Abstract
With increasing knowledge of immunologic factors and with the advent of potent immunosuppressive agents, the last several decades have seen significantly improved kidney allograft survival. However, despite overall improved short to medium-term allograft survival, long-term allograft outcomes remain unsatisfactory. A large body of literature implicates acute and chronic rejection as independent risk factors for graft loss. In this article, we review measures taken at various stages in the kidney transplant process to minimize the risk of rejection. In the pre-transplant phase, it is imperative to minimize the risk of sensitization, aim for better HLA matching including eplet matching and use desensitization in carefully selected high-risk patients. The peri-transplant phase involves strategies to minimize cold ischemia times, individualize induction immunosuppression and make all efforts for better HLA matching. In the post-transplant phase, the focus should move towards individualizing maintenance immunosuppression and using innovative strategies to increase compliance. Acute rejection episodes are risk factors for significant graft injury and development of chronic rejection thus one should strive for early detection and aggressive treatment. Monitoring for DSA development, especially in high-risk populations, should be made part of transplant follow-up protocols. A host of new biomarkers are now commercially available, and these should be used for early detection of rejection, immunosuppression modulation, prevention of unnecessary biopsies and monitoring response to rejection treatment. There is a strong push needed for the development of new drugs, especially for the management of chronic or resistant rejections, to prolong graft survival. Prevention of rejection is key for the longevity of kidney allografts. This requires a multipronged approach and significant effort on the part of the recipients and transplant centers.
1. Introduction
Our understanding of the role of the immune system in allograft survival has evolved immensely since the first human-to-human kidney transplant in 1933 which failed promptly due to acute rejection [1]. With increasing knowledge of immunologic factors and with the advent of potent immunosuppressive agents, the last several decades have seen significantly improved allograft survival. Today, median graft survival ranges from 11.7 years in deceased donor kidney transplants up to 19.2 years in living donor kidney transplants [2]. However, despite overall improved short to medium-term allograft survival, long-term allograft outcomes remain unsatisfactory [3].
A large body of literature implicates acute rejection as an independent risk factor for graft loss [4,5,6,7,8,9,10]. In addition, patients who develop acute rejection early after transplant are at higher risk of chronic allograft rejection [11,12]. With the growing burden of kidney disease and a limited organ pool, it is imperative to understand the factors contributing to allograft rejection to maximize allograft longevity. Preventing rejection in the kidney allograft is a fundamental process to minimize the risk of graft loss, prolong graft survival and decrease the need for organs required for re-transplantation, which limits the organ pool further. Thus, in this article, we review measures taken at various stages in the kidney transplant process to minimize the risk of rejection [Table 1].

Table 1.
Prevention of rejection in kidney allograft.
2. Pre-Transplant Measures
2.1. Optimization of Donor-Recipient Compatibility: HLA Matching
The human leukocyte antigen (HLA) system is a complex set of highly polymorphic antigens that are responsible for immune activation by antigen presentation and recognition of ‘self’ and ‘non-self’. Although >25,000 HLA alleles have been identified, only 60 to 70 of the most frequently occurring alleles are considered when determining compatibility for organ transplantation [13]. Mismatches in donor and recipient HLA may lead to activation of alloreactive T-cells and the production of antibodies against the allograft (DSAs) thereby limiting survival. Minimizing HLA mismatches is, therefore, one of the cornerstones of reducing allograft immunogenicity.
The benefit of better HLA matching in reducing acute rejection risk has been repeatedly demonstrated. Compared to a 0 HLA mismatch, any increase in total burden of HLA mismatches at the HLA-A, B, DR loci is associated with an incremental rate of rejection [14] and overall worse allograft survival [15]. There are now also convincing data that, in addition to HLA-ABDR mismatches, mismatches at DQ are independently associated with acute rejection, regardless of initial immunosuppression with a HR of 2.85 (95% CI, 1.05 to 7.75) when there is a two-allele mismatch [16]. The conventional HLA mismatch approach is now also being challenged by more precise analyses of HLA epitopes, which are the specific antigen binding sites of the HLA molecule and eplets, which are clusters of amino acids on the antibody biding sites [17]. There is increasing evidence that eplet mismatches rather than broad HLA mismatches alone are associated with acute rejection in kidney transplant recipients. Senev et al. observed that eplet mismatch load was associated with increased rate of de novo DSA occurrence and graft failure, especially at the DQ antigen with the odds for T cell- or antibody-mediated rejection increasing by 5% and 12%, respectively, per mismatch [18]. In another study, even within recipients who were considered low immunological risk (0–2 broad antigen HLA-ABDR mismatch), those with 20 or greater eplet mismatches experienced an increased risk of rejection compared to those with fewer than 20 mismatches (adjusted HR, 1.85; 95% CI, 1.11–3.08; p = 0.019) [19]. As such, algorithms have been developed for more precise identification of amino acids on immunogenic HLA molecules [20] to predict rejection risk and tailor immunosuppression to prevent rejection.
For deceased donor organ recipients, while the degree of HLA mismatch is not modifiable, this information can certainly guide induction immunosuppression therapy (discussed later), as well as allow the individualization of maintenance immunosuppression to minimize kidney transplant rejection. However, in the scenario of a living donation where there are multiple potential donors, the donor with the lowest antigen mismatch load may be selected preferentially. Transplant centers are increasingly enrolling HLA compatible living donor pairs in the kidney-paired donation program. A multi-center study demonstrated that 26.9% of otherwise compatible pairs enrolled in such a program and more than half successfully received an equivalent or better match [21]. Several groups have created simulations to demonstrate that HLA compatible pairs enrolled may be able to achieve significantly lower HLA class I and class II total and antibody-verified eplet mismatch load through the paired kidney donation program [22].
2.2. Desensitization in HLA-Incompatible Kidney Transplantation
Approximately 20% of the kidney transplant recipient pool is ‘sensitized’, which refers to the presence of antibodies against commonly found HLA antigens that may be donor-specific and may render the donor and recipient HLA-incompatible. Common reasons for HLA antibody development include prior blood transfusions, multiple pregnancies and prior organ transplantation [23]. Presence of even low level DSAs prior to transplantation can nearly double the risk of antibody-mediated rejection (RR 1.98; 95% CI 1.36–2.89; p < 0.001) and increase the risk for graft failure (RR, 1.76; 95% CI, 1.13–2.74; p = 0.01) [24].
However, in the case of highly sensitized patients (cPRA > 98%) that have a potential living donor or have been waiting for a deceased donor transplant for a long time, some centers opt for desensitization to improve the likelihood of transplantation and to reduce the risk of rejection across HLA-incompatibility. This entails identifying certain low-level DSAs (>3000 MFI) that may result in a positive flow cross match that are aggressively removed prior to transplantation via pheresis or immunoadsorption followed by targeted B and T-cell therapy to prevent further DSA formation.
Different combinations of therapies with varying amounts of success have been studied. IVIG therapy by itself has shown little promise [25] but IVIG in combination in rituximab alone [26] and rituximab and apheresis [27,28,29] or rituximab and immunoadsorption [30] have all been used successfully. Long term outcome studies have demonstrated a 5-year allograft survival as high as 80% in desensitized recipients of living donor kidney transplants [31]. Those who were desensitized had a clear survival benefit of 86% at 5 years [32] compared to 59.2% survival in those who remained on dialysis and 74.4% in patients who either waited or received a deceased donor kidney transplant during follow-up (p < 0.001). As such, desensitization can be a valuable tool to minimize rejection in highly sensitized individuals receiving HLA-incompatible transplants.
A wide variety of desensitization protocols have been employed by transplant centers depending on center-specific practices. Ranging from monthly IVIG for a total of 2 g/kg [33] to more aggressive therapy with 2 g/kg IVIG followed by 5–10 plasmapheresis treatments and 1–2 Rituximab doses [34].
Some studies have explored the use of novel agents for desensitization. Marks et al. examined the use of eculizumab versus standard of care (plasmapheresis +/− IVIG) and found no significant difference in treatment failure between the two groups at 9 weeks post transplantation (9.8% vs. 13.7%, p = 0.760). A French study looked at tocilizumab (IL-6 inhibitor) in comparison to standard therapy (apheresis and Rituximab) and found no difference in reduction of pre-transplant MFIs between the groups [35].
2.3. Role of Non-HLA Mismatches in Transplant Rejection
There is increasing evidence that donor-recipient mismatches at non-HLA regions may also be responsible for alloimmunity leading to chronic rejection and allograft loss. These mechanisms are discussed in detail by Jethwani et al. [36]. There are currently limited diagnostic and therapeutic options for the prevention or treatment of non-HLA antibody mediated rejection.
Key Points of pre-transplant measures
|
Now, we transition to the measures taken at and around the time of the transplant surgery.
3. Peri-Transplant Measures
3.1. Minimizing Cold-Ischemia Time/Optimizing Perfusion
A prolonged cold ischemia time of >24 h versus <12 h has been demonstrated to increase the rate of acute transplant rejection with relative risk of 1.13 (95% CI, 1.04–1.23) in first-time transplant recipients and relative risk of up to 1.66 (95% CI, 1.01–2.73) in retransplant candidates. This effect was not noted in recipients >60 years of age [37]. Strategies to minimize cold ischemia time may improve incidence of acute kidney transplant rejection rates, delayed graft function and allograft survival, especially in organs with a higher kidney donor profile index (KDPI) [38]. These strategies include simultaneous local and regional offers for higher KDPI kidneys and clear documentation by transplant centers about their organ acceptance criteria [38]. Several studies have shown improved rates of acute rejection with the use of hypothermic machine perfusion compared to static cold storage [39,40,41]. A meta-analysis comparing static cold storage to hypothermic machine perfusion did demonstrate improved DGF rates (RR 0.78, 95% CI 0.69–0.87, p < 0.0001), and improved graft survival at 3 years (RR 1.06, 95% CI 1.02–1.11, p = 0.009) but no difference in rate of acute rejection was identified [42]. Most transplant centers agree that this practice is useful to prolong the life of an allograft.
3.2. Individualizing Induction Immunosuppression
Induction immunosuppression is provided at the time of transplantation to minimize the risk of hyperacute and acute rejection. There are two categories of agents available: lymphocyte-depleting such as alemtuzumab (anti-CD52 antibody) and anti-thymocyte globulin (multi-cellular inhibition) and non-depleting such as basiliximab (IL-2 receptor antagonist) [Table 2].

Table 2.
Induction Immunosuppression Agents.
Previous studies looking at induction vs. no induction agent demonstrated an overall reduction in deceased donor graft failure with lymphocyte-depleting agents [43] with reduction in allograft failure greater in patients with panel reactive antibody ≥ 20% (ratio of adjusted rate of 0.12, 95% confidence interval, 0.03–0.44; p = 0.002) [44]. It is now common practice to use induction immunosuppression at the time of transplantation. Choice of agent depends largely on anticipated immunologic risk. The definition of ‘high risk’ is controversial, heavily debated and factors in both recipient and donor characteristics. Studies comparing graft outcomes between basiliximab and anti-thymocyte globulin (ATG) defined high risk as black ethnicity, >3 HLA antigen mismatches, > 1 HLA-DR mismatch, higher PRA (>20%), cold ischemia time >24 h, delayed graft function, prior transplant and older donor age [45]. Compared to basiliximab, the anti-thymocyte globulin group had fewer incidences of acute rejection (15.6% vs. 25.5%, p = 0.02) and of acute rejection that required treatment with antibodies (1.4% vs. 8.0%, p = 0.005) [45]. However, it was noted that there was a significantly higher rate of infection in patients who received (ATG) compared with basiliximab. Interestingly, no studies have demonstrated a difference in patient or allograft survival between the two agents.
In most studies comparing alemtuzumab to ATG, there was no significant difference in biopsy-proven acute rejection or allograft survival [46,47,48] between the two agents suggesting no benefit of using alemtuzumab over anti-thymocyte globulin. A meta-analysis comparing induction with basiliximab to induction with ATG looked at pooled results from eight randomized controlled trials and found no significant differences in 1-year acute rejection rate (OR 1.32; 95% CI 0.93–1.87; p = 0.13), 1-year graft survival rate (OR 0.73; 95% CI 0.45–1.18; p = 0.20), 1-year patient survival rate (OR 0.52; 95% CI 0.27–1.02; p = 0.06) or 1-year infection rate [49]. However, the study did note a lower risk of neoplasm in the Basiliximab group (OR 0.26; CI 0.08–0.78; p = 0.02).
Another meta-analysis that compared efficacy and safety of induction agents found that alemtuzumab (OR 0.45, CI 0.29–0.78) and rATG (OR 0.63, CI 0.42–0.95) exhibited lower incidence of biopsy-proven acute rejection than basiliximab without a difference in graft survival. This came at the cost of a higher infection rate with ATG (OR, 1.8, CI, 1.01–2.8). [50]
In conclusion, use of ATG for induction is beneficial for individuals identified as having high risk immunologic profiles to minimize the risk of acute rejection. While comparison studies do not demonstrate convincing evidence of improved allograft survival using ATG vs. basiliximab, lack of long-term follow-up remains a limitation. In addition, use of ATG in high-risk individuals minimizes the cumulative burden of immunosuppression for treatment of acute rejection episodes. Infectious risk is an important factor that weighs into this decision due to greater infection rates with ATG use.
Key points of peri-transplant measures
|
We will now take a more detailed look into the post-transplant measures to reduce the risk of rejection in the kidney allograft.
4. Post-Transplant Measures to Prevent Rejection
The post-transplant phase is critical in the prevention of both acute and chronic rejection of the kidney allograft. This can be divided into the immediate post-operative period, in which maintenance immunosuppression is introduced into a recipient’s arsenal to prevent rejection and is a critical transition period where tolerability, therapeutic drug levels and early compliance is established. This is followed by the chronic post-transplant phase where compliance is cemented and a fine balance between immunosuppression and infection is maintained.
Conventional maintenance regimens consist of a combination of immunosuppressive agents that differ by mechanism of action and side effect profiles [Table 3]. This strategy minimizes the morbidity and mortality associated with each class of agent while maximizing overall effectiveness. Such regimens may vary by patient, transplant center and geographic area. [51]

Table 3.
Maintenance Immunosuppression Agents.
The most common strategy is triple drug therapy with a combination of calcineurin inhibitor (CNI), antimetabolite (mycophenolic acid or azathioprine) and steroids. The use of non-CNI based regimens including co-stimulation blockers (Belatacept) and mTOR inhibitors (sirolimus or everolimus) is around 10%.
Serial discoveries and improvements in the drugs employed for maintenance immunosuppression have played a key role in the dramatic decrease in rates of acute rejection to <10% within the first year after transplant [52]. With excellent outcomes achieved in the short term, the aim of maintenance immunosuppression goes beyond prevention of acute rejection and includes the prevention of chronic allograft rejection and nephropathy [53].
This section will be sub-divided into the prevention of cell mediated and antibody mediated rejections.
4.1. Prevention of Cell Mediated Rejection
T-cell proliferation and signaling pathways
The key cellular mediator of rejection is the T-lymphocyte. Most immunosuppressive agents work by disrupting the keys pathways of T-cell activation. Signal 1 is initiated by the interaction of the T-cell receptor (TCR) on the T-cell with the Major Histocompatibility Complex (MHC) molecule on the Antigen Presenting Cell (APC) via the CD3 complex. Co-stimulation or Signal 2 constitutes the interaction of CD80 and CD86(B7) on the surface of APC and CD28 on T-cells. Both Signal 1 and 2 are essential to activate three signal transduction pathways: the calcium-calcineurin pathway, the RAS-mitogen activated protein (MAP) kinase pathway and the nuclear factor-kb pathway [54]. These pathways activate transcription factors that trigger the expression of many new molecules, including interlekin-2 (IL-2), CD154 and CD25. IL-2 and other cytokines activate the “target of rapamycin” pathway to provide Signal 3, the trigger for cell proliferation. Nucleotide synthesis is also required for lymphocyte proliferation and the mobilization of effector T-cells. Different classes of immunosuppressive agents target different steps of the T-cell proliferation pathways to prevent their activation and subsequent rejection of the allograft. These mechanisms have been explained in more detail in another article in this series titled Pathophysiology of rejection in kidney transplantation.
CNIs are considered the backbone of maintenance immunosuppression and are used by a vast majority of transplant centers in the United States [52] Cyclosporine and tacrolimus are the two agents of this class in clinical use and voclosporin remains an investigational agent currently [55]. CNIs bind to their binding proteins (FKBP for tacrolimus and cyclophilin for cyclosporine) and inhibit calcineurin. This inhibition blocks the dephosphorylation and activation of nuclear factor NFAT thus preventing transcription of IL-2 which is critical to lymphocyte proliferation. Cyclosporine was touted as a gamechanger after its discovery in the 1980s. However, it has largely been replaced by tacrolimus as the CNI of choice in most immunosuppression regimens. A large meta-analysis of 30 trials (4102 patients) comparing these two agents favored tacrolimus for multiple endpoints with a 44% reduction in death-censored graft failure and 31% reduction in the risk of acute rejection within 1 year of transplant [56]. This analysis also revealed a significantly higher risk of development of insulin-dependent diabetes, neurological and GI side effects with tacrolimus. Given the side effect profile and varying sensitivity to the two drugs, an individualized approach in deciding a CNI may be needed in certain patients.
Antimetabolites are an integral part of maintenance immunosuppression regimens. The most used agents are Mycophenolic acid (MPA) and azathioprine. These agents inhibit nucleotide synthesis which limits T and B-lymphocyte proliferation. MPA was approved by the FDA for the prevention of rejection in 1995. This was based on the Tricontinental Study (North America, Europe and Australia) which showed a significantly lower risk of acute rejection in kidney transplant recipients on MPA compared to Azathioprine [57]. A meta-analysis of 23 studies which included 3301 participants showed the MPA was superior to azathioprine in terms of the risk of graft loss including death (RR 0.82), death-censored graft loss (RR 0.78) and any acute rejection (RR 0.65) [58]. Thus, MPA has become the favored agent in combination with CNIs in most patients for the prevention of acute rejection. It is contra-indicated in pregnancy as it is teratogenic.
CNI Free Regimens
Although tacrolimus remains the main component of maintenance immunosuppression, it is associated with significant toxicities and thus may not be usable in all patients. In such situations, CNI free regimens have been established. Belatacept is a first in class co-stimulation blocker which interacts with Signal 2 and thus selectively blocks T-cell activation. It was approved by FDA in 2011 based on two landmark trials which compared Belatacept with cyclosporine for maintenance immunosuppression both in standard and extended criteria kidneys. Although the rate of acute rejection was higher in the Belatacept cohorts, however, this did not have an impact on long term patient and graft survival [59,60]. Subsequent meta-analysis of 5 studies that compared Belatacept and CNIs (1535 patients) have reported similar rates of death, allograft survival and acute rejection after 3 years of transplant [61]. Studies of conversion from CNI to Belatacept have also shown similar trends [62]. mTOR inhibitors engage FKBP12 to create complexes that inhibit the target of rapamycin which blocks Signal 3 by preventing cytokine receptors from activating the cell cycle. mTOR inhibitors have been evaluated in several regimens but have not shown to be superior to either CNIs or antimetabolites in prevention of rejection. However, they do have anti-viral and anti-tumor activity and thus are favored in such situations [63].
Corticosteroids remain an integral part of most immunosuppressive regimens. They inhibit production of activating cytokines and downregulate the expression of activating molecules on the surface of T-lymphocytes. However, recent trends have seen an increase in steroid free regimens to minimize long term side effects [52].
4.2. Prevention of Antibody Mediated Rejection
Antibody-mediated rejection (AMR) is a significant complication following kidney transplantation that contributes toward both short- and long-term injury in approximately 1% to 10% of kidney transplant recipients [64]. Certain factors including allo-sensitization, patient non-compliance and iatrogenic reduction in immunosuppression contribute significantly to the emergence of de novo HLA and non-HLA antibodies or persistence of pre-existing antibodies. This increases risk for chronic AMR which is thought to be a significant cause of premature graft failure [65,66,67]. The best treatment for AMR is to prevent it. Post-transplant prevention of AMR should involve a multipronged strategy.
Maintenance of immunosuppression remains a key element in this approach. Therapeutic tacrolimus levels are associated with reduced production of de novo DSA [68]. CNI minimization strategies have been proposed and used to mitigate the potential side effects of CNIs including nephrotoxicity which was thought to contribute significantly to late allograft loss. However, given the extensive research to suggest that chronic AMR is the bigger culprit [65,66,67], care providers must be careful when making changes to immunosuppression regimens, including lowering targets for CNI troughs and weighing the potential risks/benefits, especially in highly sensitized transplant patients. Mycophenolate Mofetil (MMF) is associated with decreased formation of de novo DSA and rates of biopsy proven AMR as compared to no MMF/Azathioprine in combination with a CNI [58,69]. Belatacept has also been shown to lower the incidence of donor specific antibody (DSA) formation both when used de novo and when used as a CNI conversion strategy [59,60,70]. Belatacept-based immunosuppression decreases pre-existing antibodies [71]. Thus, careful curation of immunosuppression strategies considering demographic variables, immunological risk and patient preferences should be made to minimize the risk of AMR and prevention of premature graft loss.
Another aspect of this multipronged strategy involves monitoring for the development of DSA. Currently there are no standard guidelines for this approach, and individual centers create their own protocols based on multiple factors including patient variables, HLA lab and logistical support and cost. It is debatable if lab monitoring for alloantibodies is needed in all transplant patients, but it seems justified in immunologically high-risk patients, desensitized recipients, patients with a suspicion for rejection and during treatment of an AMR to recognize allograft injury early and prevent its translation into chronic rejection [72].
4.3. Minimizing Non-Compliance
Non-compliance is a major risk factor for rejection and has been associated with premature graft loss [73,74]. It encompasses multiple aspects of transplant related care including immunosuppression medication, lab monitoring, transportation to medical visits and lifestyle modifications to minimize the risk of non-immunological graft injury and subsequent failure. Certain demographic groups are considered high risk including teenagers and young adults who are transitioning from the pediatric to adult renal services, certain social and ethnic groups and individuals known to be facing financial crises that make drugs and ongoing medical care unaffordable [74]. Given the seriousness and potential graft threatening consequences of non-adherence, every effort should be made to address this early as the likely benefits of this intervention will be diminished after emergence of de novo DSA [75]. Enhanced surveillance, especially for higher risk groups, may include interviews including additional use of telemedicine, visits from social workers, utilizing the transplant pharmacy teams to monitor prescription filling records and reiterating medication education and additional antibody screening at regular intervals especially for higher immunological risk patients.
4.4. Treatment of Rejection
Patients at risk for denovo DSA formation have preceding cellular rejections with more intense inflammation within the microvasculature and if not treated early and aggressively, it is postulated that peritubular capillaritis can lead to increased HLA expression in the microcirculation, thereby increasing the risk of allo-recognition by the recipient B-cell compartment. Moreover, when cellular rejection coincides with DSA and antibody-mediated microvascular injury, it may accelerate the time to graft dysfunction and graft loss [73]. This underscores the importance of aggressive treatment for active cellular or antibody mediated rejection to decrease the risk of chronic rejection and graft loss [73]. After addressing the acute inflammatory component with appropriate intervention, the treating physician should make sure to optimize the maintenance immunosuppression. This may include resetting target troughs for CNIs, especially if a CNI minimization strategy has been previously employed for the patient, re-introduction or increase in antimetabolite or consideration of switch to Belatacept if CNI avoidance is warranted. Treatment strategies, including details of medications used, for the same are outlined in another article of this series titled Current therapies in Kidney Transplant Rejection.
4.5. Role of Biomarkers
A multitude of novel biomarkers have been developed to assess allograft health and predict rejection before changes in GFR take place and to predict long term graft outcomes. Only a few are currently available in clinic practice. A potential early indicator for the injury and loss of allograft is donor derived cell free DNA circulating in the blood of transplanted patients. This is measured as a percent of recipient circulating DNA and increase in this fraction is a sensitive marker of allograft injury [76]. Three assays, based on NGS technology: Allosure, TRAC and Prospera are currently available for commercial use. These have been validated and are most useful for detection of AMR [77,78]. Gene expression profiling based non-invasive test available as TRUGRAF is validated for detecting subclinical rejection [79]. These tests can potentially be used for monitoring allograft function post rejection treatment and can direct immunosuppression therapy changes to balance the risk of future rejection and adverse effects. One major hindrance to their consistent use is the associated cost and thus the companies offering these tests should work towards subsidizing them over time. Other promising biomarker classes which can potentially contribute to the prevention of both acute and chronic rejection include chemokines, free microRNAs and leucocyte subclasses [80].
4.6. Emerging Therapies
Given the significant improvements in acute cellular rejection rates over time [52], there have not been any major efforts to make newer drugs affecting T-lymphocyte signaling. Novartis launched the CIRRUS-1 study in 2018 to evaluate the safety and efficacy of iscalimab (a non-B-lymphocyte depleting anti CD-40 monoclonal antibody) in kidney transplant recipients [81]. However, this was terminated early due to an interim analysis showing inferiority to tacrolimus-based regimens for rejection prevention. Clazakizumab, a monoclonal antibody against the IL-6 ligand has shown promising results in a phase 2 pilot RCT [82]. It decreased the DSA levels in patients with AMR after 1 year of transplant and showed a significantly slower decline in GFR as compared to placebo. A large phase 3 trial of clazakizumab in patients with chronic active AMR is currently ongoing [83]. Tocilizumab, a monoclonal antibody against the IL-6 receptor has shown promise both in desensitization protocols as well as for treatment of chronic AMR [84,85]. Larger RCTs are needed to confirm these findings and justify the use given significant cost associated with this drug.
Daratumumab is a monoclonal antibody against CD38 which has shown promising results in decreasing anti-HLA antibodies in AMR [86,87] and has been proposed as an agent for desensitization and treatment of AMR. However, more concrete evidence is needed before its acceptance in clinical practice.
Belimumab is a humanized, monoclonal, anti B-lymphocyte stimulator (BLyS) IgG1 antibody that prevents B-cell survival and differentiation into plasma cells. In a RCT with 28 kidney transplant recipients, there was no significant difference in the risk of major infections compared to standard immunosuppression but the IL-10/IL6 ratio of the B-cell distribution was skewed towards a regulatory profile and activated memory B-cells and plasmablasts were significantly reduced [88] This likely has the downstream effect of decreasing the risk of rejection. However, specific studies are needed to address this.
Imlifidase, an IgG degrading enzyme of Streptococcus pyogenes (IdeS), cleaves human IgG at a specific amino acid sequence within the hinge region producing Fc and F(ab)s fragments effectively blocking complement dependent cytotoxicity and antibody dependent cellular cytotoxicity [89]. It was associated with rapid reduction and even elimination of DSA [90,91]. However, rebound in DSA and anti-IdeS antibody development are significant issues associated with its use. This, along with the patient population that it will benefit the most will need to be addressed in future studies.
Berinert and Cinryze are plasma C1-esterase inhibitors that have been tested in two pilot studies and have shown a functional improvement in AMR [92,93]. More trials are currently underway and hopefully will define the role of these agents in the prevention of AMR.
A more detailed discussion on these agents is covered in a subsequent article of the series.
Key points of the post-transplant measures
|
5. Financial Impact, Practical Considerations and Real-World Challenges
Kidney transplantation is considered the best modality to manage ESRD. It has not only shown improved outcomes, both in terms of mortality and quality of life as compared to dialysis, but multiple studies have shown its cost-effectiveness and economic advantages over dialysis [94,95]. While there are certain sub-groups which incur a higher cost initially, in the long run kidney transplantation is consistently considered the superior financial option. Thus, every effort should be made to improve long-term graft outcomes and preventing rejection is a key component of the same. There are real-world challenges and practical considerations as it relates to maintaining compliance over long periods of time, including insurance coverage for medications and clinical care, logistical challenges related to transportation and an ever-increasing complexity of transplant patients which warrants a steady increase in the personnel to manage them effectively.
6. Conclusions
Prevention of rejection is key for the longevity of kidney allografts. This can be achieved at various stages in the lifetime of the graft and requires a multipronged approach and significant effort on the part of the recipients and transplant centers [Figure 1 and Figure 2]. We recognize the limitations of the current research, especially the lack of long-term follow-up for many studies of induction and maintenance immunosuppression therapy. Emerging therapies are yet to prove their worth in RCTs and long-term studies. Future research should focus on long-term graft outcomes and highlighting the comparative performance of common induction and maintenance agents as well as development and validation of newer therapies for rejection prevention. There is a lot of interest in the novel biomarkers as tools for immunosuppression modulation and prediction of allograft injury much before the usual markers of graft function like serum creatinine, urine protein, etc. are affected. However, these novel biomarkers are expensive and present significant logistical challenges including long turn-around times. Thus, a significant focus needs to be directed towards these issues.
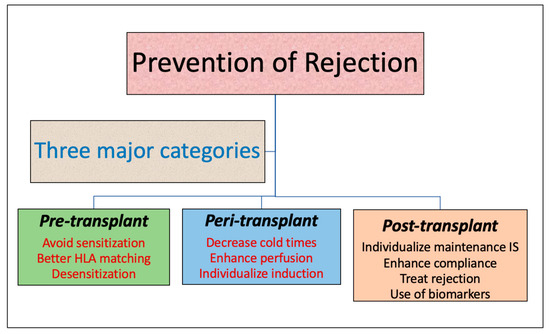
Figure 1.
Prevention of rejection.
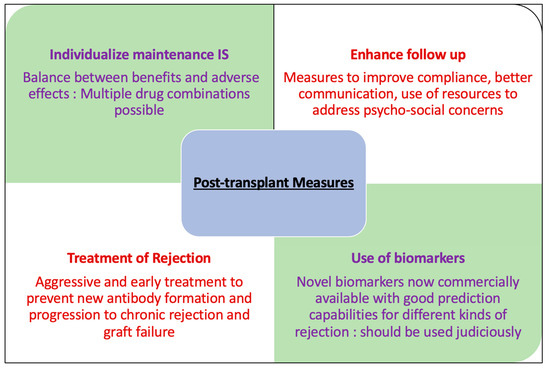
Figure 2.
Post-transplant measures to prevent rejection.
Author Contributions
Both authors contributed equally to the conceptualization, draft preparation, review and editing of this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This work was supported in part by a generous gift from Melody and Raymond Ranelli.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Voronoy, U. Blocking the reticuloendothelial system in man in some forms of mercuric chloride intoxication and the transplantation of the cadaver kidney as a method of treatment for the anuria resulting from the intoxication. Siglo Med. 1937, 97, 296. [Google Scholar]
- Poggio, E.D.; Augustine, J.J.; Arrigain, S.; Brennan, D.C.; Schold, J.D. Long-term kidney transplant graft survival-Making progress when most needed. Am. J. Transplant. 2021, 21, 2824–2832. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Smith, J.M.; Hart, A.; Miller, J.; Skeans, M.A.; Larkin, L.; Robinson, A.; Gauntt, K.; Israni, A.K.; Hirose, R.; et al. OPTN/SRTR 2020 Annual Data Report: Kidney. Am. J. Transplant. 2022, 22 (Suppl. S2), 21–136. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Gheorghian, A.; Axelrod, D.; Kalsekar, A.; L’Italien, G.; Schnitzler, M.A. The implications of acute rejection for allograft survival in contemporary U.S. kidney transplantation. Transplantation 2012, 94, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, J.; Wang, Y.; Zhang, J.; Zhu, Z.; Shou, Z.; Wang, S.; Zhang, P.; Huang, H.; He, Q. Impact of acute rejection episodes on long-term renal allograft survival. Chin. Med. J. 2003, 116, 1741–1745. [Google Scholar]
- Cole, E.H.; Johnston, O.; Rose, C.L.; Gill, J.S. Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin. J. Am. Soc. Nephrol. 2008, 3, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.M.; Mandia-Sampaio, E.L.; de Sandes-Freitas, T.V.; Felipe, C.R.; Park, S.I.; Pinheiro-Machado, P.G.; Garcia, R.; Tedesco-Silva, H., Jr.; Medina-Pestana, J.O. Risk factors associated with graft loss and patient survival after kidney transplantation. Transplant. Proc. 2009, 41, 3667–3670. [Google Scholar] [CrossRef]
- Jalalzadeh, M.; Mousavinasab, N.; Peyrovi, S.; Ghadiani, M.H. The impact of acute rejection in kidney transplantation on long-term allograft and patient outcome. Nephrourol. Mon. 2015, 7, e24439. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.U.; Ojo, A.O.; Hanson, J.A.; Cibrik, D.M.; Punch, J.D.; Leichtman, A.B.; Kaplan, B. Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation 2000, 70, 1098–1100. [Google Scholar] [CrossRef]
- Pallardó Mateu, L.M.; Sancho Calabuig, A.; Capdevila Plaza, L.; Franco Esteve, A. Acute rejection and late renal transplant failure: Risk factors and prognosis. Nephrol. Dial. Transplant. 2004, 19 (Suppl. S3), iii38–iii42. [Google Scholar] [CrossRef]
- Almond, P.S.; Matas, A.; Gillingham, K.; Dunn, D.L.; Payne, W.D.; Gores, P.; Gruessner, R.; Najarian, J.S. Risk factors for chronic rejection in renal allograft recipients. Transplantation 1993, 55, 752–756; Discussion 756–757. [Google Scholar] [CrossRef] [PubMed]
- Basadonna, G.P.; Matas, A.J.; Gillingham, K.J.; Payne, W.D.; Dunn, D.L.; Sutherland, D.E.; Gores, P.F.; Gruessner, R.W.; Najarian, J.S. Early versus late acute renal allograft rejection: Impact on chronic rejection. Transplantation 1993, 55, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.Y. The HLA system: Genetics, immunology, clinical testing, and clinical implications. Yonsei Med. J. 2007, 48, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Chadban, S.J.; Clayton, P.; Budgeon, C.A.; Murray, K.; Campbell, S.B.; Cohney, S.; Russ, G.R.; McDonald, S.P. Human leukocyte antigen mismatches associated with increased risk of rejection, graft failure, and death independent of initial immunosuppression in renal transplant recipients. Clin. Transplant. 2012, 26, E428–E437. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C.; Opelz, G.; McGarvey, C.J.; Weil, E.J.; Chakkera, H.A. The Risk of Transplant Failure with HLA Mismatch in First Adult Kidney Allografts from Deceased Donors. Transplantation 2016, 100, 1094–1102. [Google Scholar] [CrossRef]
- Lim, W.H.; Chapman, J.R.; Coates, P.T.; Lewis, J.R.; Russ, G.R.; Watson, N.; Holdsworth, R.; Wong, G. HLA-DQ Mismatches and Rejection in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2016, 11, 875–883. [Google Scholar] [CrossRef]
- Kumru Sahin, G.; Unterrainer, C.; Süsal, C. Critical evaluation of a possible role of HLA epitope matching in kidney transplantation. Transplant. Rev. 2020, 34, 100533. [Google Scholar] [CrossRef]
- Senev, A.; Coemans, M.; Lerut, E.; Van Sandt, V.; Kerkhofs, J.; Daniëls, L.; Driessche, M.V.; Compernolle, V.; Sprangers, B.; Van Loon, E.; et al. Eplet Mismatch Load and De Novo Occurrence of Donor-Specific Anti-HLA Antibodies, Rejection, and Graft Failure after Kidney Transplantation: An Observational Cohort Study. J. Am. Soc. Nephrol. 2020, 31, 2193–2204. [Google Scholar] [CrossRef]
- Do Nguyen, H.T.; Wong, G.; Chapman, J.R.; McDonald, S.P.; Coates, P.T.; Watson, N.; Russ, G.R.; D’Orsogna, L.; Lim, W.H. The Association between Broad Antigen HLA Mismatches, Eplet HLA Mismatches and Acute Rejection after Kidney Transplantation. Transplant. Direct 2016, 2, e120. [Google Scholar] [CrossRef]
- Duquesnoy, R.J. HLAMatchmaker: A molecularly based algorithm for histocompatibility determination. I. Description of the algorithm. Hum. Immunol. 2002, 63, 339–352. [Google Scholar] [CrossRef]
- Chipman, V.; Cooper, M.; Thomas, A.G.; Ronin, M.; Lee, B.; Flechner, S.; Leeser, D.; Segev, D.L.; Mandelbrot, D.A.; Lunow-Luke, T.; et al. Motivations and outcomes of compatible living donor-recipient pairs in paired exchange. Am. J. Transplant. 2022, 22, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Tafulo, S.; Malheiro, J.; Dias, L.; Lobato, L.; Ramalhete, L.; Martinho, A.; Bolotinha, C.; Costa, R.; Ivo, M. Improving HLA matching in living donor kidney transplantation using kidney paired exchange program. Transpl. Immunol. 2020, 62, 101317. [Google Scholar] [CrossRef] [PubMed]
- Abbes, S.; Metjian, A.; Gray, A.; Martinu, T.; Snyder, L.; Chen, D.-F.; Ellis, M.; Arepally, G.M.; Onwuemene, O. Human Leukocyte Antigen Sensitization in Solid Organ Transplantation: A Primer on Terminology, Testing, and Clinical Significance for the Apheresis Practitioner. Ther. Apher. Dial. 2017, 21, 441–450. [Google Scholar] [CrossRef]
- Mohan, S.; Palanisamy, A.; Tsapepas, D.; Tanriover, B.; Crew, R.J.; Dube, G.; Ratner, L.E.; Cohen, D.J.; Radhakrishnan, J. Donor-specific antibodies adversely affect kidney allograft outcomes. J. Am. Soc. Nephrol. 2012, 23, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, N.; Lonze, B.E.; Zachary, A.A.; Holechek, M.J.; Schillinger, K.; Cameron, A.M.; Desai, N.M.; Dagher, N.N.; Segev, D.L.; Montgomery, R.A.; et al. Infusion of high-dose intravenous immunoglobulin fails to lower the strength of human leukocyte antigen antibodies in highly sensitized patients. Transplantation 2012, 94, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.A.; Petrozzino, J.; Yeung, K.; Sinha, A.; Kahwaji, J.; Peng, A.; Villicana, R.; Mackowiak, J.; Jordan, S.C. Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. Transplantation 2013, 95, 852–858. [Google Scholar] [CrossRef]
- Magee, C.C.; Felgueiras, J.; Tinckam, K.; Malek, S.; Mah, H.; Tullius, S. Renal transplantation in patients with positive lymphocytotoxicity crossmatches: One center’s experience. Transplantation 2008, 86, 96–103. [Google Scholar] [CrossRef]
- Loupy, A.; Suberbielle-Boissel, C.; Zuber, J.; Anglicheau, D.; Timsit, M.O.; Martinez, F.; Thervet, E.; Bruneval, P.; Charron, D.; Hill, G.S.; et al. Combined posttransplant prophylactic IVIg/anti-CD 20/plasmapheresis in kidney recipients with preformed donor-specific antibodies: A pilot study. Transplantation 2010, 89, 1403–1410. [Google Scholar] [CrossRef]
- Amrouche, L.; Aubert, O.; Suberbielle, C.; Rabant, M.; Van Huyen, J.D.; Martinez, F.; Sberro-Soussan, R.; Scemla, A.; Tinel, C.; Snanoudj, R.; et al. Long-term Outcomes of Kidney Transplantation in Patients with High Levels of Preformed DSA: The Necker High-Risk Transplant Program. Transplantation 2017, 101, 2440–2448. [Google Scholar] [CrossRef]
- Lorenz, M.; Regele, H.; Schillinger, M.; Kletzmayr, J.; Haidbauer, B.; Derfler, K.; Druml, W.; Böhmig, G.A. Peritransplant immunoadsorption: A strategy enabling transplantation in highly sensitized crossmatch-positive cadaveric kidney allograft recipients. Transplantation 2005, 79, 696–701. [Google Scholar] [CrossRef]
- Bagnasco, S.M.; Zachary, A.A.; Racusen, L.C.; Arend, L.J.; Carter-Monroe, N.; Alachkar, N.; Nazarian, S.M.; Lonze, B.E.; Montgomery, R.A.; Kraus, E.S. Time course of pathologic changes in kidney allografts of positive crossmatch HLA-incompatible transplant recipients. Transplantation 2014, 97, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Orandi, B.J.; Luo, X.; Massie, A.B.; Garonzik-Wang, J.M.; Lonze, B.E.; Ahmed, R.; Van Arendonk, K.J.; Stegall, M.D.; Jordan, S.C.; Oberholzer, J.; et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. N. Engl. J. Med. 2016, 374, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Tyan, D.; Stablein, D.; McIntosh, M.; Rose, S.; Vo, A.; Toyoda, M.; Davis, C.; Shapiro, R.; Adey, D.; et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: Report of the NIH IG02 trial. J. Am. Soc. Nephrol. 2004, 15, 3256–3262. [Google Scholar] [CrossRef] [PubMed]
- Marks, W.H.; Mamode, N.; Montgomery, R.A.; Stegall, M.D.; Ratner, L.E.; Cornell, L.D.; Rowshani, A.T.; Colvin, R.B.; Dain, B.; Boice, J.A.; et al. Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: A randomized trial. Am. J. Transplant. 2019, 19, 2876–2888. [Google Scholar] [CrossRef]
- Jouve, T.; Daligault, M.; Noble, J.; Terrec, F.; Imerzoukene, F.; Dard, C.; Bardy, B.; Malvezzi, P.; Rostaing, L. Tocilizumab Evaluation in HLA-Desensitization before Kidney Transplantation as an Add-On Therapy to Apheresis: The TETRA Study. J. Clin. Med. 2023, 12, 424. [Google Scholar] [CrossRef]
- Jethwani, P.; Rao, A.; Bow, L.; Menon, M.C. Donor-Recipient Non-HLA Variants, Mismatches and Renal Allograft Outcomes: Evolving Paradigms. Front. Immunol. 2022, 13, 822353. [Google Scholar] [CrossRef]
- Postalcioglu, M.; Kaze, A.D.; Byun, B.C.; Siedlecki, A.; Tullius, S.G.; Milford, E.L.; Paik, J.M.; Abdi, R. Association of Cold Ischemia Time with Acute Renal Transplant Rejection. Transplantation 2018, 102, 1188–1194. [Google Scholar] [CrossRef]
- Sampaio, M.S.; Chopra, B.; Tang, A.; Sureshkumar, K.K. Impact of cold ischemia time on the outcomes of kidneys with Kidney Donor Profile Index ≥85%: Mate kidney analysis—A retrospective study. Transpl. Int. 2018, 31, 729–738. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.-H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.H.; Squifflet, J.-P.; van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Wells, A.C.; Roberts, R.J.; Akoh, J.A.; Friend, P.J.; Akyol, M.; Calder, F.R.; Allen, J.E.; Jones, M.N.; Collett, D.; et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: A UK multicenter randomized controlled trial. Am. J. Transplant. 2010, 10, 1991–1999. [Google Scholar] [CrossRef]
- Helio Tedesco-Silva, J.; Offerni, J.C.M.; Carneiro, V.A.; de Paula, M.I.; Neto, E.D.; Lemos, F.B.C.; Moura, L.R.R.; e Silva Filho, A.P.; de Morais Cunha, M.D.F.; da Silva, E.F.; et al. Randomized Trial of Machine Perfusion Versus Cold Storage in Recipients of Deceased Donor Kidney Transplants with High Incidence of Delayed Graft Function. Transplant. Direct 2017, 3, e155. [Google Scholar] [CrossRef] [PubMed]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Thompson, E.R.; Ibrahim, I.K.; Bates, L.; Talbot, D.; Wilson, C.H. Hypothermic machine perfusion is superior to static cold storage in deceased donor kidney transplantation: A meta-analysis. Clin. Transplant. 2020, 34, e13814. [Google Scholar] [CrossRef] [PubMed]
- Szczech, L.A.; Berlin, J.A.; Aradhye, S.; Grossman, R.A.; Feldman, H.I. Effect of anti-lymphocyte induction therapy on renal allograft survival: A meta-analysis. J. Am. Soc. Nephrol. 1997, 8, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Mourad, G.; Morelon, E.; Noël, C.; Glotz, D.; Lebranchu, Y. The role of Thymoglobulin induction in kidney transplantation: An update. Clin. Transplant. 2012, 26, E450–E464. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.C.; Daller, J.A.; Lake, K.D.; Cibrik, D.; Del Castillo, D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N. Engl. J. Med. 2006, 355, 1967–1977. [Google Scholar] [CrossRef]
- Lü, T.M.; Yang, S.L.; Wu, W.Z.; Tan, J.M. Alemtuzumab induction therapy in highly sensitized kidney transplant recipients. Chin. Med. J. 2011, 124, 664–668. [Google Scholar]
- Thomas, P.G.; Woodside, K.J.; Lappin, J.A.; Vaidya, S.; Rajaraman, S.; Gugliuzza, K.K. Alemtuzumab (Campath 1H) induction with tacrolimus monotherapy is safe for high immunological risk renal transplantation. Transplantation 2007, 83, 1509–1512. [Google Scholar] [CrossRef]
- Hanaway, M.J.; Woodle, E.S.; Mulgaonkar, S.; Peddi, V.R.; Kaufman, D.B.; First, M.R.; Croy, R.; Holman, J. Alemtuzumab induction in renal transplantation. N. Engl. J. Med. 2011, 364, 1909–1919. [Google Scholar] [CrossRef]
- Wang, K.; Xu, X.; Fan, M. Induction therapy of basiliximab versus antithymocyte globulin in renal allograft: A systematic review and meta-analysis. Clin. Exp. Nephrol. 2018, 22, 684–693. [Google Scholar] [CrossRef]
- Hwang, S.D.; Lee, J.H.; Lee, S.W.; Park, K.M.; Kim, J.K.; Kim, M.J.; Song, J.H. Efficacy and Safety of Induction Therapy in Kidney Transplantation: A Network Meta-Analysis. Transplant. Proc. 2018, 50, 987–992. [Google Scholar] [CrossRef]
- Marin, E.P.; Cohen, E.; Malhotra, D. Chapter 2: Immunosuppressive Therapy for Solid Organ Transplantation. In Dermatology and Solid Organ Transplantation, 1st ed.; Zeitouni, N.C., Samie, F.H., Eds.; CRC Press: Boca Raton, FL, USA, 2021; Volume 1, pp. 23–35. [Google Scholar]
- Lentine, K.L.; Smith, J.M.; Miller, J.M.; Bradbrook, K.; Larkin, L.; Weiss, S.; Handarova, D.K.; Temple, K.; Israni, A.K.; Snyder, J.J. OPTN/SRTR 2021 Annual Data Report: Kidney. Am. J. Transplant. 2023, 23, S21–S120. [Google Scholar] [CrossRef]
- Kamal, J.; Doyle, A. Immunosuppression and Kidney Transplantation. Handb. Exp. Pharmacol. 2022, 272, 165–179. [Google Scholar] [PubMed]
- Wang, D.; Matsumoto, R.; You, Y.; Che, T.; Lin, X.Y.; Gaffen, S.L.; Lin, X. CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1. Mol. Cell. Biol. 2004, 24, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Arriens, C.; Polyakova, S.; Adzerikho, I.; Randhawa, S.; Solomons, N. OP0277 Aurora Phase 3 Study demonstrates Voclosporin statistical superiority over standard of care in Lupus Nephritis (LN). Ann. Rheum. Dis. 2020, 79, 172–173. [Google Scholar] [CrossRef]
- Webster, A.C.; Woodroffe, R.C.; Taylor, R.S.; Chapman, J.R.; Craig, J.C. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: Meta-analysis and meta-regression of randomized trial data. BMJ 2005, 331, 810. [Google Scholar] [CrossRef]
- Halloran, P.; Mathew, T.; Tomlanovich, S.; Groth, C.; Hooftman, L.; Barker, C. Mycophenolate mofetil in renal allograft recipients: A pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation 1997, 63, 39–47; Erratum in Transplantation 1997, 63, 618. [Google Scholar]
- Wagner, M.; Earley, A.K.; Webster, A.C.; Schmid, C.H.; Balk, E.M.; Uhlig, K. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst. Rev. 2015, 12, CD007746. [Google Scholar] [CrossRef]
- Vincenti, F.; Rostaing, L.; Grinyo, J.; Rice, K.; Steinberg, S.; Gaite, L.; Moal, M.-C.; Mondragon-Ramirez, G.A.; Kothari, J.; Polinsky, M.S.; et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N. Engl. J. Med. 2016, 374, 333–343, Erratum in N. Engl. J. Med. 2016, 374, 698. [Google Scholar] [CrossRef]
- Durrbach, A.; Pestana, J.M.; Florman, S.; Del Carmen Rial, M.; Rostaing, L.; Kuypers, D.; Matas, A.; Wekerle, T.; Polinsky, M.; Meier-Kriesche, H.U.; et al. Long-Term Outcomes in Belatacept- Versus Cyclosporine-Treated Recipients of Extended Criteria Donor Kidneys: Final Results from BENEFIT-EXT, a Phase III Randomized Study. Am. J. Transplant. 2016, 16, 3192–3201. [Google Scholar] [CrossRef]
- Masson, P.; Henderson, L.; Chapman, J.R.; Craig, J.C.; Webster, A.C. Belatacept for kidney transplant recipients. Cochrane Database Syst. Rev. 2014, 2014, CD010699. [Google Scholar] [CrossRef]
- Rostaing, L.; Massari, P.; Garcia, V.D.; Mancilla-Urrea, E.; Nainan, G.; del Carmen Rial, M.; Steinberg, S.; Vincenti, F.; Shi, R.; Di Russo, G.; et al. Switching from calcineurin inhibitor-based regimens to a belatacept-based regimen in renal transplant recipients: A randomized phase II study. Clin. J. Am. Soc. Nephrol. 2011, 6, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.; Hodson, E.M.; A Hamiwka, L.; Lee, V.W.; Chapman, J.R.; Craig, J.C.; Webster, A.C. Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database Syst. Rev. 2019, 12, CD004290. [Google Scholar] [CrossRef] [PubMed]
- Chehade Hassib, M.D.; Pascual Manuel, M.D. The Challenge of Acute Antibody-Mediated Rejection in Kidney Transplantation. Transplantation 2016, 100, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Süsal, C.; Döhler, B.; Opelz, G. Presensitized kidney graft recipients with HLA class I and II antibodies are at increased risk for graft failure: A Collaborative Transplant Study report. Hum. Immunol. 2009, 70, 569. [Google Scholar] [CrossRef]
- Lachmann, N.; Terasaki, P.I.; Budde, K.; Liefeldt, L.; Kahl, A.; Reinke, P.; Pratschke, J.; Rudolph, B.; Schmidt, D.; Salama, A.; et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 2009, 87, 1505–1513. [Google Scholar] [CrossRef]
- Hidalgo, L.G.; Campbell, P.M.; Sis, B.; Einecke, G.; Mengel, M.; Chang, J.; Sellares, J.; Reeve, J.; Halloran, P.F. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am. J. Transplant. 2009, 9, 2532–2541. [Google Scholar] [CrossRef]
- Davis, S.; Wiebe, C.; Campbell, K.; Anobile, C.; Aubrey, M.; Stites, E.; Grafals, M.; Pomfret, E.; Nickerson, P.; Cooper, J.E. Adequate tacrolimus exposure modulates the impact of HLA class II molecular mismatch: A validation study in an American cohort. Am. J. Transplant. 2020, 21, 322–328. [Google Scholar] [CrossRef]
- Lederer, S.R.; Friedrich, N.; Banas, B.; von Welser, G.; Albert, E.D.; Sitter, T. Effects of mycophenolate mofetil on donor-specific antibody formation in renal transplantation. Clin. Transplant. 2005, 19, 168–174. [Google Scholar] [CrossRef]
- Budde, K.; Prashar, R.; Haller, H.; Rial, M.C.; Kamar, N.; Agarwal, A.; de Fijter, J.W.; Rostaing, L.; Berger, S.P.; Djamali, A.; et al. Conversion from Calcineurin Inhibitor- to Belatacept-Based Maintenance Immunosuppression in Renal Transplant Recipients: A Randomized Phase 3b Trial. J. Am. Soc. Nephrol. 2021, 32, 3252–3264. [Google Scholar] [CrossRef]
- Bray, R.A.; Gebel, H.M.; Townsend, R.; Roberts, M.E.; Polinsky, M.; Yang, L.; Meier-Kriesche, H.-U.; Larsen, C.P. Posttransplant reduction in preexisting donor-specific antibody levels after belatacept- versus cyclosporine-based immunosuppression: Post hoc analyses of BENEFIT and BENEFIT-EXT. Am. J. Transplant. 2018, 18, 1774–1782. [Google Scholar] [CrossRef]
- Morath, C.; Opelz, G.; Zeier, M.; Süsal, C. Prevention of antibody-mediated kidney transplant rejection. Transplant. Int. 2009, 25, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, C.; Gibson, I.W.; Blydt-Hansen, T.D.; Karpinski, M.; Ho, J.; Storsley, L.J.; Goldberg, A.; Birk, P.E.; Rush, D.N.; Nickerson, P.W. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am. J. Transplant. 2012, 12, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Sellarés, J.; De Freitas, D.G.; Mengel, M.; Reeve, J.; Einecke, G.; Sis, B.; Hidalgo, L.G.; Famulski, K.; Matas, A.; Halloran, P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant. 2012, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Stringer, D.; Gardner, L.; Shaw, O.; Clarke, B.; Briggs, D.; Worthington, J.; Buckland, M.; Danzi, G.; Hilton, R.; Picton, M.; et al. Optimized immunosuppression to prevent graft failure in renal transplant recipients with HLA antibodies (OuTSMART): A randomized controlled trial. eClinicalMedicine 2022, 56, 101819. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Agarwal, G.; Ahmed, F.; Julian, B.; Mannon, R.; Mehta, S.; Kumar, V.; Ong, S.; Thaduri, S.; Towns, G.; et al. Immunosurveillance with Donor Derived Cellular Free DNA Assay Plus Donor Specific Antibody, an Alternative to Renal Allograft Biopsy. Am. J. Transplant. 2020, 20, 686. [Google Scholar]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A.; et al. Cell-Free DNA and Active Rejection in Kidney Allografts. J. Am. Soc. Nephrol. 2017, 28, 2221–2232. [Google Scholar] [CrossRef]
- Sigdel, T.K.; Archila, F.A.; Constantin, T.; Prins, S.A.; Liberto, J.; Damm, I.; Towfighi, P.; Navarro, S.; Kirkizlar, E.; Demko, Z.P.; et al. Optimizing Detection of Kidney Transplant Injury by Assessment of Donor-Derived Cell-Free DNA via Massively Multiplex PCR. J. Clin. Med. 2019, 8, 19. [Google Scholar] [CrossRef]
- Friedewald, J.J.; Kurian, S.M.; Heilman, R.L.; Whisenant, T.C.; Poggio, E.D.; Marsh, C.; Baliga, P.; Odim, J.; Brown, M.M.; Ikle, D.N.; et al. Development and clinical validity of a novel blood-based molecular biomarker for subclinical acute rejection following kidney transplant. Am. J. Transplant. 2019, 19, 98–109. [Google Scholar] [CrossRef]
- Swanson, K.J.; Aziz, F.; Garg, N.; Mohamed, M.; Mandelbrot, D.; Djamali, A.; Parajuli, S. Role of novel biomarkers in kidney transplantation. World J. Transplant. 2020, 10, 230–255. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Identifier: NCT0366335.
- Doberer, K.; Duerr, M.; Halloran, P.F.; Eskandary, F.; Budde, K.; Regele, H.; Reeve, J.; Borski, A.; Kozakowski, N.; Reindl-Schwaighofer, R.; et al. A Randomized Clinical Trial of Anti-IL-6 Antibody Clazakizumab in Late Antibody-Mediated Kidney Transplant Rejection. J. Am. Soc. Nephrol. 2021, 32, 708–722. [Google Scholar] [CrossRef]
- Nickerson, P.W.; Böhmig, G.A.; Chadban, S.; Kumar, D.; Mannon, R.B.; van Gelder, T.; Lee, J.C.; Adler, S.; Chong, E.; Djamali, A. Clazakizumab for the treatment of chronic active antibody-mediated rejection (AMR) in kidney transplant recipients: Phase 3 IMAGINE study rationale and design. Trials 2022, 23, 1042. [Google Scholar] [CrossRef] [PubMed]
- Daligault, M.; Bardy, B.; Noble, J.; Bourdin, A.R.; Masson, D.; Bennani, H.N.; Bugnazet, M.B.; Malvezzi, P.; Rostaing, L.; Jouve, T. Marginal Impact of Tocilizumab Monotherapy on Anti-HLA Alloantibodies in Highly Sensitized Kidney Transplant Candidates. Transplant. Direct 2021, 7, e690. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.A.; Choi, J.; Kim, I.; Louie, S.; Cisneros, K.; Kahwaji, J.; Toyoda, M.; Ge, S.; Haas, M.; Puliyanda, D.; et al. A Phase I/II Trial of the Interleukin-6 Receptor-Specific Humanized Monoclonal (Tocilizumab) + Intravenous Immunoglobulin in Difficult to Desensitize Patients. Transplantation 2015, 99, 2356–2363. [Google Scholar] [CrossRef]
- Jordan, S.C.; Vescio, R.; Toyoda, M.; Ammerman, N.; Huang, E.; Peng, A.; Sethi, S.; Najjar, R.; Lim, K.; Vo, A. Daratumumab for Treatment of Antibody-Mediated Rejection in a Kidney Transplant Recipient [abstract]. Am. J. Transplant. 2019, 19 (Suppl. S3), 1062. [Google Scholar]
- Spica, D.; Junker, T.; Dickenmann, M.; Schaub, S.; Steiger, J.; Rüfli, T.; Halter, J.; Hopfer, H.; Holbro, A.; Hirt-Minkowski, P. Daratumumab for Treatment of Antibody-Mediated Rejection after ABO-Incompatible Kidney Transplantation. Case Rep. Nephrol. Dial. 2019, 9, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Banham, G.D.; Flint, S.M.; Torpey, N.; Lyons, P.; Shanahan, D.N.; Gibson, A.; Watson, C.; O’Sullivan, A.-M.; A Chadwick, J.; E Foster, K.; et al. Belimumab in kidney transplantation: An experimental medicine, randomized, placebo-controlled phase 2 trial. Lancet 2018, 391, 2619–2630. [Google Scholar] [CrossRef]
- Winstedt, L.; Järnum, S.; Nordahl, E.A.; Olsson, A.; Runström, A.; Bockermann, R.; Karlsson, C.; Malmström, J.; Palmgren, G.S.; Malmqvist, U.; et al. Complete Removal of Extracellular IgG Antibodies in a Randomized Dose-Escalation Phase I Study with the Bacterial Enzyme IdeS—A Novel Therapeutic Opportunity. PLoS ONE 2015, 10, e0132011. [Google Scholar] [CrossRef]
- Jordan, S.C.; Lorant, T.; Choi, J.; Kjellman, C.; Winstedt, L.; Bengtsson, M.; Zhang, X.; Eich, T.; Toyoda, M.; Eriksson, B.M.; et al. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N. Engl. J. Med. 2017, 377, 442–453. [Google Scholar] [CrossRef]
- Jordan, S.C.; Legendre, C.; Desai, N.M.; Lorant, T.; Bengtsson, M.; Lonze, B.E.; Vo, A.A.; Runström, A.M.; Laxmyr, L.; Sjöholm, K.; et al. Imlifidase Desensitization in Crossmatch-positive, Highly Sensitized Kidney Transplant Recipients: Results of an International Phase 2 Trial (Highdes). Transplantation 2021, 105, 1808–1817. [Google Scholar] [CrossRef]
- Viglietti, D.; Gosset, C.; Loupy, A.; Deville, L.; Verine, J.; Zeevi, A.; Glotz, D.; Lefaucheur, C. C1 Inhibitor in Acute Antibody-Mediated Rejection Nonresponsive to Conventional Therapy in Kidney Transplant Recipients: A Pilot Study. Am. J. Transplant. 2016, 16, 1596–1603. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Orandi, B.J.; Racusen, L.; Jackson, A.M.; Garonzik-Wang, J.M.; Shah, T.; Woodle, E.S.; Sommerer, C.; Fitts, D.; Rockich, K.; et al. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody-Mediated Rejection Following Kidney Transplantation: Results of a Randomized Double-Blind Placebo-Controlled Pilot Study. Am. J. Transplant. 2016, 16, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.A.; Schnitzler, M.A.; Xiao, H.; Irish, W.; Tuttle-Newhall, E.; Chang, S.-H.; Kasiske, B.L.; Alhamad, T.; Lentine, K.L. An economic assessment of contemporary kidney transplant practice. Am. J. Transplant. 2018, 18, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.M.; Sekercioglu, N.; Berta, W.; Coyte, P.C. Cost-effectiveness of Deceased-donor Renal Transplant Versus Dialysis to Treat End-stage Renal Disease: A Systematic Review. Transplant. Direct 2020, 6, e522. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).