P.A.; Narula, S.; McIntyre, W.F.; Whitlock, R.P.; Birnie, D.H.; Healey, J.S.; Belley-Côté, E.P. Continued versus interrupted direct oral anticoagulation for cardiac electronic device implantation: A systematic review. Pacing Clin. Electrophysiol. 2020, 43, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Gelsomino, S.; La Meir, M.; Lucà, F.; Lorusso, R.; Crudeli, E.; Vasquez, L.; Gensini, G.F.; Maessen, J. Treatment of lone atrial fibrillation: A look at the past, a view of the present and a glance at the future. Eur. J. Cardiothorac. Surg. 2012, 41, 1284–1294. [Google Scholar] [CrossRef]
- Bohnen, M.; Stevenson, W.G.; Tedrow, U.B.; Michaud, G.F.; John, R.M.; Epstein, L.M.; Albert, C.M.; Koplan, B.A. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm. 2011, 8, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Cappato, R.; Ezekowitz, M.D.; Klein, A.L.; Camm, A.J.; Ma, C.S.; Le Heuzey, J.Y.; Talajic, M.; Scanavacca, M.; Vardas, P.E.; Kirchhof, P.; et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur. Heart J. 2014, 35, 3346–3355. [Google Scholar] [CrossRef] [PubMed]
- Kupo, P.; Riesz, T.J.; Saghy, L.; Vamos, M.; Bencsik, G.; Makai, A.; Kohari, M.; Benak, A.; Miklos, M.; Pap, R. Ultrasound guidance for femoral venous access in patients undergoing pulmonary vein isolation: A quasi-randomized study. J. Cardiovasc. Electrophysiol. 2023, 34, 1177–1182. [Google Scholar] [CrossRef]
- Kupó, P.; Pap, R.; Sághy, L.; Tényi, D.; Bálint, A.; Debreceni, D.; Basu-Ray, I.; Komócsi, A. Ultrasound guidance for femoral venous access in electrophysiology procedures-systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2020, 59, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef]
- Kusano, K.; Yamane, T.; Inoue, K.; Takegami, M.; Nakao, Y.M.; Miyamoto, Y.; Shoda, M.; Nogami, A. The Japanese Catheter Ablation Registry (J-AB): A prospective nationwide multicenter registry in Japan. Annual report in 2018. J. Arrhythm. 2020, 36, 953–961. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Santangeli, P.; Mohanty, P.; Sanchez, J.E.; Horton, R.; Gallinghouse, G.J.; Themistoclakis, S.; Rossillo, A.; Lakkireddy, D.; et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: Results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014, 129, 2638–2644. [Google Scholar] [CrossRef]
- Kuwahara, T.; Abe, M.; Yamaki, M.; Fujieda, H.; Abe, Y.; Hashimoto, K.; Ishiba, M.; Sakai, H.; Hishikari, K.; Takigawa, M.; et al. Apixaban versus Warfarin for the Prevention of Periprocedural Cerebral Thromboembolism in Atrial Fibrillation Ablation: Multicenter Prospective Randomized Study. J. Cardiovasc. Electrophysiol. 2016, 27, 549–554. [Google Scholar] [CrossRef]
- Løfgren, B.; Pareek, M.; Larsen, J.M. Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation. N. Engl. J. Med. 2017, 377, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kashimura, S.; Nishiyama, T.; Katsumata, Y.; Inagawa, K.; Ikegami, Y.; Nishiyama, N.; Fukumoto, K.; Tanimoto, Y.; Aizawa, Y.; et al. Asymptomatic Cerebral Infarction During Catheter Ablation for Atrial Fibrillation: Comparing Uninterrupted Rivaroxaban and Warfarin (ASCERTAIN). JACC Clin. Electrophysiol. 2018, 4, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.R.; Allison, J.S.; Natale, A.; Weisberg, I.L.; Ellenbogen, K.A.; Richards, M.; Hsieh, W.H.; Sutherland, J.; Cannon, C.P. A Prospective Randomized Trial of Apixaban Dosing During Atrial Fibrillation Ablation: The AEIOU Trial. JACC Clin. Electrophysiol. 2018, 4, 580–588. [Google Scholar] [CrossRef]
- Kirchhof, P.; Haeusler, K.G.; Blank, B.; De Bono, J.; Callans, D.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Gentlesk, P.; Grimaldi, M.; et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur. Heart J. 2018, 39, 2942–2955. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Camm, A.J.; Cappato, R.; Diener, H.C.; Heidbüchel, H.; Mont, L.; Morillo, C.A.; Lanz, H.J.; Rauer, H.; Reimitz, P.E.; et al. Periprocedural anticoagulation in the uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation (ELIMINATE-AF) trial. Europace 2021, 23, 65–72. [Google Scholar] [CrossRef]
- Cappato, R.; Marchlinski, F.E.; Hohnloser, S.H.; Naccarelli, G.V.; Xiang, J.; Wilber, D.J.; Ma, C.S.; Hess, S.; Wells, D.S.; Juang, G.; et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur. Heart J. 2015, 36, 1805–1811. [Google Scholar] [CrossRef]
- Di Biase, L.; Callans, D.; Hæusler, K.G.; Hindricks, G.; Al-Khalidi, H.; Mont, L.; Cosedis Nielsen, J.; Piccini, J.P.; Schotten, U.; Kirchhof, P. Rationale and design of AXAFA-AFNET 5: An investigator-initiated, randomized, open, blinded outcome assessment, multi-centre trial to comparing continuous apixaban to vitamin K antagonists in patients undergoing atrial fibrillation catheter ablation. Europace 2017, 19, 132–138. [Google Scholar] [CrossRef]
- Basu-Ray, I.; Khanra, D.; Kupó, P.; Bunch, J.; Theus, S.A.; Mukherjee, A.; Shah, S.K.; Komócsi, A.; Adeboye, A.; Jefferies, J. Outcomes of uninterrupted vs interrupted Periprocedural direct oral Anticoagulants in atrial Fibrillation ablation: A meta-analysis. J. Arrhythm. 2021, 37, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Gerstenfeld, E.P.; Schilling, R.; Verma, A.; Willems, S. RE-CIRCUIT study-randomized evaluation of Dabigatran etexilate compared to warfarin in pulmonary vein ablation: Assessment of an uninterrupted periprocedural anticoagulation strategy. Am. J. Cardiol. 2015, 115, 154–155. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Camm, J.; Cappato, R.; Diener, H.C.; Heidbüchel, H.; Mont, L.; Morillo, C.A.; Abozguia, K.; Grimaldi, M.; Rauer, H.; et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: The ELIMINATE-AF trial. Eur. Heart J. 2019, 40, 3013–3021. [Google Scholar] [CrossRef]
- Nogami, A.; Soejima, K.; Morishima, I.; Hiroshima, K.; Kato, R.; Sakagami, S.; Miura, F.; Okawa, K.; Kimura, T.; Inoue, T.; et al. Real-World Investigation on Anticoagulation Management Before and After Catheter Ablation for Atrial Fibrillation in Japan—Periprocedural and Long-Term Outcomes. Circ. J. 2022, 87, 50–62. [Google Scholar] [CrossRef]
- Akagi, Y.; Iketaki, A.; Nakamura, R.; Yamamura, S.; Endo, M.; Morikawa, K.; Oikawa, S.; Ohta, T.; Tatsumi, S.; Suzuki, T.; et al. Association between Cerebral Infarction Risk and Medication Adherence in Atrial Fibrillation Patients Taking Direct Oral Anticoagulants. Healthcare 2021, 9, 1313. [Google Scholar] [CrossRef]
- Moula, A.I.; Parrini, I.; Tetta, C.; Lucà, F.; Parise, G.; Rao, C.M.; Mauro, E.; Parise, O.; Matteucci, F.; Gulizia, M.M.; et al. Obstructive Sleep Apnea and Atrial Fibrillation. J. Clin. Med. 2022, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Douketis, J.D.; Spyropoulos, A.C.; Murad, M.H.; Arcelus, J.I.; Dager, W.E.; Dunn, A.S.; Fargo, R.A.; Levy, J.H.; Samama, C.M.; Shah, S.H.; et al. Perioperative Management of Antithrombotic Therapy: An American College of Chest Physicians Clinical Practice Guideline. Chest 2022, 162, e207–e243. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, S.A.; Lucà, F.; Benvenuto, M.; Iorio, A.; Fiscella, D.; D’Ascenzo, F.; Madeo, A.; Colivicchi, F.; Di Lenarda, A.; Gulizia, M.M. Major bleeding with old and novel oral anticoagulants: How to manage it. Focus on general measures. Int. J. Cardiol. 2018, 268, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, S.A.; Lucà, F.; Benvenuto, M.; Iorio, A.; Fiscella, D.; D’Ascenzo, F.; Madeo, A.; Colivicchi, F.; Di Lenarda, A.; Gulizia, M.M. Major bleeding with old and novel oral anticoagulants: How to manage it. Focus on reversal agents. Int. J. Cardiol. 2018, 268, 75–79. [Google Scholar] [CrossRef]
- Levy, J.H.; Ageno, W.; Chan, N.C.; Crowther, M.; Verhamme, P.; Weitz, J.I. When and how to use antidotes for the reversal of direct oral anticoagulants: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Pollack, C.V., Jr.; Reilly, P.A.; van Ryn, J.; Eikelboom, J.W.; Glund, S.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kam, C.W.; et al. Idarucizumab for Dabigatran Reversal—Full Cohort Analysis. N. Engl. J. Med. 2017, 377, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Godier, A.; Martin, A.C. Specific Antidotes for Direct Oral Anticoagulant Reversal: Case Closed or Cold Case? Circulation 2019, 140, 1445–1447. [Google Scholar] [CrossRef]
- Siegal, D.; Lu, G.; Leeds, J.M.; Karbarz, M.; Castillo, J.; Mathur, V.; Hutchaleelaha, A.; Sinha, U.; Kitt, M.; McClure, M.; et al. Safety, pharmacokinetics, and reversal of apixaban anticoagulation with andexanet alfa. Blood Adv. 2017, 1, 1827–1838. [Google Scholar] [CrossRef]
- Gómez-Outes, A.; Alcubilla, P.; Calvo-Rojas, G.; Terleira-Fernández, A.I.; Suárez-Gea, M.L.; Lecumberri, R.; Vargas-Castrillón, E. Meta-Analysis of Reversal Agents for Severe Bleeding Associated with Direct Oral Anticoagulants. J. Am. Coll. Cardiol. 2021, 77, 2987–3001. [Google Scholar] [CrossRef]
- Halvorsen, S.; Storey, R.F.; Rocca, B.; Sibbing, D.; Ten Berg, J.; Grove, E.L.; Weiss, T.W.; Collet, J.P.; Andreotti, F.; Gulba, D.C.; et al. Management of antithrombotic therapy after bleeding in patients with coronary artery disease and/or atrial fibrillation: Expert consensus paper of the European Society of Cardiology Working Group on Thrombosis. Eur. Heart J. 2017, 38, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Douketis, J.D.; Spyropoulos, A.C.; Duncan, J.; Carrier, M.; Le Gal, G.; Tafur, A.J.; Vanassche, T.; Verhamme, P.; Shivakumar, S.; Gross, P.L.; et al. Perioperative Management of Patients with Atrial Fibrillation Receiving a Direct Oral Anticoagulant. JAMA Intern. Med. 2019, 179, 1469–1478. [Google Scholar] [CrossRef]
- Douketis, J.D.; Healey, J.S.; Brueckmann, M.; Eikelboom, J.W.; Ezekowitz, M.D.; Fraessdorf, M.; Noack, H.; Oldgren, J.; Reilly, P.; Spyropoulos, A.C.; et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery or procedure. Substudy of the RE-LY trial. Thromb. Haemost. 2015, 113, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Uva, M.; Head, S.J.; Milojevic, M.; Collet, J.P.; Landoni, G.; Castella, M.; Dunning, J.; Gudbjartsson, T.; Linker, N.J.; Sandoval, E.; et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur. J. Cardiothorac. Surg. 2018, 53, 5–33. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.R.; Li, N.; Vanassche, T.; Coppens, M.; Spyropoulos, A.C.; Syed, S.; Radwi, M.; Duncan, J.; Schulman, S.; Douketis, J.D. Predictors of preprocedural direct oral anticoagulant levels in patients having an elective surgery or procedure. Blood Adv. 2020, 4, 3520–3527. [Google Scholar] [CrossRef]
- Pastori, D.; Marang, A.; Bisson, A.; Menichelli, D.; Herbert, J.; Lip, G.Y.H.; Fauchier, L. Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: A nationwide cohort study. Cancer 2021, 127, 2122–2129. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Parrini, I.; Lucà, F.; Rao, C.M.; Parise, G.; Micali, L.R.; Musumeci, G.; La Meir, M.; Colivicchi, F.; Gulizia, M.M.; Gelsomino, S. Superiority of Direct Oral Anticoagulants over Vitamin K Antagonists in Oncological Patients with Atrial Fibrillation: Analysis of Efficacy and Safety Outcomes. J. Clin. Med. 2022, 11, 5712. [Google Scholar] [CrossRef]
- Mauro, E.; Lucà, F.; Tetta, C.; Parise, O.; Parrini, I.; Parise, G.; Rao, C.M.; Matteucci, F.; Micali, L.R.; Gulizia, M.M.; et al. Breast Cancer and Atrial Fibrillation. J. Clin. Med. 2022, 11, 1417. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Z.; Luo, J.; Yu, P.; Ma, J.; Yuan, P.; Zhu, W. Effectiveness and Safety of DOACs vs. VKAs in AF Patients With Cancer: Evidence from Randomized Clinical Trials and Observational Studies. Front. Cardiovasc. Med. 2021, 8, 766377. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.S.; Patel, A.; Khawaja, M.; Chen, C.; Zheng, H.; Kaczmarek, J.; Gao, F.; Karimzad, K.; Song, J.; Koutroumpakis, E.; et al. Outcomes by Class of Anticoagulant Use for Nonvalvular Atrial Fibrillation in Patients with Active Cancer. JACC CardioOncol 2022, 4, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Fanola, C.L.; Ruff, C.T.; Murphy, S.A.; Jin, J.; Duggal, A.; Babilonia, N.A.; Sritara, P.; Mercuri, M.F.; Kamphuisen, P.W.; Antman, E.M.; et al. Efficacy and Safety of Edoxaban in Patients with Active Malignancy and Atrial Fibrillation: Analysis of the ENGAGE AF-TIMI 48 Trial. J. Am. Heart Assoc. 2018, 7, e008987. [Google Scholar] [CrossRef]
- Chen, S.T.; Hellkamp, A.S.; Becker, R.C.; Berkowitz, S.D.; Breithardt, G.; Fox, K.A.A.; Hacke, W.; Halperin, J.L.; Hankey, G.J.; Mahaffey, K.W.; et al. Efficacy and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and a history of cancer: Observations from ROCKET AF. Eur. Heart J. Qual. Care Clin. Outcomes 2019, 5, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Melloni, C.; Dunning, A.; Granger, C.B.; Thomas, L.; Khouri, M.G.; Garcia, D.A.; Hylek, E.M.; Hanna, M.; Wallentin, L.; Gersh, B.J.; et al. Efficacy and Safety of Apixaban Versus Warfarin in Patients with Atrial Fibrillation and a History of Cancer: Insights from the ARISTOTLE Trial. Am. J. Med. 2017, 130, 1440–1448.e1441. [Google Scholar] [CrossRef]
- Sawant, A.C.; Kumar, A.; McCray, W.; Tetewsky, S.; Parone, L.; Sridhara, S.; Prakash, M.P.H.; Tse, G.; Liu, T.; Kanwar, N.; et al. Superior safety of direct oral anticoagulants compared to Warfarin in patients with atrial fibrillation and underlying cancer: A national veterans affairs database study. J. Geriatr. Cardiol. 2019, 16, 706–709. [Google Scholar] [CrossRef]
- Shah, S.; Norby, F.L.; Datta, Y.H.; Lutsey, P.L.; MacLehose, R.F.; Chen, L.Y.; Alonso, A. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018, 2, 200–209. [Google Scholar] [CrossRef]
- Deitelzweig, S.; Keshishian, A.V.; Zhang, Y.; Kang, A.; Dhamane, A.D.; Luo, X.; Klem, C.; Ferri, M.; Jiang, J.; Yuce, H.; et al. Effectiveness and Safety of Oral Anticoagulants Among Nonvalvular Atrial Fibrillation Patients with Active Cancer. JACC CardioOncol 2021, 3, 411–424. [Google Scholar] [CrossRef]
- Wu, V.C.; Wang, C.L.; Huang, Y.T.; Lan, W.C.; Wu, M.; Kuo, C.F.; Chen, S.W.; Chu, P.H.; Wen, M.S.; Kuo, C.C.; et al. Novel Oral Anticoagulant versus Warfarin in Cancer Patients with Atrial Fibrillation: An 8-Year Population-Based Cohort Study. J. Cancer 2020, 11, 92–99. [Google Scholar] [CrossRef]
- Deng, Y.; Tong, Y.; Deng, Y.; Zou, L.; Li, S.; Chen, H. Non-Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Patients with Cancer and Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012540. [Google Scholar] [CrossRef]
- Sabatino, J.; De Rosa, S.; Polimeni, A.; Sorrentino, S.; Indolfi, C. Direct Oral Anticoagulants in Patients with Active Cancer: A Systematic Review and Meta-Analysis. JACC CardioOncol 2020, 2, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Ameri, P.; Alings, M.; Colivicchi, F.; Collins, R.; De Luca, L.; Di Nisio, M.; Fabbri, G.; Gabrielli, D.; Janssens, S.; Maggioni, A.P.; et al. Baseline characteristics of patients with atrial fibrillation and cancer enrolled in the BLITZ-AF Cancer registry. Eur. Heart J. 2022, 43. [Google Scholar] [CrossRef]
- Lin, Y.S.; Kuan, F.C.; Chao, T.F.; Wu, M.; Chen, S.W.; Chen, M.C.; Chung, C.M.; Chu, P.H.; Lip, G.Y.H.; Wu, V.C. Mortality associated with the use of non-vitamin K antagonist oral anticoagulants in cancer patients: Dabigatran versus rivaroxaban. Cancer Med. 2021, 10, 7079–7088. [Google Scholar] [CrossRef]
- Ardeshirrouhanifard, S.; An, H.; Goyal, R.K.; Raji, M.A.; Segal, J.B.; Alexander, G.C.; Mehta, H.B. Use of oral anticoagulants among individuals with cancer and atrial fibrillation in the United States, 2010–2016. Pharmacotherapy 2022, 42, 375–386. [Google Scholar] [CrossRef]
- Boriani, G.; Lee, G.; Parrini, I.; Lopez-Fernandez, T.; Lyon, A.R.; Suter, T.; Van der Meer, P.; Cardinale, D.; Lancellotti, P.; Zamorano, J.L.; et al. Anticoagulation in patients with atrial fibrillation and active cancer: An international survey on patient management. Eur. J. Prev. Cardiol. 2021, 28, 611–621. [Google Scholar] [CrossRef]
- Mosarla, R.C.; Vaduganathan, M.; Qamar, A.; Moslehi, J.; Piazza, G.; Giugliano, R.P. Anticoagulation Strategies in Patients with Cancer: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Peixoto de Miranda, É.J.F.; Takahashi, T.; Iwamoto, F.; Yamashiro, S.; Samano, E.; Macedo, A.V.S.; Ramacciotti, E. Drug-Drug Interactions of 257 Antineoplastic and Supportive Care Agents with 7 Anticoagulants: A Comprehensive Review of Interactions and Mechanisms. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620936325. [Google Scholar] [CrossRef]
- Lucà, F.; Parrini, I.; Abrignani, M.G.; Rao, C.M.; Piccioni, L.; Di Fusco, S.A.; Ceravolo, R.; Bisceglia, I.; Riccio, C.; Gelsomino, S.; et al. Management of Acute Coronary Syndrome in Cancer Patients: It’s High Time We Dealt with It. J. Clin. Med. 2022, 11, 1792. [Google Scholar] [CrossRef]
- Rietbrock, S.; Heeley, E.; Plumb, J.; van Staa, T. Chronic atrial fibrillation: Incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age > 75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am. Heart J. 2008, 156, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Cipolletta, L.; Di Fusco, S.A.; Iorio, A.; Pozzi, A.; Rao, C.M.; Ingianni, N.; Benvenuto, M.; Madeo, A.; Fiscella, D.; et al. Remote monitoring: Doomed to let down or an attractive promise? Int. J. Cardiol. Heart Vasc. 2019, 24, 100380. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Colivicchi, F.; Oliva, F.; Abrignani, M.; Caretta, G.; Di Fusco, S.A.; Giubilato, S.; Cornara, S.; Di Nora, C.; Pozzi, A.; et al. Management of oral anticoagulant therapy after intracranial hemorrhage in patients with atrial fibrillation. Front. Cardiovasc. Med. 2023, 10, 1061618. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Wallentin, L.; Connolly, S.J.; Ezekowitz, M.; Healey, J.S.; Oldgren, J.; Yang, S.; Alings, M.; Kaatz, S.; Hohnloser, S.H.; et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: An analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011, 123, 2363–2372. [Google Scholar] [CrossRef]
- Galvain, T.; Hill, R.; Donegan, S.; Lisboa, P.; Lip, G.Y.H.; Czanner, G. Efficacy and Safety of Anticoagulants in Patients with Atrial Fibrillation and History of Falls or Risk of Falls: A Systematic Review and Multilevel Meta-Analysis. Drug Saf. 2022, 45, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Giugliano, R.P.; Braunwald, E.; Murphy, S.A.; Mercuri, M.; Choi, Y.; Aylward, P.; White, H.; Zamorano, J.L.; Antman, E.M.; et al. Edoxaban Versus Warfarin in Atrial Fibrillation Patients at Risk of Falling: ENGAGE AF-TIMI 48 Analysis. J. Am. Coll. Cardiol. 2016, 68, 1169–1178. [Google Scholar] [CrossRef]
- Rao, M.P.; Vinereanu, D.; Wojdyla, D.M.; Alexander, J.H.; Atar, D.; Hylek, E.M.; Hanna, M.; Wallentin, L.; Lopes, R.D.; Gersh, B.J.; et al. Clinical Outcomes and History of Fall in Patients with Atrial Fibrillation Treated with Oral Anticoagulation: Insights from the ARISTOTLE Trial. Am. J. Med. 2018, 131, 269–275.e2. [Google Scholar] [CrossRef]
- Martin, K.; Beyer-Westendorf, J.; Davidson, B.L.; Huisman, M.V.; Sandset, P.M.; Moll, S. Use of the direct oral anticoagulants in obese patients: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 1308–1313. [Google Scholar] [CrossRef]
- Boriani, G.; Ruff, C.T.; Kuder, J.F.; Shi, M.; Lanz, H.J.; Antman, E.M.; Braunwald, E.; Giugliano, R.P. Edoxaban versus Warfarin in Patients with Atrial Fibrillation at the Extremes of Body Weight: An Analysis from the ENGAGE AF-TIMI 48 Trial. Thromb. Haemost. 2021, 121, 140–149. [Google Scholar] [CrossRef]
- Boonyawat, K.; Caron, F.; Li, A.; Chai-Adisaksopha, C.; Lim, W.; Iorio, A.; Lopes, R.D.; Garcia, D.; Crowther, M.A. Association of body weight with efficacy and safety outcomes in phase III randomized controlled trials of direct oral anticoagulants: A systematic review and meta-analysis. J. Thromb. Haemost. 2017, 15, 1322–1333. [Google Scholar] [CrossRef]
- Barakat, A.F.; Jain, S.; Masri, A.; Alkukhun, L.; Senussi, M.; Sezer, A.; Wang, Y.; Thoma, F.; Bhonsale, A.; Saba, S.; et al. Outcomes of Direct Oral Anticoagulants in Atrial Fibrillation Patients Across Different Body Mass Index Categories. JACC Clin. Electrophysiol. 2021, 7, 649–658. [Google Scholar] [CrossRef]
- Deitelzweig, S.; Keshishian, A.; Kang, A.; Dhamane, A.D.; Luo, X.; Li, X.; Balachander, N.; Rosenblatt, L.; Mardekian, J.; Pan, X.; et al. Effectiveness and Safety of Oral Anticoagulants among NVAF Patients with Obesity: Insights from the ARISTOPHANES Study. J. Clin. Med. 2020, 9, 1633. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.S.; Laliberté, F.; Kharat, A.; Lejeune, D.; Moore, K.T.; Jung, Y.; Lefebvre, P.; Ashton, V. Real-world effectiveness and safety of rivaroxaban versus warfarin among non-valvular atrial fibrillation patients with obesity in a US population. Curr. Med. Res. Opin. 2021, 37, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Rocca, B.; Fox, K.A.A.; Ajjan, R.A.; Andreotti, F.; Baigent, C.; Collet, J.P.; Grove, E.L.; Halvorsen, S.; Huber, K.; Morais, J.; et al. Antithrombotic therapy and body mass: An expert position paper of the ESC Working Group on Thrombosis. Eur. Heart J. 2018, 39, 1672–1686f. [Google Scholar] [CrossRef]
- Brar, T.; Chua, D. Direct-Acting Oral Anticoagulant Choice for Stroke Prevention in Obese Patients with Atrial Fibrillation. Can. J. Cardiol. 2021, 37, 1489–1492. [Google Scholar] [CrossRef]
- Pandey, A.K.; Eikelboom, J.W. Direct Oral Anticoagulant Dosing in Extremes of Body Weight: Time to Revisit the Guidelines? Thromb. Haemost. 2021, 121, 118–120. [Google Scholar] [CrossRef]
- Wiethorn, E.E.; Bell, C.M.; Wiggins, B.S. Effectiveness and Safety of Direct Oral Anticoagulants in Patients with Nonvalvular Atrial Fibrillation and Weighing ≥ 120 Kilograms versus 60–120 Kilograms. Am. J. Cardiovasc. Drugs 2021, 21, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Mocini, D.; Di Fusco, S.A.; Mocini, E.; Donini, L.M.; Lavalle, C.; Di Lenarda, A.; Riccio, C.; Caldarola, P.; De Luca, L.; Gulizia, M.M.; et al. Direct Oral Anticoagulants in Patients with Obesity and Atrial Fibrillation: Position Paper of Italian National Association of Hospital Cardiologists (ANMCO). J. Clin. Med. 2021, 10, 4185. [Google Scholar] [CrossRef]
- Yoon, M.; Yang, P.S.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Sung, J.H.; Pak, H.N.; Lee, M.H.; et al. Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 1695–1703. [Google Scholar] [CrossRef]
- Boriani, G.; Savelieva, I.; Dan, G.A.; Deharo, J.C.; Ferro, C.; Israel, C.W.; Lane, D.A.; La Manna, G.; Morton, J.; Mitjans, A.M.; et al. Chronic kidney disease in patients with cardiac rhythm disturbances or implantable electrical devices: Clinical significance and implications for decision making-a position paper of the European Heart Rhythm Association endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1169–1196. [Google Scholar] [CrossRef]
- Malavasi, V.L.; Pettorelli, D.; Fantecchi, E.; Zoccali, C.; Laronga, G.; Trenti, T.; Lip, G.Y.H.; Boriani, G. Variations in clinical management of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation according to different equations for estimating renal function: Post hoc analysis of a prospective cohort. Intern. Emerg. Med. 2018, 13, 1059–1067. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [PubMed]
- Bansilal, S.; Castellano, J.M.; Garrido, E.; Wei, H.G.; Freeman, A.; Spettell, C.; Garcia-Alonso, F.; Lizano, I.; Arnold, R.J.; Rajda, J.; et al. Assessing the Impact of Medication Adherence on Long-Term Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2016, 68, 789–801. [Google Scholar] [CrossRef]
- van Breugel, H.N.; Gelsomino, S.; Lozekoot, P.W.; Accord, R.E.; Lucà, F.; Parise, O.; Crijns, H.J.; Maessen, J.G. Guideline adherence in antithrombotic treatment after concomitant ablation surgery in atrial fibrillation patients. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 313–320. [Google Scholar] [CrossRef]
- Raparelli, V.; Proietti, M.; Cangemi, R.; Lip, G.Y.; Lane, D.A.; Basili, S. Adherence to oral anticoagulant therapy in patients with atrial fibrillation. Focus on non-vitamin K antagonist oral anticoagulants. Thromb. Haemost. 2017, 117, 209–218. [Google Scholar] [CrossRef]
- Teppo, K.; Jaakkola, J.; Biancari, F.; Halminen, O.; Linna, M.; Haukka, J.; Putaala, J.; Tiili, P.; Lehtonen, O.; Niemi, M.; et al. Association of income and educational levels with adherence to direct oral anticoagulant therapy in patients with incident atrial fibrillation: A Finnish nationwide cohort study. Pharmacol. Res. Perspect. 2022, 10, e00961. [Google Scholar] [CrossRef]
- Mitrovic, D.; Veeger, N.; van Roon, E. Impact of minor bleeds on confidence in anticoagulation therapy, adherence to treatment and quality of life in patients using a non-vitamin K antagonist oral anticoagulant for atrial fibrillation. Curr. Med. Res. Opin. 2022, 38, 1485–1488. [Google Scholar] [CrossRef]
- Keita, I.; Aubin-Auger, I.; Lalanne, C.; Aubert, J.P.; Chassany, O.; Duracinsky, M.; Mahé, I. Assessment of quality of life, satisfaction with anticoagulation therapy, and adherence to treatment in patients receiving long-course vitamin K antagonists or direct oral anticoagulants for venous thromboembolism. Patient Prefer. Adherence 2017, 11, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.J.; Shinde, M.U.; Kwong, W.J.; Fu, A.C.; Tan, H.; Weintraub, W.S. Comparison of claims vs patient-reported adherence measures and associated outcomes among patients with nonvalvular atrial fibrillation using oral anticoagulant therapy. Patient Prefer. Adherence 2018, 12, 105–117. [Google Scholar] [CrossRef]
- Chen, P.T.; Wang, T.J.; Hsieh, M.H.; Liu, J.C.; Liu, C.Y.; Wang, K.Y.; Laio, W.C. Anticoagulation adherence and its associated factors in patients with atrial fibrillation: A cross-sectional study. BMJ Open 2019, 9, e029974. [Google Scholar] [CrossRef] [PubMed]
- Ungar, L.; Rodriguez, F.; Hellkamp, A.S.; Becker, R.C.; Berkowitz, S.D.; Breithardt, G.; Fox, K.A.A.; Hacke, W.; Halperin, J.L.; Hankey, G.J.; et al. Patient-Reported Satisfaction and Study Drug Discontinuation: Post-Hoc Analysis of Findings from ROCKET AF. Cardiol. Ther. 2019, 8, 283–295. [Google Scholar] [CrossRef]
- Benzimra, M.; Bonnamour, B.; Duracinsky, M.; Lalanne, C.; Aubert, J.P.; Chassany, O.; Aubin-Auger, I.; Mahé, I. Real-life experience of quality of life, treatment satisfaction, and adherence in patients receiving oral anticoagulants for atrial fibrillation. Patient Prefer. Adherence 2018, 12, 79–87. [Google Scholar] [CrossRef]
- van Breugel, H.; Parise, O.; Nieman, F.H.M.; Accord, R.E.; Lucà, F.; Lozekoot, P.; Kumar, N.; van Mastrigt, G.; Nijs, J.; Vrakking, R.; et al. Does sinus rhythm conversion after cardiac surgery affect postoperative health- related quality of life? J. Cardiothorac. Surg. 2016, 11, 75. [Google Scholar] [CrossRef]
- Holthuis, E.; Smits, E.; Spentzouris, G.; Beier, D.; Enders, D.; Gini, R.; Bartolini, C.; Mazzaglia, G.; Penning-van Beest, F.; Herings, R. Increased Risk of Stroke Due to Non-adherence and Non-persistence with Direct Oral Anticoagulants (DOACs): Real-World Analyses Using a Nested Case-Control Study from The Netherlands, Italy and Germany. Drugs Real. World Outcomes 2022, 9, 597–607. [Google Scholar] [CrossRef]
- Xiang, X.; Cao, Y.; Sun, K.; Song, J.; Tian, Y.; Yin, Q.; Juan, J.; Hu, Y. Real world adherence to oral anticoagulant in non-valvular atrial fibrillation patients in China. Curr. Med. Res. Opin. 2018, 34, 255–261. [Google Scholar] [CrossRef]
- Moudallel, S.; van den Bemt, B.J.F.; Zwikker, H.; de Veer, A.; Rydant, S.; Dijk, L.V.; Steurbaut, S. Association of conflicting information from healthcare providers and poor shared decision making with suboptimal adherence in direct oral anticoagulant treatment: A cross-sectional study in patients with atrial fibrillation. Patient Educ. Couns. 2021, 104, 155–162. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.S.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Sung, J.H.; Pak, H.N.; Lee, M.H.; et al. The optimal drug adherence to maximize the efficacy and safety of non-vitamin K antagonist oral anticoagulant in real-world atrial fibrillation patients. Europace 2020, 22, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Yagi, N.; Suzuki, S.; Nagai, K.; Tanaka, T.; Nagahama, T.; Arita, T.; Otsuka, T.; Yamashita, T. Current status of oral anticoagulant adherence in Japanese patients with atrial fibrillation: A claims database analysis. J. Cardiol. 2021, 78, 150–156. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S.R.; Park, H.S.; Lee, Y.S.; Ahn, J.H.; Choi, J.I.; Shin, D.G.; Kim, D.K.; Park, J.S.; Hwang, K.W.; et al. Adherence to dabigatran and the influence of dabigatran-induced gastrointestinal discomfort in the real-world practice. Int. J. Cardiol. 2021, 323, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Komen, J.J.; Heerdink, E.R.; Klungel, O.H.; Mantel-Teeuwisse, A.K.; Forslund, T.; Wettermark, B.; Hjemdahl, P. Long-term persistence and adherence with non-vitamin K oral anticoagulants in patients with atrial fibrillation and their associations with stroke risk. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, f72–f80. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Bider, Z.; Luong, T.Q.; Cheetham, T.C.; Lang, D.T.; Fischer, H.; Reynolds, K. Long-Term Medication Adherence Trajectories to Direct Oral Anticoagulants and Clinical Outcomes in Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e021601. [Google Scholar] [CrossRef]
- Toorop, M.M.A.; van Rein, N.; Nierman, M.C.; Vermaas, H.W.; Huisman, M.V.; van der Meer, F.J.M.; Cannegieter, S.C.; Lijfering, W.M. Self-reported therapy adherence and predictors for nonadherence in patients who switched from vitamin K antagonists to direct oral anticoagulants. Res. Pract. Thromb. Haemost. 2020, 4, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Bartoli-Abdou, J.K.; Patel, J.P.; Crawshaw, J.; Vadher, B.; Brown, A.; Roberts, L.N.; Patel, R.K.; Arya, R.; Auyeung, V. Exploration of adherence and patient experiences with DOACs one year after switching from vitamin-K antagonists- insights from the switching study. Thromb. Res. 2018, 162, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Mostaza, J.M.; Suárez Fernández, C.; Castilla Guerra, L.; Suriñach, J.M.; Tamarit, J.J.; Diaz Diaz, J.L.; García Polo, I.; Santamaria, E.F.; Fidalgo Fernández, M.A.; de la Guerra Acebal, C.; et al. Type and doses of oral anticoagulants and adherence to anticoagulant treatment in elderly patients with atrial fibrillation: The ESPARTA study. J. Comp. Eff. Res. 2018, 7, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Smet, L.; Heggermont, W.A.; Goossens, E.; Eeckloo, K.; Vander Stichele, R.; De Potter, T.; De Backer, T. Adherence, knowledge, and perception about oral anticoagulants in patients with atrial fibrillation at high risk for thromboembolic events after radiofrequency ablation. J. Adv. Nurs. 2018, 74, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Kjekshus, V.H.R.; Schuster, P. Adherence to oral anticoagulant treatment and risk factor assessment six months after DC-conversion of atrial fibrillation. Scand. Cardiovasc. J. 2020, 54, 179–185. [Google Scholar] [CrossRef]
- Salmasi, S.; Safari, A.; Kapanen, A.; Adelakun, A.; Kwan, L.; MacGillivray, J.; Andrade, J.G.; Deyell, M.W.; Loewen, P. Oral anticoagulant adherence and switching in patients with atrial fibrillation: A prospective observational study. Res. Soc. Adm. Pharm. 2022, 18, 3920–3928. [Google Scholar] [CrossRef]
- Perreault, S.; de Denus, S.; White-Guay, B.; Côté, R.; Schnitzer, M.E.; Dubé, M.P.; Dorais, M.; Tardif, J.C. Oral Anticoagulant Prescription Trends, Profile Use, and Determinants of Adherence in Patients with Atrial Fibrillation. Pharmacotherapy 2020, 40, 40–54. [Google Scholar] [CrossRef]
- Salmasi, S.; De Vera, M.A.; Safari, A.; Lynd, L.D.; Koehoorn, M.; Barry, A.R.; Andrade, J.G.; Deyell, M.W.; Rush, K.; Zhao, Y.; et al. Longitudinal Oral Anticoagulant Adherence Trajectories in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2021, 78, 2395–2404. [Google Scholar] [CrossRef]
- Rodriguez-Bernal, C.L.; Peiró, S.; Hurtado, I.; García-Sempere, A.; Sanfélix-Gimeno, G. Primary Nonadherence to Oral Anticoagulants in Patients with Atrial Fibrillation: Real-World Data from a Population-Based Cohort. J. Manag. Care Spec. Pharm. 2018, 24, 440–448. [Google Scholar] [CrossRef]
- Zielinski, G.D.; van Rein, N.; Teichert, M.; Klok, F.A.; Rosendaal, F.R.; van der Meer, F.J.M.; Huisman, M.V.; Cannegieter, S.C.; Lijfering, W.M. Persistence of oral anticoagulant treatment for atrial fibrillation in the Netherlands: A surveillance study. Res. Pract. Thromb. Haemost. 2020, 4, 141–153. [Google Scholar] [CrossRef]
- Shani, M.; Comaneshter, D.; Lustman, A. Adherence to Oral Anticoagulant Medications. Isr. Med. Assoc. J. 2021, 23, 580–583. [Google Scholar] [PubMed]
- Simonyi, G.; Paksy, A.; Várnai, R.; Medvegy, M. Real-world adherence to oral anticoagulants in atrial fibrillation. Orv. Hetil. 2020, 161, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y.S.; Kim, T.H.; Cha, M.J.; Lee, J.M.; Park, J.; Park, J.K.; Kang, K.W.; Shim, J.; Uhm, J.S.; et al. A prospective survey of the persistence of warfarin or NOAC in nonvalvular atrial fibrillation: A COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF). Korean J. Intern. Med. 2020, 35, 99–108. [Google Scholar] [CrossRef]
- Bennaghmouch, N.; de Veer, A.; Zivelonghi, C.; van Dijk, L.; Ten Berg, J.M. First report of a comparative patient-oriented perspective on the use of non-vitamin-K oral anticoagulants or vitamin-K antagonists in atrial fibrillation: Patients’ experiences, side-effects and practical problems leading to non-adherence. Neth. Heart J. 2019, 27, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Benedetto, V.; Gichuru, P.; Burnell, J.; Antoniou, S.; Schilling, R.J.; Strain, W.D.; Ryan, R.; Watkins, C.; Marshall, T.; et al. Adherence and persistence to direct oral anticoagulants in atrial fibrillation: A population-based study. Heart 2020, 106, 119–126. [Google Scholar] [CrossRef]
- Schaefer, J.K.; Li, M.; Wu, Z.; Basu, T.; Dorsch, M.P.; Barnes, G.D.; Carrier, M.; Griggs, J.J.; Sood, S.L. Anticoagulant medication adherence for cancer-associated thrombosis: A comparison of LMWH to DOACs. J. Thromb. Haemost. 2021, 19, 212–220. [Google Scholar] [CrossRef]
- Prentice, A.; Ruiz, I.; Weeda, E.R. Medication adherence to rivaroxaban and dabigatran in patients with non-valvular atrial fibrillation: A meta-analysis. J. Thromb. Thrombolysis 2020, 49, 360–364. [Google Scholar] [CrossRef]
- Paquette, M.; Riou França, L.; Teutsch, C.; Diener, H.C.; Lu, S.; Dubner, S.J.; Ma, C.S.; Rothman, K.J.; Zint, K.; Halperin, J.L.; et al. Persistence with Dabigatran Therapy at 2 Years in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 1573–1583. [Google Scholar] [CrossRef]
- Chiu, P.E.; Tsao, H.M.; Tsai, C.H. Discrepancy Among Self-Reported Adherence, Prescription Refills, and Actual Anticoagulant Control. J. Nurs. Res. 2020, 28, e63. [Google Scholar] [CrossRef]
- Obamiro, K.O.; Chalmers, L.; Lee, K.; Bereznicki, B.J.; Bereznicki, L.R. Adherence to Oral Anticoagulants in Atrial Fibrillation: An Australian Survey. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 337–343. [Google Scholar] [CrossRef]
- Barcellona, D.; Mameli, A.; Cornacchini, S.; Perra, F.; Diovaldi, M.; Farci, N.; Moledda, V.; Marongiu, F. Patients’ adherence to oral anticoagulants therapy: Comparison between vitamin K antagonists and direct oral anticoagulants. Int. J. Cardiol. 2021, 333, 162–166. [Google Scholar] [CrossRef]
- Lucà, F.; Abrignani, M.G.; Parrini, I.; Di Fusco, S.A.; Giubilato, S.; Rao, C.M.; Piccioni, L.; Cipolletta, L.; Passaretti, B.; Giallauria, F.; et al. Update on Management of Cardiovascular Diseases in Women. J. Clin. Med. 2022, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Majd, Z.; Trinh, T.; Paranjpe, R.; Abughosh, S.M. Group based trajectory modeling to assess adherence to oral anticoagulants among atrial fibrillation patients with comorbidities: A retrospective study. Int. J. Clin. Pharm. 2022, 44, 966–974. [Google Scholar] [CrossRef]
- Bartoli-Abdou, J.K.; Patel, J.P.; Vadher, B.; Brown, A.; Roberts, L.N.; Patel, R.K.; Arya, R.; Auyeung, V. Long-term adherence to direct acting oral anticoagulants and the influence of health beliefs after switching from vitamin-K antagonists: Findings from the Switching Study. Thromb. Res. 2021, 208, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, X.; Yang, J. Analysis of Influencing Factors of Compliance with Non-Vitamin K Antagonist Oral Anticoagulant in Patients with Nonvalvular Atrial Fibrillation and Correlation with the Severity of Ischemic Stroke. Evid. Based Complement. Altern. Med. 2021, 2021, 1021127. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, C.G.; Kogut, S.; Willey, C. Real-World Health Care Costs Based on Medication Adherence and Risk of Stroke and Bleeding in Patients Treated with Novel Anticoagulant Therapy. J. Manag. Care Spec. Pharm. 2018, 24, 430–439. [Google Scholar] [CrossRef]
- Taylor, E.C.; O’Neill, M.; Hughes, L.D.; Bennett, P.; Moss-Morris, R. Examining Adherence to Medication in Patients With Atrial Fibrillation: The Role of Medication Beliefs, Attitudes, and Depression. J. Cardiovasc. Nurs. 2020, 35, 337–346. [Google Scholar] [CrossRef]
- Rossi, A.P.; Facchinetti, R.; Ferrari, E.; Nori, N.; Sant, S.; Masciocchi, E.; Zoico, E.; Fantin, F.; Mazzali, G.; Zamboni, M. Predictors of self-reported adherence to direct oral anticoagulation in a population of elderly men and women with non-valvular atrial fibrillation. J. Thromb. Thrombolysis 2018, 46, 139–144. [Google Scholar] [CrossRef]
- Gebreyohannes, E.A.; Salter, S.M.; Chalmers, L.; Bereznicki, L.; Lee, K. Reasons for non-adherence to thromboprophylaxis prescribing guidelines in atrial fibrillation in Western Australia: A qualitative descriptive study of general practitioners’ views. Thromb. Res. 2021, 208, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Rome, B.N.; Gagne, J.J.; Avorn, J.; Kesselheim, A.S. Non-warfarin oral anticoagulant copayments and adherence in atrial fibrillation: A population-based cohort study. Am. Heart J. 2021, 233, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Shehab, A.; Bhagavathula, A.S.; Abebe, T.B.; Abegaz, T.M.; Elnour, A.A.; Sabbour, H.M.; Uzzafer, M.; Hersi, A.; Hamad, A.K. Patient Adherence to Novel Oral Anticoagulants (NOACs) for the Treatment of Atrial Fibrillation and Occurrence of Associated Bleeding Events: A Systematic Review and Meta-analysis. Curr. Vasc. Pharmacol. 2019, 17, 341–349. [Google Scholar] [CrossRef]
- Solla-Ruiz, I.; Villanueva-Benito, I.; Paredes-Galán, E.; Salterain-González, N.; Oria-González, G.; De La Cuesta-Arzamendi, F.; Querejeta-Iraola, R. Differences between patient-driven adherence to vitamin K antagonists and direct oral anticoagulants. Do few missed doses matter? ACO-MEMS Study. Thromb. Res. 2019, 179, 20–27. [Google Scholar] [CrossRef]
- El-Hajj, M.; Ajrouche, R.; Zein, S.; Rachidi, S.; Awada, S.; Al-Hajje, A. Evaluation of risk factors and drug adherence in the occurrence of stroke in patients with atrial fibrillation. Pharm Pract. (Granada) 2020, 18, 1860. [Google Scholar] [CrossRef]
- Yamashiro, K.; Kurita, N.; Tanaka, R.; Ueno, Y.; Miyamoto, N.; Hira, K.; Nakajima, S.; Urabe, T.; Hattori, N. Adequate Adherence to Direct Oral Anticoagulant is Associated with Reduced Ischemic Stroke Severity in Patients with Atrial Fibrillation. J. Stroke Cerebrovasc. Dis. 2019, 28, 1773–1780. [Google Scholar] [CrossRef]
- Komatsu, Y.; Yokoyama, S.; Hosomi, K.; Takada, M. Impact of Medication Adherence on the Association Between Oral Anticoagulant Use and Risk of Dementia: A Retrospective Cohort Study using the Japanese Claims Database. Drugs Real. World Outcomes 2022, 9, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Lane, D.A. The Compelling Issue of Nonvitamin K Antagonist Oral Anticoagulant Adherence in Atrial Fibrillation Patients: A Systematic Need for New Strategies. Thromb. Haemost. 2020, 120, 369–371. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Branda, M.E.; Kunneman, M.; Hargraves, I.G.; Sivly, A.L.; Brito, J.P.; Burnett, B.; Zeballos-Palacios, C.; Linzer, M.; Suzuki, T.; et al. Effect of Shared Decision-Making for Stroke Prevention on Treatment Adherence and Safety Outcomes in Patients with Atrial Fibrillation: A Randomized Clinical Trial. J. Am. Heart Assoc. 2022, 11, e023048. [Google Scholar] [CrossRef] [PubMed]
- Haché, J.; Bonsu, K.O.; Chitsike, R.; Nguyen, H.; Young, S. Assessment of a Pharmacist-Led Direct Oral Anticoagulant Monitoring Clinic. Can. J. Hosp. Pharm. 2021, 74, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Contreras, E.; Gil-Guillén, V.F.; De La Figuera-Von Wichmann, M.; Franch-Nadal, J.; Llisterri-Caro, J.L.; Martell-Claros, N.; Martin-De Pablos, J.L.; Casado-Martinez, J.J.; Bertomeu-Gonzalez, V.; Pertusa Martínez, S.; et al. Non-compliance and inertia in hypertensive Spaniards at high cardiovascular risk: CUMPLE study. Curr. Med. Res. Opin. 2014, 30, 11–17. [Google Scholar] [CrossRef]
- Toscos, T.; Drouin, M.; Pater, J.A.; Flanagan, M.; Wagner, S.; Coupe, A.; Ahmed, R.; Mirro, M.J. Medication adherence for atrial fibrillation patients: Triangulating measures from a smart pill bottle, e-prescribing software, and patient communication through the electronic health record. JAMIA Open 2020, 3, 233–242. [Google Scholar] [CrossRef]
- Boehme, P.; Wienand, P.; Herrmann, M.; Truebel, H.; Mondritzki, T. New digital adherence devices could prevent millions of strokes from atrial fibrillation by the end of the next century. Med. Hypotheses 2017, 108, 46–50. [Google Scholar] [CrossRef]
- Musinguzi, N.; Muganzi, C.D.; Boum, Y., 2nd; Ronald, A.; Marzinke, M.A.; Hendrix, C.W.; Celum, C.; Baeten, J.M.; Bangsberg, D.R.; Haberer, J.E. Comparison of subjective and objective adherence measures for preexposure prophylaxis against HIV infection among serodiscordant couples in East Africa. Aids 2016, 30, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.B.; Sams, J.; Tatsuoka, C.; Cassidy, K.A.; Sajatovic, M. Use of automated medication adherence monitoring in bipolar disorder research: Pitfalls, pragmatics, and possibilities. Ther. Adv. Psychopharmacol. 2015, 5, 76–87. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, M.J.; Schmidt, H. mHealth for Tuberculosis Treatment Adherence: A Framework to Guide Ethical Planning, Implementation, and Evaluation. Glob. Health Sci. Pract. 2016, 4, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Robiner, W.N.; Flaherty, N.; Fossum, T.A.; Nevins, T.E. Desirability and feasibility of wireless electronic monitoring of medications in clinical trials. Transl. Behav. Med. 2015, 5, 285–293. [Google Scholar] [CrossRef][Green Version]
- Carnicelli, A.P.; Hong, H.; Connolly, S.J.; Eikelboom, J.; Giugliano, R.P.; Morrow, D.A.; Patel, M.R.; Wallentin, L.; Alexander, J.H.; Cecilia Bahit, M.; et al. Direct Oral Anticoagulants Versus Warfarin in Patients with Atrial Fibrillation: Patient-Level Network Meta-Analyses of Randomized Clinical Trials with Interaction Testing by Age and Sex. Circulation 2022, 145, 242–255. [Google Scholar] [CrossRef]
- Chan, N.; Sobieraj-Teague, M.; Eikelboom, J.W. Direct oral anticoagulants: Evidence and unresolved issues. Lancet 2020, 396, 1767–1776. [Google Scholar] [CrossRef]
- Capranzano, P.; Angiolillo, D.J. Evidence and Recommendations for Uninterrupted Versus Interrupted Oral Anticoagulation in Patients Undergoing Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2021, 14, 764–767. [Google Scholar] [CrossRef]
- Harkness, W.; Pipitone, O.; Joss, J.; Schiedler, M.; Shagavah, S.; Moore, R.; Hsing, J. Observed Apixaban Anti-Xa Levels in Obese Patients. Ann. Pharmacother. 2022, 56, 1215–1221. [Google Scholar] [CrossRef]
- Proulx, L.-A.B.; Potter, B.J.; Perreault, S. Comparative effectiveness and safety of doac versus warfarin among obese with atrial fibrillation. J. Am. Coll. Cardiol. 2022, 79, 52. [Google Scholar] [CrossRef]
- Wańkowicz, P.; Staszewski, J.; Dębiec, A.; Nowakowska-Kotas, M.; Szylińska, A.; Rotter, I. Ischemic Stroke Risk Factors in Patients with Atrial Fibrillation Treated with New Oral Anticoagulants. J. Clin. Med. 2021, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Sanada, F.; Muratsu, J.; Otsu, R.; Shimizu, H.; Koibuchi, N.; Uchida, K.; Taniyama, Y.; Yoshimura, S.; Rakugi, H.; Morishita, R. Local Production of Activated Factor X in Atherosclerotic Plaque Induced Vascular Smooth Muscle Cell Senescence. Sci. Rep. 2017, 7, 17172. [Google Scholar] [CrossRef] [PubMed]

| Drugs | Strong | Moderate to Weak |
|---|---|---|
| Pgp or combined CYP3A4/5/Pgp inhibitors | Clarithromycin, Cobicistat, Ketoconazole, Itraconazole, Dronedarone, Erythromycin, Posaconazole, Ritonavir, Voriconazole | Amiodarone, Cyclosporine, Diltiazem, Ticagrelor, Verapamil, Quinidine |
| CYP3A4/5 inhibitors | Boceprevir, Grapefruit Juice | Fluconazole |
| P-gp inducers | Rifampin | |
| CYP3A4/5 inducers | Phenytoin | |
| CYP3A4/5 inducer+combined P-gp inducer | Apalutamide, Bosentan, Carbamazepine, Phenobarbital, St. John’s Wort |
| Dose for Each DOAC According to GFR | ||||
|---|---|---|---|---|
| GFR (mL/min) | Dabigatran | Rivaroban | Apixaban | Edoxaban |
| >50 | 150/110 mg BID | 20 mg OD | 2.5/5 mg BID | 60 mg OD |
| 50–30 | 150/110 mg BID | 15 mg OD | 2.5/5 mg BID | 30 mg OD |
| 30–15 | Contraindicated | 15 mg OD | 2.5 mg BID | 30 mg OD |
| <15 or dialysis | Contraindicated | Contraindicated | Contraindicated | Contraindicated |
| DOAC | CrCl | |||||
|---|---|---|---|---|---|---|
| ≥80 mL/min | 50–79 mL/min | 30–49 mL/min | 15–29 mL/min | <15 mL/min | ||
| Dabigatran | Low BR | ≥24 h | ≥36 h | ≥48 h | ||
| High BR | ≥48 h | ≥72 h | ≥96 h | |||
| Apixaban Edoxaban Rivaroxaban | Low BR | ≥24 h | ≥36 h | |||
| High BR | ≥48 h | |||||
 Contraindicated.
Contraindicated.| Study | ≥75 Years | Overall Relative Risk vs. VKAs for Stroke/SE, RR (95% CI) | Overall Relative Risk vs. VKAs for Primary Safety, RR (95% CI) | |
|---|---|---|---|---|
| Dabigatran | RE-LY [40] | 7258 (40%) | 110 mg, 0.91 (0.74–1.11) 150 mg, 0.66 (0.53–0.82) | 110 mg, 0.80 (0.69–0.93) 150 mg, 0.93 (0.81–1.07) |
| Rivaroxaban | ROCKET-AF [42] | 6229 (44%) | 0.88 (0.75–1.03) | 1.03 (0.96–1.11) |
| Apixaban | ARISTOTLE [41] | 5678 (31%) | 0.79 (0.66–0.95) | 0.69 (0.60–0.80) |
| Edoxaban | ENGAGE-AF [21] | 5668 (40%) | 0.87 (0.73–1.04) | 0.80 (0.71–0.91) |
| Tools for Evaluating Quality of Life or Satisfaction in Anticoagulated Patients |
|---|
| AF-related symptom subscale of the AF Severity Scale |
| Knowledge of Warfarin Anticoagulation Treatment Scale |
| Satisfaction Scale about Service and Warfarin Treatment |
| Perceived benefits subscale of the Beliefs about Anticoagulation Survey |
| Concerns about Anticoagulation Therapy Scale |
| Self-efficacy for Appropriate Medication Use Scale |
| Short-form Adherence to Refills and Medications Scale |
| Short-form Adherence to Refills and Medications Scale |
| Perception of Anticoagulant Treatment Questionnaire |
| Anti-Clot Treatment Scale |
| Treatment Satisfaction Questionnaire for Medication version II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucà, F.; Oliva, F.; Abrignani, M.G.; Di Fusco, S.A.; Parrini, I.; Canale, M.L.; Giubilato, S.; Cornara, S.; Nesti, M.; Rao, C.M.; et al. Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios. J. Clin. Med. 2023, 12, 5955. https://doi.org/10.3390/jcm12185955
Lucà F, Oliva F, Abrignani MG, Di Fusco SA, Parrini I, Canale ML, Giubilato S, Cornara S, Nesti M, Rao CM, et al. Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios. Journal of Clinical Medicine. 2023; 12(18):5955. https://doi.org/10.3390/jcm12185955
Chicago/Turabian StyleLucà, Fabiana, Fabrizio Oliva, Maurizio Giuseppe Abrignani, Stefania Angela Di Fusco, Iris Parrini, Maria Laura Canale, Simona Giubilato, Stefano Cornara, Martina Nesti, Carmelo Massimiliano Rao, and et al. 2023. "Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios" Journal of Clinical Medicine 12, no. 18: 5955. https://doi.org/10.3390/jcm12185955
APA StyleLucà, F., Oliva, F., Abrignani, M. G., Di Fusco, S. A., Parrini, I., Canale, M. L., Giubilato, S., Cornara, S., Nesti, M., Rao, C. M., Pozzi, A., Binaghi, G., Maloberti, A., Ceravolo, R., Bisceglia, I., Rossini, R., Temporelli, P. L., Amico, A. F., Calvanese, R., ... Gulizia, M. M. (2023). Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios. Journal of Clinical Medicine, 12(18), 5955. https://doi.org/10.3390/jcm12185955








