Abstract
Objective: To investigate and identify risk factors and predictors for the difference in functional outcome and complications between total hip arthroplasty (THA) through minimally invasive and conventional approaches, using a meta-regression analysis of randomized controlled trials (RCTs). Methods: A systematic review of the literature up to 31 July 2022 was performed. A meta-regression was conducted based on a random effects meta-analysis using the Hartung–Knapp–Sidik–Jonkman method. Results: A total of 41 RCTs with 3607 patients were found. The following predictors of HHS ≥ 6 months postoperatively were identified: patient age (predictor estimate = 0.14; p < 0.01), avascular necrosis of the femoral head (predictor estimate = −0.03; p = 0.04); incision length (predictor estimate = −0.82; p < 0.01). The following predictors of complication rate were identified: osteoarthritis (predictor estimate = 0.02; p = 0.02); femoral neck fracture (predictor estimate = −0.02; p = 0.02); SuperPATH (predictor estimate = −1.72; p < 0.01). Conclusions: Patient age, avascular necrosis of the femoral head, and incision length were identified as predictors of the effect size of the HHS ≥ 6 months postoperatively; and osteoarthritis, femoral neck fracture, and SuperPATH as predictors of the effect size of the complication rate. Based on these findings, we recommend that more frequent use of minimally invasive THA in elderly patients should be considered. Level of evidence I: a systematic review of all relevant randomized controlled trials. Registered in PROSPERO on 10 August 2022 (CRD42022350287).
1. Introduction
Total hip arthroplasty (THA) is one of the most effective and successful procedures in orthopaedic surgery [1]. THA relieves pain, restores function to the hip joint, and improves the patient’s overall quality of life. There are several indications for THA: symptomatic hip osteoarthritis, avascular necrosis of the femoral head (ANFH), hip dysplasia, and inflammatory arthritic conditions. As the world’s population ages, the number of hip joint disorders is increasing [2]. Approximately 240 million people worldwide have symptomatic osteoarthritis [3,4]. Almost 10% of patients over the age of 45 have radiographic evidence of symptomatic hip osteoarthritis [5]. The prevalence of ANFH is two per 100,000 people [6]. It is more common in males, with the highest prevalence in men aged 25 to 44 years and women aged 55 to 75 years [7]. The absolute number of femoral neck fractures is expected to increase to 2.6 million in 2025 and 4.5 million in 2050 [8]. According to guidelines, the operating surgeon can choose between endoprosthetic and femoral head-preserving procedures for surgical treatment [9]. Femoral neck fractures in elderly patients are increasingly treated with THA.
Surgical approaches to the hip joint are divided into six types based on the anatomical relationship to the greater trochanter: anterior, anterolateral, lateral (transgluteal or transtrochanteric), posterior, posterolateral, and superior. Minimally invasive or muscle-sparing surgical approaches to the hip joint are modifications of these conventional approaches that must satisfy two conditions: the preservation of musculotendinous structures, and a short incision length (≤10 cm). The direct anterior approach (Smith-Petersen), the anterolateral approach (modified Watson–Jones), the direct superior approach with SuperPATH technique and the two-incision approach are described as minimally invasive [10,11,12,13]. The advantages of minimally invasive approaches include less pain, lower blood loss, and faster recovery due to less surgical tissue trauma [14]. Limited visibility during exposure has been highlighted as a disadvantage of minimally invasive approaches. Sometimes, this disadvantage has to be compensated for by excessive intraoperative wound retraction or by an unphysiological positioning of the leg, which in some cases can lead to complications [15,16].
Unfortunately, not all patients benefit to the same extent after THA. The reasons for this are not yet clear. There are several studies [17,18,19,20,21,22,23,24] and systematic reviews [25,26,27,28,29] that have shed light on some risk factors and predictors of THA outcome, but there is still no meta-regression analysis on this topic in the specialist literature. In particular, there is no single study that examines the risk factors and predictors of differences in outcome between minimally invasive and conventional approach THA.
We aimed to investigate and identify risk factors and predictors for the effect size of functional outcome and complications after THA through minimally invasive and conventional approaches by performing a meta-regression analysis of randomized controlled trials (RCTs).
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
Our study protocol was registered in PROSPERO on 10 August 2022 (CRD42022350287). Two independent reviewers (NR,PL) searched the following databases for relevant manuscripts up to 31 July 2022: PubMed, China National Knowledge Infrastructure (CNKI), The Cochrane Library, Clinical trials, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Embase. We used the MeSH terms ‘minimally invasive’, ‘muscle-sparing’, ‘SuperPATH’, ‘direct anterior’, ‘two incision’, ‘conventional approaches’, ‘THA’, ‘THR’, ‘hip arthroplasty’, and ‘hip replacement’ with a BOOLEAN search strategy and adapted them to the syntax of the databases. There were no restrictions on publication language. We did not include old RCTs, published before 2010. The reason for this is that there have been many improvements in THA over the last decades. Older THA methods have lost their importance today, making it difficult to compare them with newer THA methods in a meta-regression analysis. A Chinese-speaking reviewer (KL) helped by translating Chinese articles. In addition to this search strategy, we performed a manual review of the reference lists of relevant systematic reviews. We did not review grey literature. Inclusion criteria were: (i) randomized controlled trials (RCT) with (ii) human participants (without demographic restrictions such as patient age, sex, body mass index (BMI), etc.) with hip disease (osteoarthritis, dysplasia, ANFH) or femoral neck fracture, who were treated with (iii) THA through minimally invasive approaches or conventional approaches. Exclusion criteria were: no outcome of interest, hip replacement with hemiarthroplasty, unclear identification of the approach as minimally invasive. Hip approaches for THA were accepted as minimally invasive approaches under at least one of the following two conditions: (1) If the approaches were minimally invasive by definition; this means that the approach per se is known to be muscle and tendon sparing and has an incision length ≤ 10 cm. (2) In other cases, we referred to the authors’ assessment if an approach was explicitly described as minimally invasive in their RCT. The literature search is presented in a PRISMA flowchart diagram (Figure 1).
2.2. Data Extraction
Two independent reviewers extracted the following information from each RCT, according to the PRISMA guidelines: RCT details (e.g., study design, treatment protocol, duration, number of patients, number of hips operated, year of publication, and risk of bias), primary patient characteristics (e.g., age, sex, BMI, preoperative Harris Hip Score (HHS), and indication for surgery), intervention (e.g., approach, use of bone cement, table position, use of traction table, operation time, incision length, intraoperative blood loss, cup inclination angle, laboratory parameters), patient outcome (e.g., postoperative HHS and complication rate). In the case of missing data, a letter requesting additional information was sent to the corresponding authors. If information on standard deviation was missing, it was calculated by imputation [30].
2.3. Outcome Parameters
We focused on the two main THA outcome parameters: the functional outcome parameter ‘HHS’ and the ‘complication rate’. The HHS was developed to assess the outcome of hip surgery [31]. This score accumulates points from the assessment of four aspects: pain, function, degree of deformity, and range of motion of the hip. The higher the total score, the better the outcome, with a range of total scores from 0 to 100. The second outcome parameter was the complication rate. We considered the following relevant types of complications: dislocation, infection, intraoperative periprosthetic fracture, deep vein thrombosis of the lower extremity, and haematoma. To obtain more consistent data, we summarized the reported data into HHS ≤ 3 months postoperatively and HHS ≥ 6 months postoperatively. If the RCT reported more than one value for one outcome parameter, we used the most recent record of short-term HHS and short-term complications.
2.4. RCT Quality Assessment
Risk of bias and level of evidence were assessed according to the Cochrane’s risk of bias 2 (RoB 2) tool [32] and the recommendations of the GRADE system [33]. We assessed the RCTs for publication bias, by using the Egger’s regression intercept test for asymmetry of the funnel plots. Statistical significance was set at a p-value < 0.05. We presented the results in funnel plots to find evidence of publication bias. In the funnel plot, the horizontal axis (‘x-axis’) shows the estimated effect size of the RCTs and the ‘y-axis’ shows the estimated standard error of the RCTs (=measure of the uncertainty of the estimated effect size). The dashed vertical line is the overall effect estimated from the meta-analysis of all RCTs. Ideally, the RCTs should be symmetrically distributed within the triangle.
2.5. Data Synthesis and Statistical Analysis
We performed a meta-regression based on a random effects meta-analysis using the Hartung–Knapp–Sidik–Jonkman method for both continuous and nominal study level covariates [34]. We fitted regression models with single covariates and assessed heterogeneity using Cochrane’s test (p value < 0.10 indicates heterogeneity). The effect of covariates was assessed using the Wald-type test on model coefficients.
The forest plots show the measures of treatment effect between minimally invasive THA and conventional approach THA, labelled ‘experimental group’ and ‘control group’ respectively. Mean differences (MDs) with 95% confidence intervals (CIs) were calculated for the continuous outcome parameter HHS and odds ratios (ORs) with 95% CIs were calculated for the dichotomous outcome parameter complication rate. A positive MD and an OR of less than 1 favoured the experimental group. In addition, the results of the common effect model are also shown in the forest plots.
The bubble plots illustrate the meta-regression results. The value of the predictor for each RCT is plotted on the horizontal axis (‘x-axis’) and the effect size is plotted on the vertical axis (‘y-axis’). Each bubble in the bubble plots corresponds to one RCT. The size of the bubbles indicates the weight with which the study contributes to the overall result. The black solid line is the regression line. The slope of the regression line corresponds exactly to the effect size of the predictor on the outcome variable. If the predictor has a strong influence on the outcome variable, then the slope of the regression line is large (steep line). If the predictor has no influence on the outcome variable, the regression line is flat and more or less parallel to the horizontal zero line.
A professional statistician (RH) performed all statistic calculations using the R packages meta and metaphor, with minor assistance from the first author (NR). We reported THAs rather than patients because in some RCTs patients received bilateral THAs.
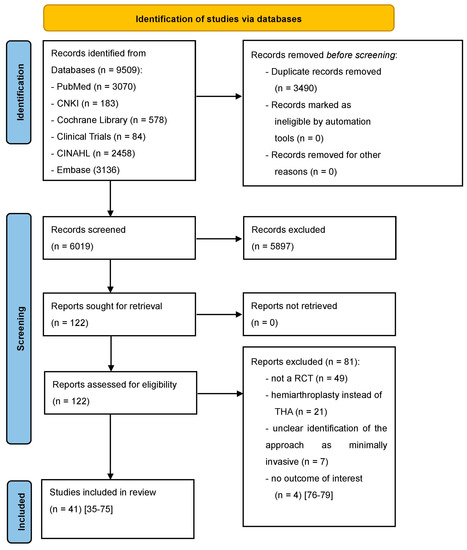
Figure 1.
PRISMA flow diagram of the search results and selection according to our inclusion criteria. CNKI: China National Knowledge Infrastructure; CINAHL: Cumulative Index to Nursing and Allied Health Literature; RCT: randomized controlled trial; THA: total hip arthroplasty [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79].
3. Results
According to our inclusion criteria, we found a total of 41 RCTs [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75] in the systematic review of the literature (Figure 1). The RCTs included 3630 THAs in 3607 patients. Of these 3607 patients, 1734 (48.07%) underwent minimally invasive THA surgery and 1873 (51.93%) underwent conventional THA surgery. Of these 1734 patients in the minimally invasive group, 765 (44.12%) patients from 16 RCTs were operated through a direct anterior approach [35,36,37,38,39,40,53,54,55,57,60,62,63,65,66,75], 83 (4.79%) patients from 1 RCT were operated through a MicroHip approach [41], 143 (8.25%) patients from 3 RCTs were operated through a minimally invasive posterior approach [42,46,69], 140 (8.07%) patients from 4 RCTs were operated through a minimally invasive anterolateral approach [51,56,64,72], 25 (1.44%) patients from 1 RCT were operated through a minimally invasive lateral approach [67], and 578 (33.33%) patients from 16 RCTs were operated through SuperPATH [43,44,45,47,48,49,50,52,58,59,61,68,70,71,73,74]. The mean age of the patients was 64.93 years (range: 51.00-89.10), and 1766 patients (48.96%) were male. The mean BMI of the patients was 26.34 kg/m2 (range: 21.50–34.60). The mean preoperative HHS was 46.30 points (range: 15.40–84.00). The most common surgical indications were osteoarthritis with 2287 (65.56%), femoral neck fracture with 665 (19.07%), ANFH with 505 (14.48%) and dysplasia with 30 (0.86%) of a total of 3487 diagnoses. Only 24 [35,36,37,39,40,41,42,46,51,53,54,55,56,57,60,62,63,64,66,67,69,70,72,73] of the 41 RCTs provided information on the use of bone cement. Of these 24 RCTs, 10 RCTs [36,40,41,42,46,51,53,54,60,63] used cemented prosthesis anchorage. These 10 RCTs included 1125 THAs, of which 687 THAs (61.07%) were operated with the use of bone cement. No bone cement was used in the remaining 14 RCTs. The mean operation time was 77.91 min. (range: 36.00–125.30). The mean incision length was 10.98 cm (range: 5.80–19.30). The mean incision length of conventional approach THA was 12.86 cm, and the mean incision length of minimally invasive THA was 9.10 cm. The mean intraoperative blood loss was 340.09 mL (range: 71.90–1644.00). The mean acetabular cup inclination angle was 42.60° (range: 37.00–50.10). The mean C-reactive protein (CRP) level 1–3 days postoperatively was 80.61 mg/L (range: 11.40–178.00). The mean creatine kinase (CK) level 1–3 days postoperatively was 594.34 mg/L (range: 203.20–1035.25). Further details are shown in Table 1 and Table 2. Two of the RCTs had identical patient cohorts, providing different outcome parameters with different follow-up times [53,54]. In addition, two RCTs [52,71] included patients with bilateral THA (see Table 1). Two RCTs [52,64] did not report data on BMI and patient age separately for minimally invasive THA and conventional approach THA, but summarized them for the whole patient cohort (see Table 1).

Table 1.
Main characteristics of the included RCTs and patients (See continuation in Table 2). RCT: randomized controlled trial; THA: total hip arthroplasty; SD: standard deviation; BMI: body mass index; HHS: Harris Hip Score; MI: minimally invasive; AL: anterolateral; DAA: direct anterior approach; L: lateral; MH: MicroHip; P: posterior; PL: posterolateral; S: SuperPATH; CA: conventional approach; TT: traction table; Lat: lateral decubitus position; NR: not reported; * This RCT divided the patient cohort according to their BMI; ** This RCT divided the patient cohort according to their diagnosis; *** Both RCTs used identical patient cohorts, giving different outcome parameters.

Table 2.
Main characteristics of the patients (Continuation of Table 1). RCT: randomized controlled trial; ANFH: avascular necrosis of the femoral head; SD: standard deviation; CRP: c-reactive protein; CK: creatine kinase; NR: not reported * This RCT divided the patient cohort according to their BMI; ** This RCT divided the patient cohort according to their diagnosis; *** Both RCTs used identical patient cohorts, giving different outcome parameters.
3.1. Quality Assessment
The outcome parameter HHS ≤ 3 months postoperatively showed a high risk of publication bias (Egger’s p-value = 0.03, Figure 2). In the corresponding funnel plot, there are many RCTs [43,44,45,49,50,70,72] outside of the triangle, especially in the upper right area. These RCTs show a relatively large positive effect with a relatively small standard error (i.e., with a relatively large apparent certainty). The outcome parameters HHS ≥ 6 months postoperatively and complication rate showed a low risk of publication bias (Egger’s p-value = 0.2, Figure 3; Egger’s p-value = 0.83, Figure 4, respectively). The risk of bias and the level of evidence assessment are shown in Table 3 and Table 4. According to the Cochrane’s Risk of Bias 2 (RoB 2) tool [32], 19 [35,37,38,40,41,43,45,47,48,49,57,59,60,61,63,64,67,68,73] out of 41 RCTs had a high risk of bias, 8 RCTs [44,51,56,65,66,69,71,74] had a moderate risk of bias, and 14 RCTs [36,39,42,46,50,52,53,54,55,58,62,70,72,75] had a low risk of bias. According to the recommendations of the GRADE system [33], the outcome parameters HHS ≤ 3 months postoperatively and complication rate showed low quality of evidence, and the outcome parameter HHS ≥ 6 months postoperatively showed moderate quality of evidence.
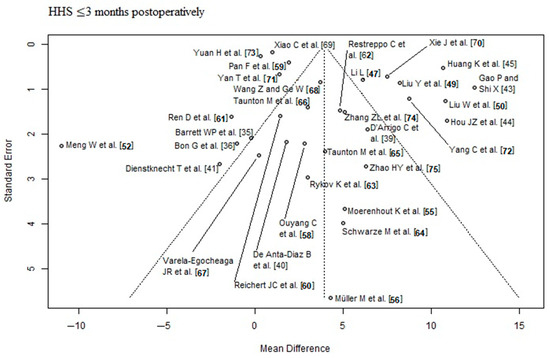
Figure 2.
Funnel plot of the HHS ≤ 3 months postoperatively. Many RCTs [43,44,45,49,50,70,72] lie outside of the funnel plot triangle, especially in the upper right area, which detects a high risk of publication bias (Egger’s p-value = 0.03). HHS: Harris hip score [35,36,39,40,41,43,44,45,47,49,50,52,55,56,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75].
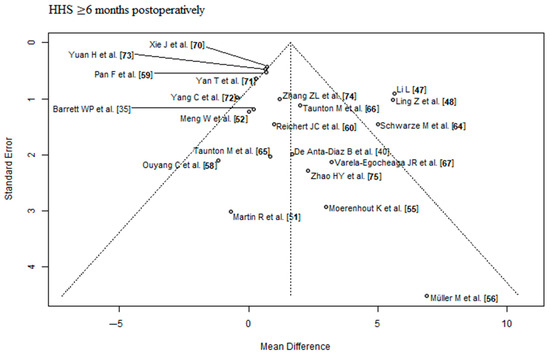
Figure 3.
Funnel plot of the HHS ≥ 6 months postoperatively. Most of the RCTs lie inside of the funnel plot triangle, which detects a low risk of publication bias. HHS: Harris hip score [35,40,47,48,51,52,55,56,58,59,60,64,65,66,67,70,71,72,73,74,75].

Figure 4.
Funnel plot of the complication rate. Most of the RCTs lie inside of the funnel plot triangle, which detects a low risk of publication bias [35,36,37,38,39,41,42,43,44,46,48,49,54,55,57,58,59,60,65,66,70,74,75].

Table 3.
Risk of bias assessment. (+): low risk of bias; (?): moderate risk of bias; (−): high risk of bias.

Table 4.
Level of evidence assessment according to GRADE recommendations. RCT: randomized controlled trial; HHS: Harris Hip Score; SD: standard deviation.
3.2. Meta-Analysis
3.2.1. HHS ≤ 3 Months Postoperatively
Data on 2690 THAs were pooled from 32 RCTs (I2 = 96%, p < 0.01, Figure 5). The HHS ≤ 3 months postoperatively of minimally invasive THA was 3.93 points higher than the HHS ≤ 3 months postoperatively of conventional approach THA (MD = 3.93, 95% CI 2.22 to 5.64).
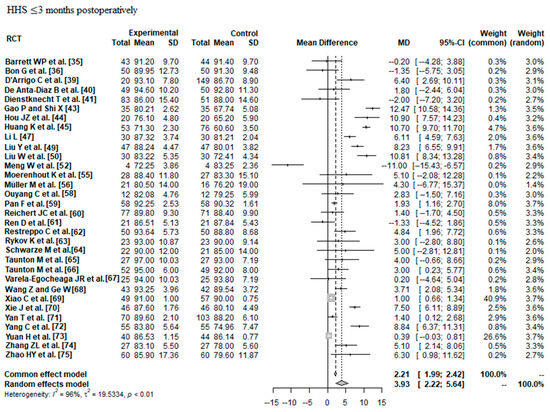
Figure 5.
Forest plot of the HHS ≤ 3 months postoperatively. The MD of the summary measure has positive values, which favours minimally invasive THA (MD = 3.93, 95% CI 2.22 to 5.64). RCT: randomized controlled trial; SD: standard deviation; MD: mean difference; CI: confidence interval; HHS: Harris hip score [35,36,39,40,41,43,44,45,47,49,50,52,55,56,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75].
3.2.2. HHS ≥ 6 Months Postoperatively
Data on 1698 THAs were pooled from 21 RCTs (I2 = 69%, p < 0.01, Figure 6). The HHS ≥ 6 months postoperatively of minimally invasive THA was 1.62 points higher than the HHS ≥ 6 months postoperatively of conventional approach THA (MD = 1.62, 95% CI 0.67 to 2.57).
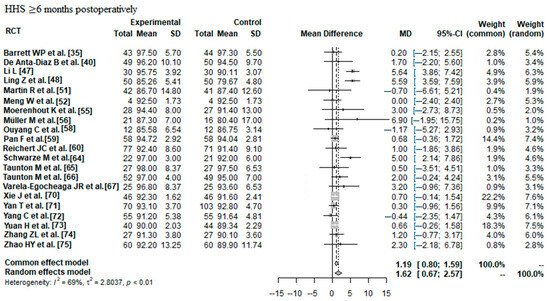
Figure 6.
Forest plot of the HHS ≥ 6 months postoperatively. The MD of the summary measure has positive values, which favours minimally invasive THA (MD = 1.62, 95% CI 0.67 to 2.57). RCT: randomized controlled trial; SD: standard deviation; MD: mean difference; CI: confidence interval; HHS: Harris hip score [35,40,47,48,51,52,55,56,58,59,60,64,65,66,67,70,71,72,73,74,75].
3.2.3. Complication Rate
Data on 2152 THAs were pooled from 23 RCTs (I2 = 66%, p < 0.01, Figure 7). The complication risk of minimally invasive THA was indifferent compared with the complication risk of conventional approach THA (OR = 1.21, 95% CI 0.56 to 2.59).
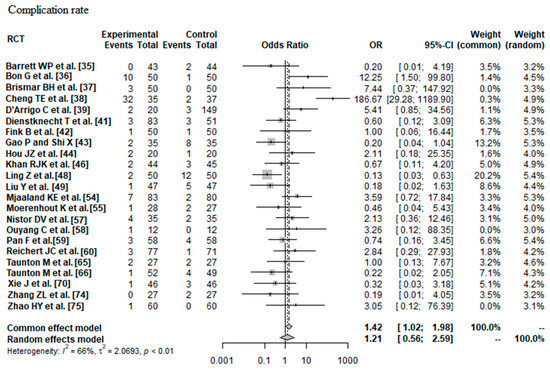
Figure 7.
Forest plot of the complication rate. The 95% CI of the OR summary measure have a <1 and a >1 value, which means that there was no difference between minimally invasive and conventional approach THA (OR = 1.21, 95% CI 0.56 to 2.59). RCT: randomized controlled trial; SD: standard deviation; OR: odds ratio; CI: confidence interval; THA: total hip arthroplasty [35,36,37,38,39,41,42,43,44,46,48,49,54,55,57,58,59,60,65,66,70,74,75].
3.3. Meta-Regression Analysis
3.3.1. Risk Factors and Predictors of HHS ≤ 3 Months Postoperatively
The following predictors and risk factors were examined for their influence on HHS ≤ 3 months postoperatively: patient age, BMI, preoperative HHS, sex, osteoarthritis, femoral neck fracture, dysplasia, ANFH, surgical approach, operation time, incision length, intraoperative blood loss, acetabular cup inclination, CRP 1–3 days postoperatively, CK 1–3 days postoperatively, and use of bone cement. None of these predictors and risk factors were statistically significant (see Table 5).

Table 5.
All results of the meta-regression analysis for all risk factors and predictors and all outcome parameters. *: statistically significant; **: The calculation is N/A because with a number of < 3 RCTs included, no meta-regression can be performed; RCT: randomized controlled trial; THA: total hip arthroplasty; HHS: Harris hip score; BMI: body mass index; ANFH: avascular necrosis of the femoral neck; CRP: C-reactive protein; CK: creatine kinase.
3.3.2. Risk Factors and Predictors of HHS ≥ 6 Months Postoperatively
Patient age: Of the 21 RCTs [35,40,47,48,51,52,55,56,58,59,60,64,65,66,67,70,71,72,73,74,75] with 1698 THAs reporting patient age demographics, a positive association (predictor estimate = 0.14) was found between patient age and HHS ≥ 6 months postoperatively. For each 1-year increase in mean patient age, the effect size for HHS ≥ 6 months postoperatively increased by an average of 0.14 points (p < 0.01; Figure 8).
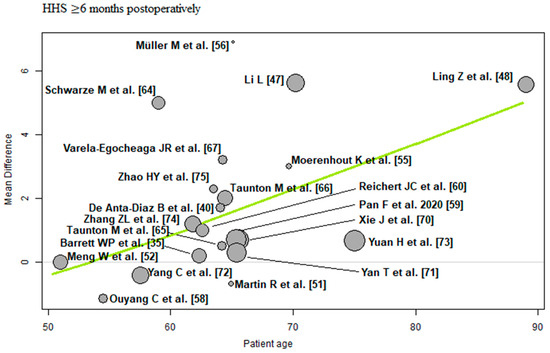
Figure 8.
Bubble plot of the predictor patient age and the outcome parameter HHS ≤ 3 months postoperatively. The slope of the regression line (in green) is large (steep line), which shows that the predictor has a strong influence on the outcome variable. HHS: Harris hip score [35,40,47,48,51,52,55,56,58,59,60,64,65,66,67,70,71,72,73,74,75].
ANFH: Of the 19 RCTs [35,40,48,51,52,56,58,59,60,64,65,66,67,70,71,72,73,74,75] with 1594 THAs reporting ANFH, a negative association (predictor estimate = −0.03) was found between ANFH and HHS ≥ 6 months postoperatively. For every 1 percentage point increase in the incidence of ANFH, the effect size for HHS ≥ 6 months postoperatively decreased by an average of 0.03 points (p = 0.04; Figure 9).
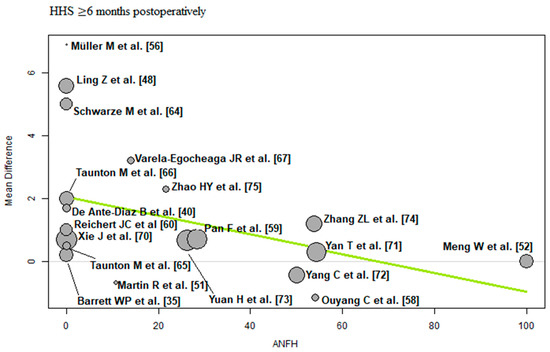
Figure 9.
Bubble plot of the predictor ANFH and the outcome parameter HHS ≤ 3 months postoperatively. The slope of the regression line (in green) is large (steep line), which shows that the predictor has a strong influence on the outcome variable. HHS: Harris hip score; ANFH: avascular necrosis of the femoral head [35,40,48,51,52,56,58,59,60,64,65,66,67,70,71,72,73,74,75].
Incision length: Of the 13 RCTs [35,40,48,51,52,56,58,59,70,71,72,73,75] with 1133 THAs reporting incision length, there was a negative association (predictor estimate = −0.82) between incision length and HHS ≥ 6 months postoperatively. For each 1 cm increase in mean incision length, the effect size for HHS ≥ 6 months postoperatively decreased by an average of 0.82 points (p < 0.01; Figure 10).
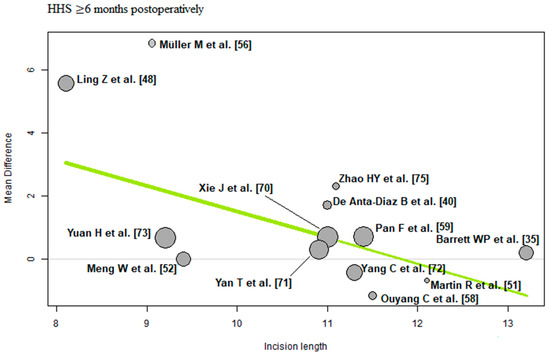
Figure 10.
Bubble plot of the predictor incision length and the outcome parameter HHS ≤ 3 months postoperatively. The slope of the regression line (in green) is large (steep line), which shows that the predictor has a strong influence on the outcome variable. HHS: Harris hip score [35,40,48,51,52,56,58,59,70,71,72,73,75].
The remaining risk factors and predictors (BMI, preoperative HHS, sex, osteoarthritis, femoral neck fracture, dysplasia, surgical approach, operation time, intraoperative blood loss, acetabular cup inclination, CRP 1–3 days postoperatively, CK 1–3 days postoperatively, and use of bone cement) were not statistically significant (see Table 5).
3.3.3. Risk Factors and Predictors of Complication Rate
Osteoarthritis: Of the 22 RCTs [35,36,37,38,39,41,42,43,44,46,48,49,54,57,58,59,60,65,66,70,74,75] with 2097 THAs reporting osteoarthritis, a positive association (predictor estimate = 0.02) was found between osteoarthritis and the complication rate. For each 1 percentage point increase in the incidence of osteoarthritis, the effect size for the complication rate increased by an average of 0.02 (p = 0.02; Figure 11).
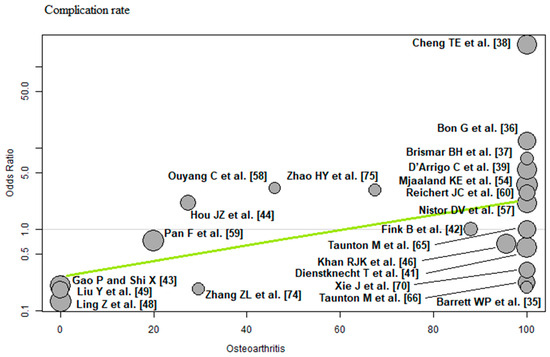
Figure 11.
Bubble plot of the predictor osteoarthritis and the outcome parameter complication rate. The slope of the regression line (in green) is large (steep line), which shows that the predictor has a strong influence on the outcome variable [35,36,37,38,39,41,42,43,44,46,48,49,54,57,58,59,60,65,66,70,74,75].
Femoral neck fracture: Of the 23 RCTs [35,36,37,38,39,41,42,43,44,46,48,49,54,55,57,58,59,60,65,66,70,74,75] with 2152 THAs reporting femoral neck fracture, a negative association (predictor estimate = −0.02) was found between femoral neck fracture and the complication rate. For each 1 percentage point increase in the incidence of femoral neck fracture, the effect size for the complication rate decreased by an average of 0.02 (p = 0.02; Figure 12).
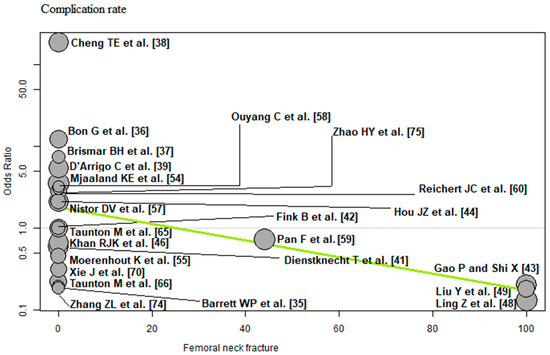
Figure 12.
Bubble plot of the predictor femoral neck fracture and the outcome parameter complication rate. The slope of the regression line (in green) is large (steep line), which shows that the predictor has a strong influence on the outcome variable [35,36,37,38,39,41,42,43,44,46,48,49,54,55,57,58,59,60,65,66,70,74,75].
Surgical approach: Of the 23 RCTs [35,36,37,38,39,41,42,43,44,46,48,49,54,55,57,58,59,60,65,66,70,74,75] with 2152 THAs reporting the surgical approach, a negative association (predictor estimate = −1.72) was found between SuperPATH and the complication rate. For each 1 percentage point increase in the frequency of SuperPATH surgical technique, the effect size for the complication rate decreased by an average of 1.72 (p < 0.01; Figure 13).
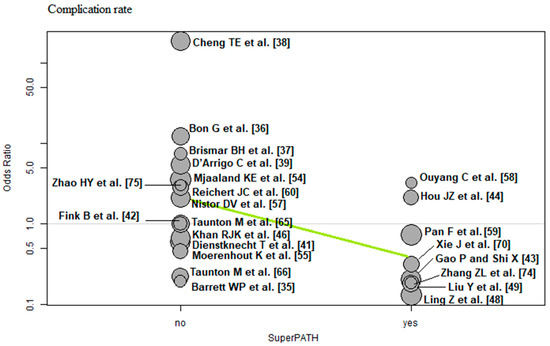
Figure 13.
Bubble plot of the predictor SuperPATH and the outcome parameter complication rate. The slope of the regression line (in green) is large (steep line), which shows that the predictor has a strong influence on the outcome variable [35,36,37,38,39,41,42,43,44,46,48,49,54,55,57,58,59,60,65,66,70,74,75].
The remaining risk factors and predictors (patient age, BMI, preoperative HHS, sex, dysplasia, ANFH, other surgical approach, except SuperPATH, operation time, incision length, intraoperative blood loss, acetabular cup inclination, CRP 1–3 days postoperatively, CK 1–3 days postoperatively, and use of bone cement) were not statistically significant. All results of the meta-regression analysis for all risk factors and predictors and all outcome parameters are shown in Table 5.
4. Discussion
Our systematic review and meta-regression analysis examined risk factors and predictors for the effect size of the functional outcome and complication rate of minimally invasive and conventional approach THA, including 3630 THAs in 3607 patients from 41 RCTs. For a better understanding, it must be emphasized once again that the present study does not simply examine the factors influencing THA, but examines which factors influence the differences in the outcome between the minimally invasive and conventional approach THA. To the best of our knowledge, this is the first study of its kind. The main results of our study showed that minimally invasive THA had a better short-term functional outcome than conventional approach THA. There was no difference in short-term complications. We identified patient age, ANFH, and incision length as predictors of the effect size of the HHS ≥ 6 months postoperatively. We identified osteoarthritis, femoral neck fracture, and SuperPATH surgical technique as predictors of the effect size of the complication rate.
The outcome parameter HHS ≤ 3 months postoperatively showed a high risk of publication bias, while the outcome parameters HHS ≥ 6 months postoperatively and the complication rate showed a low risk of publication bias. A total of 19 out of 45 RCTs had a high risk of bias, 8 RCTs had a moderate risk of bias and 14 RCTs had a low risk of bias. The outcome parameters HHS ≤ 3 months postoperatively and complication rate showed low quality of evidence, the outcome parameter HHS ≥ 6 months postoperatively showed moderate quality of evidence. The meta-analysis of the outcome parameters did not show relevant differences between THA through minimally invasive approaches compared with THA through conventional approaches. Minimally invasive THA had 3.93 and 1.62 points higher HHS ≤ 3 months postoperatively and ≥ 6 months postoperatively, respectively, than the conventional approach THA. These small differences in hip function are clinically irrelevant. Minimally invasive THA was indifferent compared with the complication risk of conventional approach THA. We considered 15 potential risk factors and predictors. We did not identify any risk factors and predictors for the effect size of the outcome parameter HHS ≤ 3 months postoperatively. The effect size of the outcome parameter HHS ≥ 6 months postoperatively was influenced by the patient age, ANFH, and incision length. The effect size of the complication rate was influenced by osteoarthritis, femoral neck fracture, and SuperPATH surgical technique.
Patient age and HHS ≥ 6 months postoperatively showed a positive association. For each 1-year increase in mean age, the effect size for HHS ≥ 6 months postoperatively increased by an average of 0.14 points. This means that the difference found in the meta-analysis between minimally invasive and conventional approach THA changes by 0.14 HHS points per one patient-year in favour of the minimally invasive approaches. Elderly patients therefore benefit more from minimally invasive THA than from conventional approach THA compared with younger patients. This finding can be explained by the fact that minimally invasive approaches are muscle-sparing and less traumatic. In general, younger patients are better able to compensate for tissue trauma than elderly patients. The specialist literature is conflicting on this issue. A 2016 systematic review by Buirs et al. [25] found a significant negative association between patient age and functional outcome. Another systematic review by Hofstede et al. [26] found no relevant influence of patient age on THA outcome. A retrospective study of 1806 patients by Huddleston et al. [23] showed that increasing patient age is a risk factor for experiencing any adverse event. A matched case-control study by Melloh et al. [24] showed that elderly patients had a lower risk of cemented stem loosening, with the odds decreasing by 3.00% per year of age. A systematic review by Prokopetz et al. [27] showed a statistically significant association between patient age and revision risk. The younger patients had an increased risk of revision, while the risk generally decreased with each additional decade of age. A meta-analysis by Ren et al. [28] showed that age was not strongly associated with the infection risk.
The ANFH and HHS ≥ 6 months postoperatively showed a negative association. For every 1 percentage point increase in the incidence of ANFH, the effect size for HHS ≥ 6 months postoperatively decreased by an average of 0.03 points. This means that the difference found in the meta-analysis between minimally invasive and conventional approach THA levels off as the incidence of ANFH increases. The finding that the choice between minimally invasive or conventional approach does not play a relevant role in ANFH is important for surgical practice. This finding has not yet been described in the specialist literature. The systematic review by Prokopetz et al. [27] showed a higher revision risk in patients diagnosed with ANFH as compared with osteoarthritis.
Incision length and HHS ≥ 6 months postoperatively showed a negative association. For each 1 cm increase in mean incision length, the effect size for HHS ≥ 6 months postoperatively decreased by an average of 0.82 points. This means that the difference found in the meta-analysis between minimally invasive and conventional approach THA changes by 0.82 points for every 1 cm increase in incision length to the disadvantage of the minimally invasive approaches. In our patient cohort, the mean incision length of THA through conventional approaches was 12.86 cm, and the mean incision length of THA through minimally invasive approaches was 9.10 cm. In general, minimally invasive THA aims to achieve an incision length ≤ 10 cm, which explains why the difference in the HHS levels off as the incision length increases.
Osteoarthritis and the complication rate showed a positive association. For every 1 percentage point increase in the incidence of osteoarthritis, the effect size for the complication rate increased by an average of 0.02. Femoral neck fracture and the complication rate showed a negative association. For every 1 percentage point increase in the incidence of femoral neck fracture, the effect size for the complication rate decreased by an average of 0.02. These changes in the difference in the complication rate between minimally invasive and conventional approach THA do not appear to be clinically relevant. The meta-analysis by Ren et al. [28] found a higher infection risk in patients with femoral neck fractures.
SuperPATH surgical technique and the complication rate showed a negative association. For each 1 percentage point increase in the frequency of SuperPATH, the effect size for the complication rate decreased by an average of 1.72. The more often SuperPATH was used as a minimally invasive approach, the more significantly the complication rate of minimally invasive THA decreased compared with the conventional approach. A recently published meta-analysis by Ramadanov [80] showed no differences in complication rates between SuperPATH and conventional approaches in THA.
Based on these findings, we recommend that more frequent use of minimally invasive THA in elderly patients should be considered. However, there are still some potential disadvantages of the minimally invasive THA that should be taken into account when choosing the approach and technique for a particular patient. The minimally invasive approach aims to achieve a shorter incision length, which results in a limited view of the surgical field, making it more difficult to identify anatomical abnormalities or complications during surgery. There is an increased risk of nerve and vessel injury, which can be minimized by the technical skill of the surgeon. In addition, minimally invasive approaches have a longer operation time than conventional approaches due to the need to use specialized instruments and the complexity of the surgical technique itself. Longer operation times may increase the risk of bacterial contamination and infection. With regard to the latter, prosthesis infection can also result from the complex interaction of several factors: bacteria, prosthesis, and host weakness. Again, it depends on the surgical skill of the surgeon to keep the operation time as short as possible in minimally invasive THA, since excessive operation times of more than 180 min. after joint replacement are associated with significant blood loss (more than 800 mL), blood transfusion, excessive tissue trauma, the presence of nosocomial bacterial strains, and failure to follow the rules of asepsis and antisepsis [81].
We identified the following strengths and limitations of our meta-regression analysis: (1) We used an intention-to-treat (ITT) analysis, so a certain number of patients were lost to follow-up. (2) The medium-term and long-term THA outcomes were not considered. (3) Some risk factors and predictors were reported less frequently than others. Further meta-regression analyses with larger data sets are needed to draw definitive conclusions on these risk factors and predictors. (4) In some cases, information on standard deviation was not reported, and it was inserted via imputation. (5) We examined a wide range of risk factors and predictors and performed a meta-regression analysis, which has not been performed before on this topic.
5. Conclusions
We identified patient age, ANFH, and incision length as predictors of the effect size of the HHS ≥ 6 months postoperatively; and osteoarthritis, femoral neck fracture, and SuperPATH surgical technique as predictors for the effect size of the complication rate. Elderly patients seem to benefit from minimally invasive THA. SuperPATH seems to reduce the complication rate of minimally invasive THA compared with conventional approach THA. Based on these findings, and taking into account our limitations, we recommend that more frequent use of minimally invasive THA in elderly patients should be considered.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12185895/s1, Raw data set.
Author Contributions
N.R.: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing—original draft; writing—review and editing. M.O.: Writing—review and editing. P.L.: Data curation; formal analysis; investigation; methodology. K.L.: Data curation; formal analysis; investigation; methodology; translation from Chinese. R.H.: Formal analysis; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing—review and editing. P.M.-K.: Writing—review and editing. D.D.: Writing—review & editing. R.B.: Writing—review & editing; supervision; validation. All authors have read and agreed to the published version of the manuscript.
Funding
Funded by the Brandenburg Medical School publication fund supported by the German Research Foundation and the Ministry of Science, Research and Cultural Affairs of the State of Brandenburg.
Institutional Review Board Statement
Ethical review and approval was waived for this study as it is a systematic review of previously published data.
Informed Consent Statement
Patient consent was waived for this study as it is a systematic review of previously published data.
Data Availability Statement
Raw data set is available in the supplement.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ANFH | avascular necrosis of the femoral head |
| BMI | body mass index |
| CI | confidence interval |
| CINAHL | Cumulative Index to Nursing and Allied Health Literature |
| CK | creatine kinase |
| CNKI | China National Knowledge Infrastructure |
| CRP | C-reactive protein |
| HHS | Harris Hip Score |
| ITT | intention-to-treat |
| MD | mean difference |
| OR | odds ratio |
| THA | total hip arthroplasty |
References
- Varacallo, M.A.; Herzog, L.; Toossi, N.; Johanson, N.A. Ten-Year Trends and Independent Risk Factors for Unplanned Readmission Following Elective Total Joint Arthroplasty at a Large Urban Academic Hospital. J. Arthroplast. 2017, 32, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 3–6. [Google Scholar] [PubMed]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.M.; Helmick, C.G.; Renner, J.B.; Luta, G.; Dragomir, A.D.; Woodard, J.; Fang, F.; Schwartz, T.A.; Nelson, A.E.; Abbate, L.M.; et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: The Johnston County Osteoarthritis Project. J. Rheumatol. 2009, 36, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Steinbuch, M.; Stevenson, R.; Miday, R.; Watts, N.B. The epidemiology of osteonecrosis: Findings from the GPRD and THIN databases in the UK. Osteoporos. Int. 2010, 21, 569–577. [Google Scholar] [CrossRef]
- Zhao, D.W.; Yu, M.; Hu, K.; Wang, W.; Yang, L.; Wang, B.J.; Gao, X.H.; Guo, Y.M.; Xu, Y.Q.; Wei, Y.S.; et al. Prevalence of Nontraumatic Osteonecrosis of the Femoral Head and its Associated Risk Factors in the Chinese Population: Results from a Nationally Representative Survey. Chin. Med. J. 2015, 128, 2843–2850. [Google Scholar] [CrossRef]
- Rosell, P.A.; Parker, M.J. Functional outcome after hip fracture. A 1-year prospective outcome study of 275 patients. Injury 2003, 34, 529–532. [Google Scholar] [CrossRef]
- Bonnaire, F.; Weber, A. S2e-Leitlinie Schenkelhalsfraktur des Erwachsenen. 2015. Available online: https://www.awmf.org/uploads/tx_szleitlinien/012-001l_S2e_Schenkelhalsfraktur_2015-10_01.pdf (accessed on 1 January 2023).
- Bender, B.; Nogler, M.; Hozack, W.J. Direct anterior approach for total hip arthroplasty. Orthop. Clin. N. Am. 2009, 40, 321e8. [Google Scholar] [CrossRef]
- Graf, R.; Mohajer, M.A. The Stolzalpe technique: A modified Watson-Jones approach. Int. Orthop. 2007, 31 (Suppl. 1), S21e4. [Google Scholar] [CrossRef]
- Berger, R.A. The technique of minimally invasive total hip arthroplasty using the two-incision approach. Instr. Course Lect. 2004, 53, 149e55. [Google Scholar]
- Della Torre, P.K.; Fitch, D.A.; Chow, J.C. Supercapsular percutaneously-assisted total hip arthroplasty: Radiographic outcomes and surgical technique. Ann. Transl. Med. 2015, 3, 180. [Google Scholar] [PubMed]
- Howell, J.R.; Garbuz, D.S.; Duncan, C.P. Minimally invasive hip replacement: Rationale, applied anatomy, and instrumentation. Orthop. Clin. N. Am. 2004, 35, 107e18. [Google Scholar] [CrossRef]
- Berry, D.J.; Berger, R.A.; Callaghan, J.J.; Dorr, L.D.; Duwelius, P.J.; Hartzband, M.A.; Lieberman, J.R.; Mears, D.C. Minimally invasive total hip arthroplasty. Development, early results, and a critical analysis. Presented at the Annual Meeting of the American Orthopaedic Association, Charleston, South Carolina, USA, June 14, 2003. J. Bone Joint Surg. Am. 2003, 85, 2235–2246. [Google Scholar] [CrossRef]
- Cheng, T.; Feng, J.G.; Liu, T.; Zhang, X.L. Minimally invasive total hip arthroplasty: A systematic review. Int. Orthop. 2009, 33, 1473e81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nilsdotter, A.-K.; Petersson, I.F.; Roos, E.M.; Lohmander, L.S. Predictors of patient relevant outcome after total hip replacement for osteoarthritis: A prospective study. Ann. Rheum. Dis. 2003, 62, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Johanson, N.A.; Abzug, J.M.; Gallus, C.A.; Charlson, M.E. Baseline function and comorbidity predict outcome in total hip arthroplasty. UPOJ 2009, 194, 1–7. [Google Scholar]
- Davis, A.M.; Agnidis, Z.; Bradley, E.; Kiss, A.; Waddell, J.P.; Gross, A.E. Predictors of functional outcome two years following revision hip arthroplasty. J. Bone Joint Surg. Am. 2006, 88-A, 685–691. [Google Scholar]
- Cushnaghan, J.; Coggon, D.; Reading, I.; Croft, P.; Byng, P.; Cox, K.; Dieppe, P.; Cooper, C. Long-term outcome following total hip arthroplasty: A controlled longitudinal study. Arthritis Rheum. 2007, 57, 1375–1380. [Google Scholar] [CrossRef]
- Biring, G.S.; Masri, B.A.; Greidanus, N.V.; Duncan, C.P.; Garbuz, D.S. Predictors of quality of life outcomes after revision total hip replacement. J. Bone Joint Surg. Br. 2007, 89-B, 1446–1451. [Google Scholar] [CrossRef]
- Smith, G.H.; Johnson, S.; Ballantyne, J.A.; Dunstan, E.; Brenkel, I.J. Predictors of excellent early outcome after total hip arthroplasty. J. Orthop. Surg. Res. 2012, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.I.; Wang, Y.; Uquillas, C.; Herndon, J.H.; Maloney, W.J. Age and obesity are risk factors for adverse events after total hip arthroplasty. Clin. Orthop. Relat. Res. 2012, 470, 490–496. [Google Scholar] [CrossRef]
- Melloh, M.; Eggli, S.; Busato, A.; Roder, C. Predictors of early stem loosening after total hip arthroplasty: A case-control study. J. Orthop. Surg. 2011, 19, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Buirs, L.D.; Van Beers, L.W.; Scholtes, V.A.; Pastoors, T.; Sprague, S.; Poolman, R.W. Predictors of physical functioning after total hip arthroplasty: A systematic review. BMJ Open 2016, 6, e010725. [Google Scholar] [CrossRef] [PubMed]
- Hofstede, S.N.; Gademan, M.G.; Vliet Vlieland, T.P.; Nelissen, R.G.; Marang-van de Mheen, P.J. Preoperative predictors for outcomes after total hip replacement in patients with osteoarthritis: A systematic review. BMC Musculoskelet. Disord. 2016, 17, 212. [Google Scholar] [CrossRef]
- Prokopetz, J.J.; Losina, E.; Bliss, R.L.; Wright, J.; Baron, J.A.; Katz, J.N. Risk factors for revision of primary total hip arthroplasty: A systematic review. BMC Musculoskelet. Disord. 2012, 13, 251. [Google Scholar] [CrossRef]
- Ren, X.; Ling, L.; Qi, L.; Liu, Z.; Zhang, W.; Yang, Z.; Wang, W.; Tu, C.; Li, Z. Patients’ risk factors for periprosthetic joint infection in primary total hip arthroplasty: A meta-analysis of 40 studies. BMC Musculoskelet. Disord. 2021, 22, 776. [Google Scholar] [CrossRef]
- Hewlett-Smith, N.; Pope, R.; Furness, J.; Simas, V.; Hing, W. Prognostic factors for inpatient functional recovery following total hip and knee arthroplasty: A systematic review. Acta Orthop. 2020, 91, 313–318. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Deeks, J.; Altman, D. Special topics in statistics. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011; Chapter 16. [Google Scholar]
- Harris, W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: Treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J. Bone Joint Surg. Am. 1969, 51, 737–755. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Berkey, C.S.; Hoaglin, D.C.; Antczak-Bouckoms, A.; Mosteller, F.; Colditz, G.A. Meta-analysis of multiple outcomes by regression with random effects. Stat. Med. 1998, 17, 2537–2550. [Google Scholar] [CrossRef]
- Barrett, W.P.; Turner, S.E.; Leopold, J.P. Prospective randomized study of direct anterior vs postero-lateral approach for total hip arthroplasty. J. Arthroplast. 2013, 28, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Bon, G.; Kacem, E.B.; Lepretre, P.M.; Weissland, T.; Mertl, P.; Dehl, M.; Gabrion, A. Does the direct anterior approach allow earlier recovery of walking following total hip arthroplasty? A randomized prospective trial using accelerometry. Orthop. Traumatol. Surg. Res. 2019, 105, 445–452. [Google Scholar] [CrossRef]
- Brismar, B.H.; Hallert, O.; Tedhamre, A.; Lindgren, J.U. Early gain in pain reduction and hip function, but more complications following the direct anterior minimally invasive approach for total hip arthroplasty: A randomized trial of 100 patients with 5 years of follow up. Acta Orthop. 2018, 89, 484–489. [Google Scholar] [CrossRef]
- Cheng, T.E.; Wallis, J.A.; Taylor, N.F.; Holden, C.T.; Marks, P.; Smith, C.L.; Armstrong, M.S.; Singh, P.J. A Prospective Randomized Clinical Trial in Total Hip Arthroplasty-Comparing Early Results Between the Direct Anterior Approach and the Posterior Approach. J. Arthroplast. 2017, 32, 883–890. [Google Scholar] [CrossRef]
- D’Arrigo, C.; Speranza, A.; Monaco, E.; Carcangiu, A.; Ferretti, A. Learning curve in tissue sparing total hip replacement: Comparison between different approaches. J. Orthop. Traumatol. 2009, 10, 47–54. [Google Scholar] [CrossRef]
- De Anta-Díaz, B.; Serralta-Gomis, J.; Lizaur-Utrilla, A.; Benavidez, E.; López-Prats, F.A. No differences between direct anterior and lateral approach for primary total hip arthroplasty related to muscle damage or functional outcome. Int. Orthop. 2016, 40, 2025–2030. [Google Scholar] [CrossRef]
- Dienstknecht, T.; Lüring, C.; Tingart, M.; Grifka, J.; Sendtner, E. A minimally invasive approach for total hip arthroplasty does not diminish early post-operative outcome in obese patients: A prospective, randomised trial. Int. Orthop. 2013, 37, 1013–1018. [Google Scholar] [CrossRef]
- Fink, B.; Mittelstaedt, A.; Schulz, M.S.; Sebena, P.; Singer, J. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty A prospective and comparative study. J. Orthop. Surg. Res. 2010, 5, 46. [Google Scholar] [CrossRef]
- Gao, P.; Shi, X. The effect of total hip replacement with minimally invasive SuperPATH approach in the treatment of femoral neck fractures in the elderly. Hen. Med. Res. 2020, 29, 3715–3717. (In Chinese) [Google Scholar]
- Hou, J.Z.; Bao, H.; Cheng, Y. Early effect observation of total hip arthroplasty by using SuperPATH technique. J. Clin. Orthop. 2017, 20, 50–53. (In Chinese) [Google Scholar]
- Huang, K.; Xie, K.; Shi, Y.; Lu, X.; Chen, J.; Lu, L.; Liu, J.; Lu, M.; Pan, S.; Tang, Y. Analysis of early clinical efficacy of SuperPATH approach and lateral approach for initial total hip arthroplasty. Youjiang Med. J. 2021, 49, 9. [Google Scholar]
- Khan, R.J.; Maor, D.; Hofmann, M.; Haebich, S. A comparison of a less invasive piriformis-sparing approach versus the standard posterior approach to the hip: A randomised controlled trial. J. Bone Joint Surg. Br. 2012, 94, 43–50. [Google Scholar] [CrossRef]
- Li, L. SuperPATH minimally invasive total hip replacement surgery treatment: Analysis of clinical efficacy of aseptic necrosis of femoral head. Chin. J. Mod. Drug Appl. 2020, 14, 84–86. (In Chinese) [Google Scholar]
- Ling, Z.; Zhou, P.; Fu, Y. Analysis of the effect of total hip replacement via SuperPATH approach on the prognosis of elderly patients with femoral neck fracture. Chin. J. Front. Med. Sc. 2020, 12, 66–70. (In Chinese) [Google Scholar]
- Liu, Y.; Hu, P.; Zhu, J.; She, H.; Zhang, Y. Efficacy of minimally invasive total hip arthroplasty in the treatment of elderly femoral neck fractures. Prac. J. Med. Pharm. 2021, 38, 226–231. [Google Scholar]
- Liu, W.; Liu, X.; Gao, H.; Wang, G.; Li, J. Comparison of the curative effect, pain degree, and hip joint function between SuperPATH hip replacement and total hip replacement. Mod. Chin. Docs. 2022, 60, 78–84. [Google Scholar]
- Martin, R.; Clayson, P.E.; Troussel, S.; Fraser, B.P.; Docquier, P.L. Anterolateral minimally invasive total hip arthroplasty: A prospective randomized controlled study with a follow-up of 1 year. J. Arthroplast. 2011, 26, 1362–1372. [Google Scholar] [CrossRef]
- Meng, W.; Huang, Z.; Wang, H.; Wang, D.; Luo, Z.; Bai, Y.; Gao, L.; Wang, G.; Zhou, Z. Supercapsular percutaneously-assisted total hip (SuperPath) versus posterolateral total hip arthroplasty in bilateral osteonecrosis of the femoral head: A pilot clinical trial. BMC Musculoskelet. Disord. 2019, 21, 2. [Google Scholar] [CrossRef]
- Mjaaland, K.E.; Kivle, K.; Svenningsen, S.; Pripp, A.H.; Nordsletten, L. Comparison of markers for muscle damage, inflammation, and pain using minimally invasive direct anterior versus direct lateral approach in total hip arthroplasty: A prospective, randomized, controlled trial. J. Orthop. Res. 2015, 33, 1305–1310. [Google Scholar] [CrossRef]
- Mjaaland, K.E.; Kivle, K.; Svenningsen, S.; Nordsletten, L. Do Postoperative Results Differ in a Randomized Trial Between a Direct Anterior and a Direct Lateral Approach in THA? Clin. Orthop. Relat. Res. 2019, 477, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Moerenhout, K.; Derome, P.; Laflamme, G.Y.; Leduc, S.; Gaspard, H.S.; Benoit, B. Direct anterior versus posterior approach for total hip arthroplasty: A multicentre, prospective, randomized clinical trial. Can. J. Surg. 2020, 63, E412–E417. [Google Scholar] [CrossRef]
- Müller, M.; Tohtz, S.; Springer, I.; Dewey, M.; Perka, C. Randomized controlled trial of abductor muscle damage in relation to the surgical approach for primary total hip replacement: Minimally invasive anterolateral versus modified direct lateral approach. Arch. Orthop. Trauma Surg. 2011, 131, 179–189. [Google Scholar] [CrossRef]
- Nistor, D.V.; Caterev, S.; Bolboacă, S.D.; Cosma, D.; Lucaciu, D.O.G.; Todor, A. Transitioning to the direct anterior approach in total hip arthroplasty. Is it a true muscle sparing approach when performed by a low volume hip replacement surgeon? Int. Orthop. 2017, 41, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Wang, H.; Meng, W.; Luo, Z.; Wang, D.; Pei, F.; Zhou, Z. Randomized controlled trial of comparison between the SuperPATH and posterolateral approaches in total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 1500–1506. (In Chinese) [Google Scholar] [PubMed]
- Pan, Y.; Zhang, J.; Yan, X.; Chang, X.; Li, J.; Tang, B. Comparison of SuperPATH and posterolateral total hip replacement. Orthop. J. China 2020, 28, 1176–1180. (In Chinese) [Google Scholar]
- Reichert, J.C.; von Rottkay, E.; Roth, F.; Renz, T.; Hausmann, J.; Kranz, J.; Rackwitz, L.; Nöth, U.; Rudert, M. A prospective randomized comparison of the minimally invasive direct anterior and the transgluteal approach for primary total hip arthroplasty. BMC Musculoskelet. Disord. 2018, 19, 241. [Google Scholar] [CrossRef]
- Ren, D.; Yang, G.; Zhao, H.; Zha, J.; Lu, S.; Xu, Y. Effect of SuperPath minimally invasive incision total hip arthroplasty on femoral head necrosis and the quality of life. J. Hebei Med. Univ. 2016, 37, 1416–1419. (In Chinese) [Google Scholar]
- Restrepo, C.; Parvizi, J.; Pour, A.E.; Hozack, W.J. Prospective randomized study of two surgical approaches for total hip arthroplasty. J. Arthroplast. 2010, 25, 671–679.e1. [Google Scholar] [CrossRef]
- Rykov, K.; Reininga, I.H.F.; Sietsma, M.S.; Knobben, B.A.S.; Ten Have BLEF. Posterolateral vs Direct Anterior Approach in Total Hip Arthroplasty (POLADA Trial): A Randomized Controlled Trial to Assess Differences in Serum Markers. J. Arthroplast. 2017, 32, 3652–3658.e1. [Google Scholar] [CrossRef]
- Schwarze, M.; Budde, S.; von Lewinski, G.; Windhagen, H.; Keller, M.C.; Seehaus, F.; Hurschler, C.; Floerkemeier, T. No effect of conventional vs. minimally invasive surgical approach on clinical outcome and migration of a short stem total hip prosthesis at 2-year follow-up: A randomized controlled study. Clin. Biomech. 2018, 51, 105–112. [Google Scholar] [CrossRef]
- Taunton, M.J.; Mason, J.B.; Odum, S.M.; Springer, B.D. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: A prospective, randomized clinical trial. J. Arthroplast. 2014, 29 (Suppl. 9), 169–172. [Google Scholar] [CrossRef] [PubMed]
- Taunton, M.J.; Trousdale, R.T.; Sierra, R.J.; Kaufman, K.; Pagnano, M.W. John Charnley Award: Randomized Clinical Trial of Direct Anterior and Miniposterior Approach THA: Which Provides Better Functional Recovery? Clin. Orthop. Relat. Res. 2018, 476, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Varela-Egocheaga, J.R.; Suárez-Suárez, M.A.; Fernández-Villán, M.; González-Sastre, V.; Varela-Gómez, J.R.; Murcia-Mazón, A. Minimally invasive hip surgery: The approach did not make the difference. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 47–52. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, W. SuperPATH approach total hip replacement for elderly patients with femoral neck fracture: Impact of hip function. Clin. Med. 2021, 41, 27–29. (In Chinese) [Google Scholar]
- Xiao, C.; Gao, Z.; Zhang, S.; Long, N.; Yao, K.; Cai, P.; He, F.; Liu, L.; Jiang, Y. Comparative prospective randomized study of minimally invasive transpiriformis approach versus conventional posterolateral approach in total hip arthroplasty as measured by biology markers. Int. Orthop. 2021, 45, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Wang, L.; Yao, X.; Pan, Z.; Jiang, Q. Comparison of supercapsular percutaneously assisted approach total hip versus conventional posterior approach for total hip arthroplasty: A prospective, randomized controlled trial. J. Orthop. Surg. Res. 2017, 12, 138. [Google Scholar] [CrossRef]
- Yan, T.; Tian, S.; Wang, Y.; Yang, X.; Li, T.; Liu, J.; Pan, P.; Wang, R.; Wang, D.; Sun, K. Comparison of early effectiveness between SuperPATH approach and Hardinge approach in total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2017, 31, 17–24. (In Chinese) [Google Scholar] [PubMed]
- Yang, C.; Zhu, Q.; Han, Y.; Zhu, J.; Wang, H.; Cong, R.; Zhang, D. Minimally-invasive total hip arthroplasty will improve early postoperative outcomes: A prospective, randomized, controlled trial. Ir. J. Med. Sci. 2010, 179, 285–290. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, J.; Sun, Z.; Zhang, Z. Comparison of effectiveness between SuperPATH approach and posterolateral approach in total hip arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 14–19. (In Chinese) [Google Scholar] [PubMed]
- Zhang, Z.L.; Lin, J.; Xia, B. Clinical research on joint function and life quality through SuperPath approach in total hip arthroplasty. China J. Integr. Trad. Chin. West. Med. 2019, 25, 709–714. (In Chinese) [Google Scholar]
- Zhao, H.Y.; Kang, P.D.; Xia, Y.Y.; Shi, X.J.; Nie, Y.; Pei, F.X. Comparison of Early Functional Recovery After Total Hip Arthroplasty Using a Direct Anterior or Posterolateral Approach: A Randomized Controlled Trial. J. Arthroplast. 2017, 32, 3421–3428. [Google Scholar] [CrossRef]
- Landgraeber, S.; Quitmann, H.; Güth, S.; Haversath, M.; Kowalczyk, W.; Kecskeméthy, A.; Heep, H.; Jäger, M. A prospective randomized peri- and post-operative comparison of the minimally invasive anterolateral approach versus the lateral approach. Orthop. Rev. 2013, 5, e19. [Google Scholar]
- Li, X.; Ma, L.; Wang, Q.; Rong, K. Comparison of total hip arthroplasty with minimally invasive SuperPath approach vs. conventional posterolateral approach in elderly patients: A one-year follow-up randomized controlled research. Asian J. Surg. 2021, 44, 531–536. [Google Scholar] [CrossRef]
- Parvizi, J.; Restrepo, C.; Maltenfort, M.G. Total Hip Arthroplasty Performed Through Direct Anterior Approach Provides Superior Early Outcome: Results of a Randomized, Prospective Study. Orthop. Clin. N. Am. 2016, 47, 497–504. [Google Scholar] [CrossRef]
- Zhao, S. Minimally invasive SuperPATH approach for hip replacement in elderly patients. Analysis of clinical efficacy in patients with bone neck fractures. Mod. Diagn. Treat. 2021, 32, 3593. [Google Scholar]
- Ramadanov, N. An Updated Meta-Analysis of Randomized Controlled Trials on Total Hip Arthroplasty through SuperPATH versus Conventional Approaches. Orthop. Surg. 2022, 14, 807–823. [Google Scholar] [CrossRef]
- Basile, G.; Gallina, M.; Passeri, A.; Gaudio, R.M.; Castelnuovo, N.; Ferrante, P.; Calori, G.M. Prosthetic joint infections and legal disputes: A threat to the future of prosthetic orthopedics. J. Orthop. Traumatol. 2021, 22, 44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).