MCP1 Inverts the Correlation between FGF23 and Omega 6/3 Ratio: Is It Also True in Renal Transplantation?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Fatty Acid Analysis
2.3. Enzyme-Linked Immunosorbent Assay (ELISA) for Monocyte Chemoattractant Protein 1 (MCP1) and FGF23 Intact/c-Terminal
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laupacis, A.; Keown, P.; Pus, N.; Krueger, H.; Ferguson, B.; Wong, C.; Muirhead, N. A Study of the Quality of Life and Cost-Utility of Renal Transplantation. Kidney Int. 1996, 50, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Hansrivijit, P.; Leeaphorn, N.; Acharya, P.; Torres-Ortiz, A.; Kaewput, W.; Kovvuru, K.; Kanduri, S.R.; Bathini, T.; Cheungpasitporn, W. Recent Advances and Clinical Outcomes of Kidney Transplantation. J. Clin. Med. 2020, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic Review: Kidney Transplantation Compared with Dialysis in Clinically Relevant Outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kriesche, H.U.; Schold, J.D.; Srinivas, T.R.; Reed, A.; Kaplan, B. Kidney Transplantation Halts Cardiovascular Disease Progression in Patients with End-Stage Renal Disease. Am. J. Transplant. 2004, 4, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.C.; Lu, L.; Cao, Y.; Sun, R.R.; Chen, S.; Zhang, P.Y. Cardiovascular Disease and Its Relationship with Chronic Kidney Disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2918–2926. [Google Scholar]
- Mattinzoli, D.; Turolo, S.; Alfieri, C.M.; Ikehata, M.; Caldiroli, L.; Armelloni, S.; Montini, G.; Agostoni, C.; Messa, P.; Vettoretti, S.; et al. MCP1 Could Mediate FGF23 and Omega 6/Omega 3 Correlation Inversion in CKD. J. Clin. Med. 2022, 11, 7099. [Google Scholar] [CrossRef]
- Rodríguez, M.; López, I.; Muñoz, J.; Aguilera-Tejero, E.; Almaden, Y. FGF23 and Mineral Metabolism, Implications in CKD-MBD. Nefrologia 2012, 32, 275–278. [Google Scholar] [CrossRef]
- Batra, J.; Buttar, R.S.; Kaur, P.; Kreimerman, J.; Melamed, M.L. FGF-23 and Cardiovascular Disease: Review of Literature. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 423–429. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, S.; Poveda, J.; Navarro-García, J.A.; González-Lafuente, L.; Rodríguez-Sánchez, E.; Ruilope, L.M.; Ruiz-Hurtado, G. An Overview of FGF-23 as a Novel Candidate Biomarker of Cardiovascular Risk. Front. Physiol. 2021, 12, 632260. [Google Scholar] [CrossRef] [PubMed]
- Mattinzoli, D.; Li, M.; Castellano, G.; Ikehata, M.; Armelloni, S.; Elli, F.M.; Molinari, P.; Tsugawa, K.; Alfieri, C.M.; Messa, P. Fibroblast Growth Factor 23 Level Modulates the Hepatocyte’s Alpha-2-HS-Glycoprotein Transcription through the Inflammatory Pathway TNFα/NFκB. Front. Med. 2022, 9, 1038638. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 Induces Left Ventricular Hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [PubMed]
- Silswal, N.; Touchberry, C.D.; Daniel, D.R.; McCarthy, D.L.; Zhang, S.; Andresen, J.; Stubbs, J.R.; Wacker, M.J. FGF23 Directly Impairs Endothelium-Dependent Vasorelaxation by Increasing Superoxide Levels and Reducing Nitric Oxide Bioavailability. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E426–E436. [Google Scholar] [CrossRef]
- Mattinzoli, D.; Rastaldi, M.P.; Ikehata, M.; Armelloni, S.; Pignatari, C.; Giardino, L.A.; Li, M.; Alfieri, C.M.; Regalia, A.; Riccardi, D.; et al. FGF23-Regulated Production of Fetuin-A (AHSG) in Osteocytes. BONE 2018, 83, 35–47. [Google Scholar] [CrossRef]
- Tagliabracci, V.S.; Engel, J.L.; Wiley, S.E.; Xiao, J.; Gonzalez, D.J.; Appaiah, H.N.; Koller, A.; Nizet, V.; White, K.E.; Dixon, J.E. Dynamic Regulation of FGF23 by Fam20C Phosphorylation, GalNAc-T3 Glycosylation, and Furin Proteolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 5520–5525. [Google Scholar] [CrossRef]
- Wolf, M.; White, K.E. Coupling Fibroblast Growth Factor 23 Production and Cleavage: Iron Deficiency, Rickets, and Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 411–419. [Google Scholar] [CrossRef]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and Functional Iron Deficiency Regulate Fibroblast Growth Factor 23 Production. Kidney Int. 2016, 89, 135–146. [Google Scholar] [CrossRef]
- Niu, J.; Kolattukudy, P.E. Role of MCP-1 in Cardiovascular Disease: Molecular Mechanisms and Clinical Implications. Clin. Sci. 2009, 117, 95–109. [Google Scholar] [CrossRef]
- Vianna, H.R.; Soares, C.M.B.M.; Silveira, K.D.; Elmiro, G.S.; Mendes, P.M.; De Sousa Tavares, M.; Teixeira, M.M.; Miranda, D.M.; Simões E Silva, A.C. Cytokines in Chronic Kidney Disease: Potential Link of MCP-1 and Dyslipidemia in Glomerular Diseases. Pediatr. Nephrol. 2013, 28, 463–469. [Google Scholar] [CrossRef]
- Piemonti, L.; Calori, G.; Lattuada, G.; Mercalli, A.; Ragogna, F.; Garancini, M.P.; Ruotolo, G.; Luzi, L.; Perseghin, G. Association between Plasma Monocyte Chemoattractant Protein-1 Concentration and Cardiovascular Disease Mortality in Middle-Aged Diabetic and Nondiabetic Individuals. Diabetes Care 2009, 32, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, N.; Driianska, V.; Savchenko, S. Dyslipidemia and Intraperitoneal Inflammation Axis in Peritoneal Dialysis Patients: A Cross-Sectional Pilot Study. Kidney Dis. 2020, 6, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Gosling, J.; Slaymaker, S.; Gu, L.; Tseng, S.; Zlot, C.H.; Young, S.G.; Rollins, B.J.; Charo, I.F. MCP-1 Deficiency Reduces Susceptibility to Atherosclerosis in Mice That Overexpress Human Apolipoprotein B. J. Clin. Investig. 1999, 103, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Noels, H.; Lehrke, M.; Vanholder, R.; Jankowski, J. Lipoproteins and Fatty Acids in Chronic Kidney Disease: Molecular and Metabolic Alterations. Nat. Rev. Nephrol. 2021, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Yu, Q.; Song, S.; Brenna, J.T.; Shen, Y.; Ye, K. Higher Ratio of Plasma Omega-6/Omega-3 Fatty Acids Is Associated with Greater Risk of All-Cause, Cancer, and Cardiovascular Mortality: A Population-Based Cohort Study in UK Biobank. medRxiv 2023. [Google Scholar] [CrossRef]
- Nowalany-Kozielska, E.; Wojnicz, R. Immunosuppressive Therapy for Heart Failure: A Guide to Patient Selection. Am. J. Cardiovasc. Drugs 2003, 3, 321–324. [Google Scholar] [CrossRef]
- Miller, L.W. Cardiovascular Toxicities of Immunosuppressive Agents. Am. J. Transplant. 2002, 2, 807–818. [Google Scholar] [CrossRef]
- Kalnins, A.; Thomas, M.N.; Andrassy, M.; Müller, S.; Wagner, A.; Pratschke, S.; Rentsch, M.; Klussmann, S.; Kauke, T.; Angele, M.K.; et al. Spiegelmer Inhibition of MCP-1/CCR2—Potential as an Adjunct Immunosuppressive Therapy in Transplantation. Scand. J. Immunol. 2015, 82, 102–109. [Google Scholar] [CrossRef]
- Rangaswami, J.; Mathew, R.O.; Parasuraman, R.; Tantisattamo, E.; Lubetzky, M.; Rao, S.; Yaqub, M.S.; Birdwell, K.A.; Bennett, W.; Dalal, P.; et al. Cardiovascular Disease in the Kidney Transplant Recipient: Epidemiology, Diagnosis and Management Strategies. Nephrol. Dial. Transplant. 2019, 34, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Gul, A.; Sarnak, M.J. Cardiovascular Risk Factors in Chronic Kidney Disease. Kidney Int. 2005, 68, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Dickerson, J.A.; Tan, L.C.; Hebert, C.A. Activation of Nuclear Factor-Kappa B Correlates with MCP-1 Expression by Human Mesangial Cells. Kidney Int. 1995, 48, 1263–1271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, S.; Grabner, A.; Yanucil, C.; Schramm, K.; Czaya, B.; Krick, S.; Czaja, M.J.; Bartz, R.; Abraham, R.; Di Marco, G.S.; et al. Fibroblast Growth Factor 23 Directly Targets Hepatocytes to Promote Inflammation in Chronic Kidney Disease. Kidney Int. 2016, 90, 985–996. [Google Scholar] [CrossRef]
- Vafadari, R.; Kraaijeveld, R.; Weimar, W.; Baan, C.C. Tacrolimus Inhibits NF-ΚB Activation in Peripheral Human T Cells. PLoS ONE 2013, 8, e60784. [Google Scholar] [CrossRef]
- Bendíčková, K.; Tidu, F.; De Zuani, M.; Kohoutková, M.H.; Andrejčinová, I.; Pompeiano, A.; Bělášková, S.; Forte, G.; Zelante, T.; Frič, J. Calcineurin Inhibitors Reduce NFAT-Dependent Expression of Antifungal Pentraxin-3 by Human Monocytes. J. Leukoc. Biol. 2020, 107, 497–508. [Google Scholar] [CrossRef]
- Du, S.; Hiramatsu, N.; Hayakawa, K.; Kasai, A.; Okamura, M.; Huang, T.; Yao, J.; Takeda, M.; Araki, I.; Sawada, N.; et al. Suppression of NF-KappaB by Cyclosporin a and Tacrolimus (FK506) via Induction of the C/EBP Family: Implication for Unfolded Protein Response. J. Immunol. 2009, 182, 7201–7211. [Google Scholar] [CrossRef]
- Lin, H.Y.H.; Chang, K.T.; Hung, C.C.; Kuo, C.H.; Hwang, S.J.; Chen, H.C.; Hung, C.H.; Lin, S.F. Effects of the MTOR Inhibitor Rapamycin on Monocyte-Secreted Chemokines. BMC Immunol. 2014, 15, 37. [Google Scholar] [CrossRef]
- Chaintreuil, P.; Kerreneur, E.; Bourgoin, M.; Savy, C.; Favreau, C.; Robert, G.; Jacquel, A.; Auberger, P. The Generation, Activation, and Polarization of Monocyte-Derived Macrophages in Human Malignancies. Front. Immunol. 2023, 14, 1178337. [Google Scholar] [CrossRef]
- Jiao, B.; An, C.; Du, H.; Tran, M.; Wang, P.; Zhou, D.; Wang, Y. Stat6 Deficiency Attenuates Myeloid Fibroblast Activation and Macrophage Polarization in Experimental Folic Acid Nephropathy. Cells 2021, 10, 3057. [Google Scholar] [CrossRef]
- Jiao, B.; An, C.; Tran, M.; Du, H.; Wang, P.; Zhou, D.; Wang, Y. Pharmacological Inhibition of STAT6 Ameliorates Myeloid Fibroblast Activation and Alternative Macrophage Polarization in Renal Fibrosis. Front. Immunol. 2021, 12, 735014. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Jiao, B.; Du, H.; Tran, M.; Song, B.; Wang, P.; Zhou, D.; Wang, Y. Jumonji Domain-Containing Protein-3 (JMJD3) Promotes Myeloid Fibroblast Activation and Macrophage Polarization in Kidney Fibrosis. Br. J. Pharmacol. 2023, 180, 2250–2265. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, L.; Yang, J.; King, G.; Xiao, Z.; Quarles, L.D. Counter-Regulatory Paracrine Actions of FGF-23 and 1,25(OH)2D in Macrophages. FEBS Lett. 2016, 590, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, M.; Sul, O.J.; Choi, E.K.; Kim, J.E.; Suh, J.H.; Choi, H.S. MCP-1 Deficiency Enhances Browning of Adipose Tissue via Increased M2 Polarization. J. Endocrinol. 2019, 242, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, X.; Li, Y.; Geng, X.; Jia, X.; Zhang, L.; Yang, H. Arachidonic Acid Metabolism Controls Macrophage Alternative Activation Through Regulating Oxidative Phosphorylation in PPARγ Dependent Manner. Front. Immunol. 2021, 12, 618501. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of Arachidonic Acid Metabolism: A Review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Martinelli, N.; Girelli, D.; Malerba, G.; Guarini, P.; Illig, T.; Trabetti, E.; Sandri, M.; Friso, S.; Pizzolo, F.; Schaeffer, L.; et al. FADS Genotypes and Desaturase Activity Estimated by the Ratio of Arachidonic Acid to Linoleic Acid Are Associated with Inflammation and Coronary Artery Disease. Am. J. Clin. Nutr. 2008, 88, 941–949. [Google Scholar] [CrossRef]
- Krüger, B.; Schröppel, B.; Ashkan, R.; Marder, B.; Zülke, C.; Murphy, B.; Krämer, B.K.; Fischereder, M. A Monocyte Chemoattractant Protein-1 (MCP-1) Polymorphism and Outcome after Renal Transplantation. J. Am. Soc. Nephrol. 2002, 13, 2585–2589. [Google Scholar] [CrossRef]
- Jang, H.R.; Kim, M.; Hong, S.; Lee, K.; Park, M.Y.; Yang, K.E.; Lee, C.J.; Jeon, J.; Lee, K.W.; Lee, J.E.; et al. Early Postoperative Urinary MCP-1 as a Potential Biomarker Predicting Acute Rejection in Living Donor Kidney Transplantation: A Prospective Cohort Study. Sci. Rep. 2021, 11, 18832. [Google Scholar] [CrossRef] [PubMed]
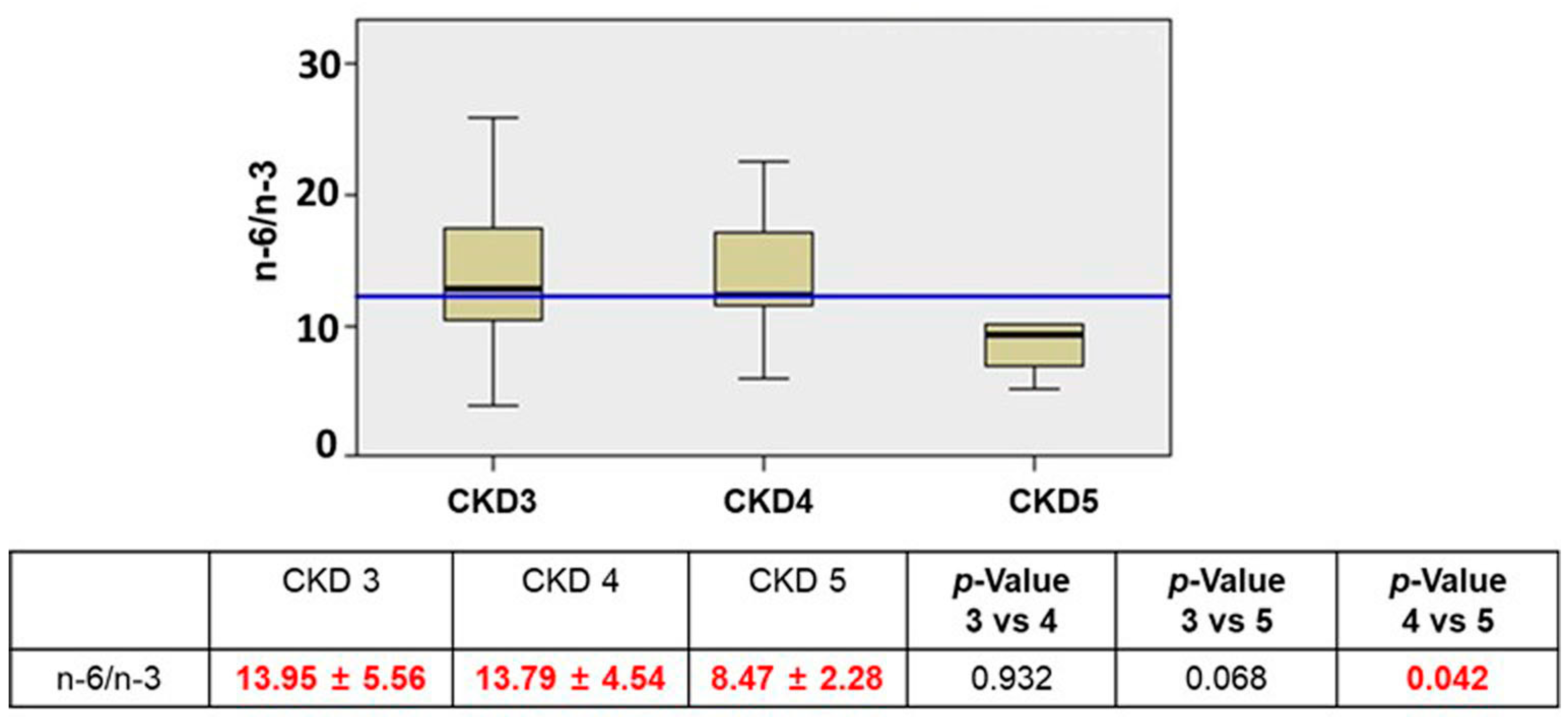
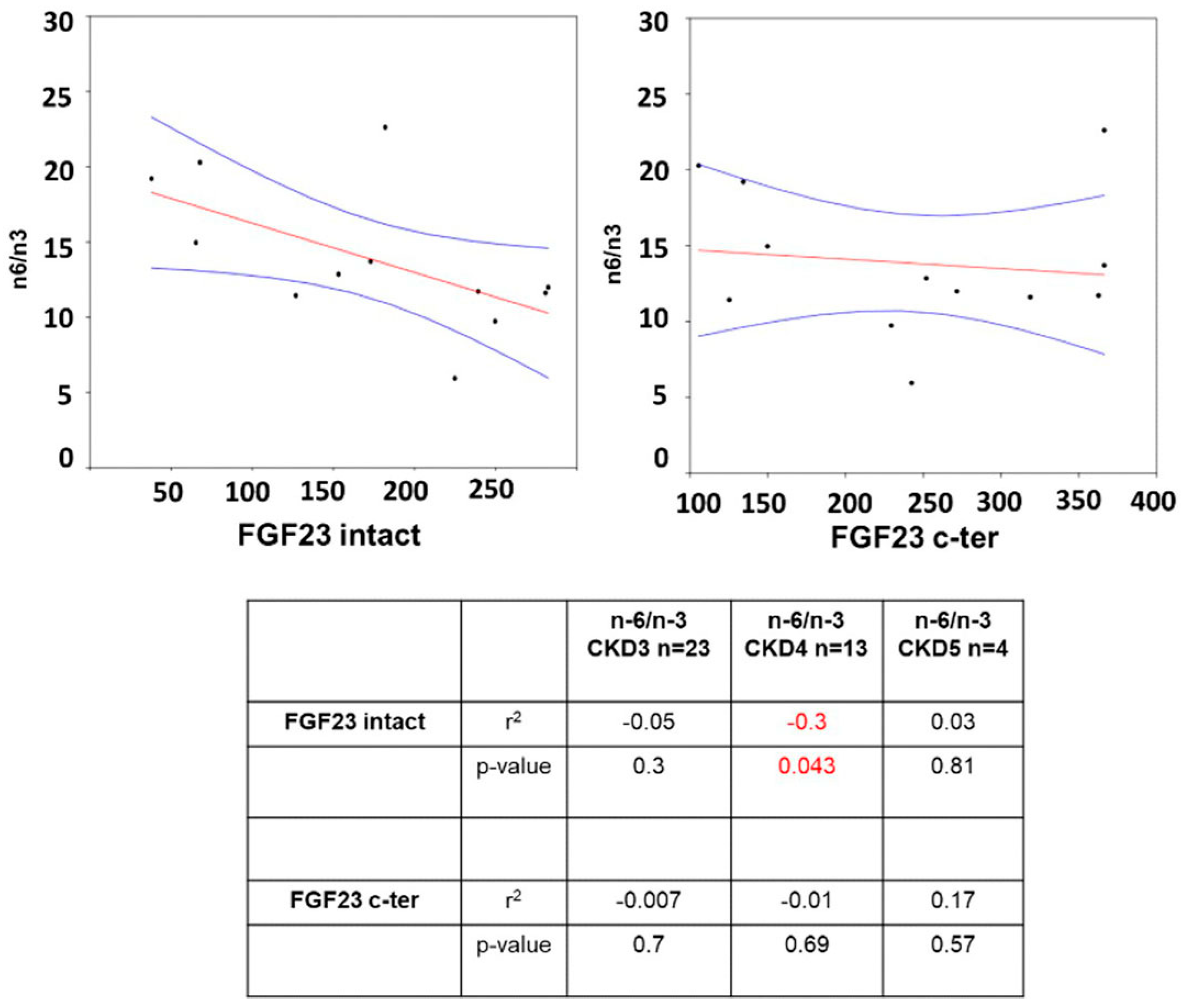
| Total n = 40 | CKD 3 n = 23 | CKD 4 n = 13 | CKD 5n = 4 | p-Value 3 vs. 4 | p-Value 3 vs. 5 | p-Value 4 vs. 5 | |
|---|---|---|---|---|---|---|---|
| Demographic Data | |||||||
| Age (years) | 55 ± 16 | 53 ± 17 | 57 ± 17 | 62 ± 9 | 0.46 | 0.30 | 0.60 |
| Gender m/f (N) | 22/18 | 18/5 | 3/10 | 1/3 | |||
| Clinical Data | |||||||
| eGFR (mL/min/1.73 m2) | 34.44 ± 16.54 | 45.52 ± 13.66 | 23.48 ± 4.20 | 11.98 ± 3.06 | <0.0001 | <0.0001 | <0.0001 |
| P-Creat (mg/dL) | 2.24 ± 0.96 | 1.73 ± 0.41 | 2.44 ± 0.44 | 4.33 ± 1.30 | <0.0001 | <0.0001 | <0.0001 |
| COL tot (mg/dL) | 192.91 ± 30.12 | 187.26 ± 28.04 | 194.31 ± 33.28 | 215.25 ± 23.77 | 0.522 | 0.078 | 0.265 |
| TGL (mg/dL) | 164.77 ± 64.75 | 156.68 ± 51.65 | 163.54 ± 78.03 | 207.25 ± 75.71 | 0.766 | 0.114 | 0.340 |
| HDL (mg/dL) | 55.30 ± 13.42 | 55.21 ± 15.84 | 57.46 ± 9.58 | 48.75 ± 12.31 | 0.651 | 0.454 | 0.155 |
| LDL (mg/dL) | 101.55 ± 30.47 | 100.09 ± 22.17 | 96.47 ± 39.62 | 125.05 ± 27.29 | 0.743 | 0.062 | 0.202 |
| Na (mmol/L) | 141.63 ± 2.69 | 141.95 ± 1.78 | 141.62 ± 2.99 | 140.25 ± 1.50 | 0.696 | 0.091 | 0.400 |
| K (mmol/L) | 4.44 ± 0.46 | 4.48 ± 0.39 | 4.39 ± 0.49 | 4.40 ± 0.75 | 0.562 | 0.744 | 0.981 |
| Biomarker Data | |||||||
| Ca (mg/dL) | 9.46 ± 0.72 | 9.63 ± 0.69 | 9.50 ± 0.57 | 8.52 ± 0.79 | 0.563 | 0.009 | 0.015 |
| S-phosphorus (mg/dL) | 3.43 ± 0.87 | 3.15 ± 0.59 | 3.62 ± 0.73 | 4.23 ± 1.80 | 0.055 | 0.036 | 0.320 |
| FGF23 INT (pg/mL) | 152.8 ± 113.90 | 108.0 ± 60.05 | 169.4 ± 82.97 | 345.0 ± 214.25 | 0.017 | <0.0001 | 0.023 |
| FGF23 CT (RU/mL) | 242.6 ± 191.80 | 200.5 ± 191.70 | 239.3 ± 94.82 | 485.3 ± 284.57 | 0.502 | 0.018 | 0.013 |
| MCP1 (pg/mL) | 241.4 ± 104.10 | 247.7 ± 122.40 | 242.4 ± 77.56 | 203.2 ± 78.89 | 0.889 | 0.494 | 0.393 |
| CKD 3 | CKD 4 | CKD 5 | p-Value 3 vs. 4 | p-Value 3 vs. 5 | p-Value 4 vs. 5 | |
|---|---|---|---|---|---|---|
| PUFA | 35.37 ± 4.12 | 34.95 ± 3.82 | 33.80 ± 3.56 | 0.769 | 0.486 | 0.603 |
| PUFA n-3 | 2.76 ± 1.37 | 2.61 ± 1.04 | 3.73 ± 1.01 | 0.739 | 0.192 | 0.076 |
| ALA (18:3 n-3) | 0.27 ± 0.10 | 0.35 ± 0.16 | 0.41 ± 0.06 | 0.101 | 0.012 | 0.435 |
| EPA (20:5 n-3) | 0.09 ± 0.14 | 0.06 ± 0.03 | 0.03 ± 0.01 | 0.607 | 0.411 | 0.060 |
| DPA (22:5 n-3) | 0.32 ± 0.17 | 0.31 ± 0.12 | 0.52 ± 0.19 | 0.796 | 0.049 | 0.019 |
| DHA (22:6 n-3) | 2.15 ± 1.22 | 1.94 ± 0.9 | 2.79 ± 0.81 | 0.596 | 0.333 | 0.116 |
| PUFA n-6 | 32.53 ± 4.18 | 32.27 ± 3.41 | 30.00 ± 3.71 | 0.849 | 0.272 | 0.272 |
| LA (18:2 n-6) | 24.47 ± 4.65 | 24.10 ± 3.96 | 23.08 ± 1.87 | 0.814 | 0.568 | 0.631 |
| GLA (18:3 n-6) | 0.08 ± 0.07 | 0.09 ± 0.07 | 0.05 ± 0.03 | 0.776 | 0.411 | 0.319 |
| DGLA (20:3 n-6) | 1.44± 0.56 | 1.19 ± 0.39 | 1.08 ± 0.53 | 0.176 | 0.257 | 0.669 |
| AA (20:4 n-6) | 6.62 ± 2.03 | 6.97 ± 2.1 | 5.83 ± 2.43 | 0.632 | 0.495 | 0.373 |
| AdA (22:4 n-6) | 0.29 ± 0.12 | 0.28 ± 0.14 | 0.21 ± 0.10 | 0.853 | 0.251 | 0.388 |
| Osbond (22:5 n-6) | 0.08 ± 0.06 | 0.07 ± 0.07 | 0.05 ± 0.03 | 0.688 | 0.367 | 0.572 |
| AA/LA | 0.28 ± 0.11 | 0.33 ± 0.15 | 0.25 ± 0.11 | 0.240 | 0.700 | 0.370 |
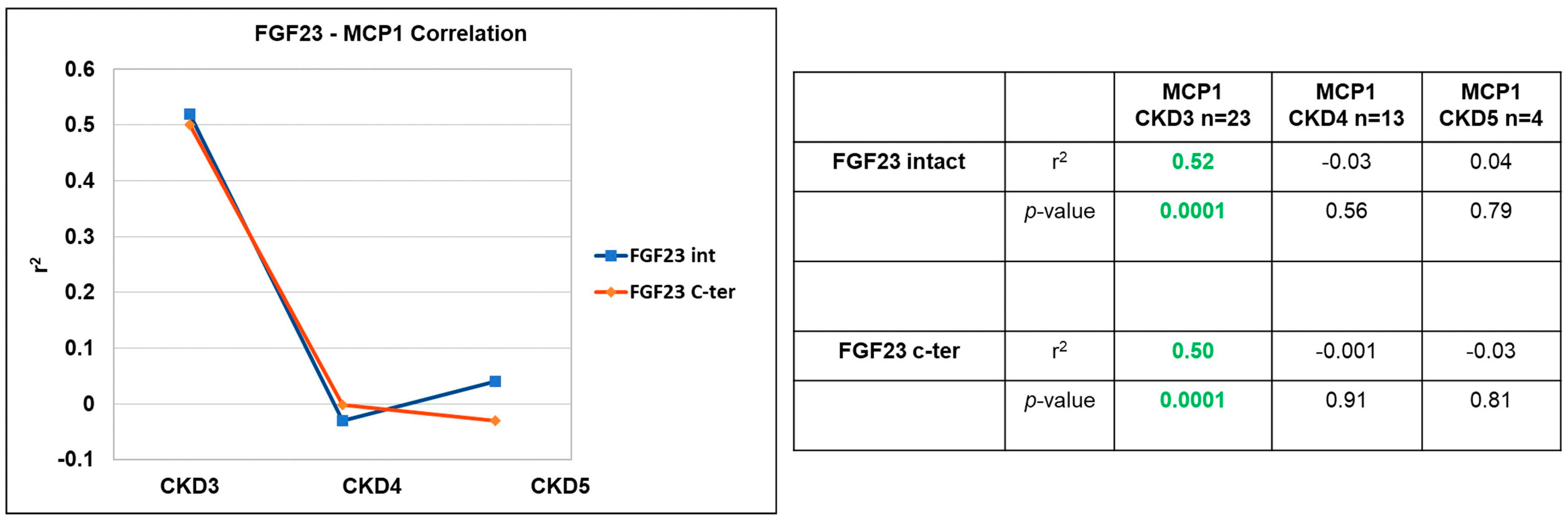
| Fatty Acid | MCP1 CKD3 n = 23 | MCP1 CKD 4 n = 13 | MCP1 CKD5 n = 4 | |||
|---|---|---|---|---|---|---|
| r2 | p-Value | r2 | p-Value | r2 | p-Value | |
| PUFA | −0.330 | 0.144 | 0.070 | 0.829 | <0.001 | 1.000 |
| PUFA n-3 | 0.087 | 0.708 | 0.130 | 0.688 | 0.400 | 0.600 |
| ALA (18:3 n-3) | −0.081 | 0.726 | −0.040 | 0.901 | −0.400 | 0.600 |
| EPA (20:5 n-3) | 0.030 | 0.400 | −0.090 | 0.760 | −0.010 | 0.890 |
| DPA (22:5 n-3) | 0.113 | 0.626 | −0.344 | 0.274 | 0.800 | 0.200 |
| DHA (22:6 n-3) | 0.118 | 0.610 | 0.144 | 0.656 | 0.400 | 0.600 |
| PUFA n-6 | −0.425 | 0.055 | −0.098 | 0.762 | −0.400 | 0.600 |
| LA (18:2 n-6) | −0.403 | 0.070 | −0.154 | 0.632 | −0.200 | 0.800 |
| GLA (18:3 n-6) | <−0.001 | 0.910 | −0.090 | 0.340 | −0.770 | 0.120 |
| DGLA (20:3 n-6) | −0.179 | 0.437 | −0.259 | 0.416 | <0.001 | 1.000 |
| AA (20:4 n-6) | 0.066 | 0.775 | 0.242 | 0.449 | −0.400 | 0.600 |
| AdA (22:4 n-6) | −0.030 | 0.380 | −0.010 | 0.700 | −0.040 | 0.790 |
| Osbond (22:5 n-6) | 0.140 | 0.078 | −0.070 | 0.390 | −0.530 | 0.260 |
| n-6/n-3 | −0.153 | 0.507 | −0.123 | 0.704 | −0.400 | 0.600 |
| AA/LA | 0.170 | 0.060 | <0.001 | 0.990 | 0.010 | 0.880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattinzoli, D.; Turolo, S.; Ikehata, M.; Vettoretti, S.; Montini, G.; Agostoni, C.; Conti, C.; Benedetti, M.; Messa, P.; Alfieri, C.M.; et al. MCP1 Inverts the Correlation between FGF23 and Omega 6/3 Ratio: Is It Also True in Renal Transplantation? J. Clin. Med. 2023, 12, 5928. https://doi.org/10.3390/jcm12185928
Mattinzoli D, Turolo S, Ikehata M, Vettoretti S, Montini G, Agostoni C, Conti C, Benedetti M, Messa P, Alfieri CM, et al. MCP1 Inverts the Correlation between FGF23 and Omega 6/3 Ratio: Is It Also True in Renal Transplantation? Journal of Clinical Medicine. 2023; 12(18):5928. https://doi.org/10.3390/jcm12185928
Chicago/Turabian StyleMattinzoli, Deborah, Stefano Turolo, Masami Ikehata, Simone Vettoretti, Giovanni Montini, Carlo Agostoni, Costanza Conti, Matteo Benedetti, Piergiorgio Messa, Carlo Maria Alfieri, and et al. 2023. "MCP1 Inverts the Correlation between FGF23 and Omega 6/3 Ratio: Is It Also True in Renal Transplantation?" Journal of Clinical Medicine 12, no. 18: 5928. https://doi.org/10.3390/jcm12185928
APA StyleMattinzoli, D., Turolo, S., Ikehata, M., Vettoretti, S., Montini, G., Agostoni, C., Conti, C., Benedetti, M., Messa, P., Alfieri, C. M., & Castellano, G. (2023). MCP1 Inverts the Correlation between FGF23 and Omega 6/3 Ratio: Is It Also True in Renal Transplantation? Journal of Clinical Medicine, 12(18), 5928. https://doi.org/10.3390/jcm12185928







