Epidemiology and Diagnosis of Post-Thrombotic Syndrome: Qualitative Synthesis with a Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
- Human study participants: adult patients (18 or older), both sexes, all ethnicities, all nationalities.
- Consensus statements, recommendations, standards of practice.
- Original articles relevant to post-thrombotic syndrome (or post-phlebitic syndrome, venous stress disorder, and venous thrombosis complication/sequelae) secondary to DVT reporting incidence and method of disease assessment.
- English-language publications.
2.2. Exclusion Criteria
- Conference abstracts, case reports, studies with fewer than 10 patients.
2.3. Study Outcomes
2.4. Literature Search Strategy
2.5. Study Selection
2.6. Data Extraction and Critical Appraisal
2.7. Data Synthesis and Statistical Analysis
2.8. Patient and Public Involvement
3. Results
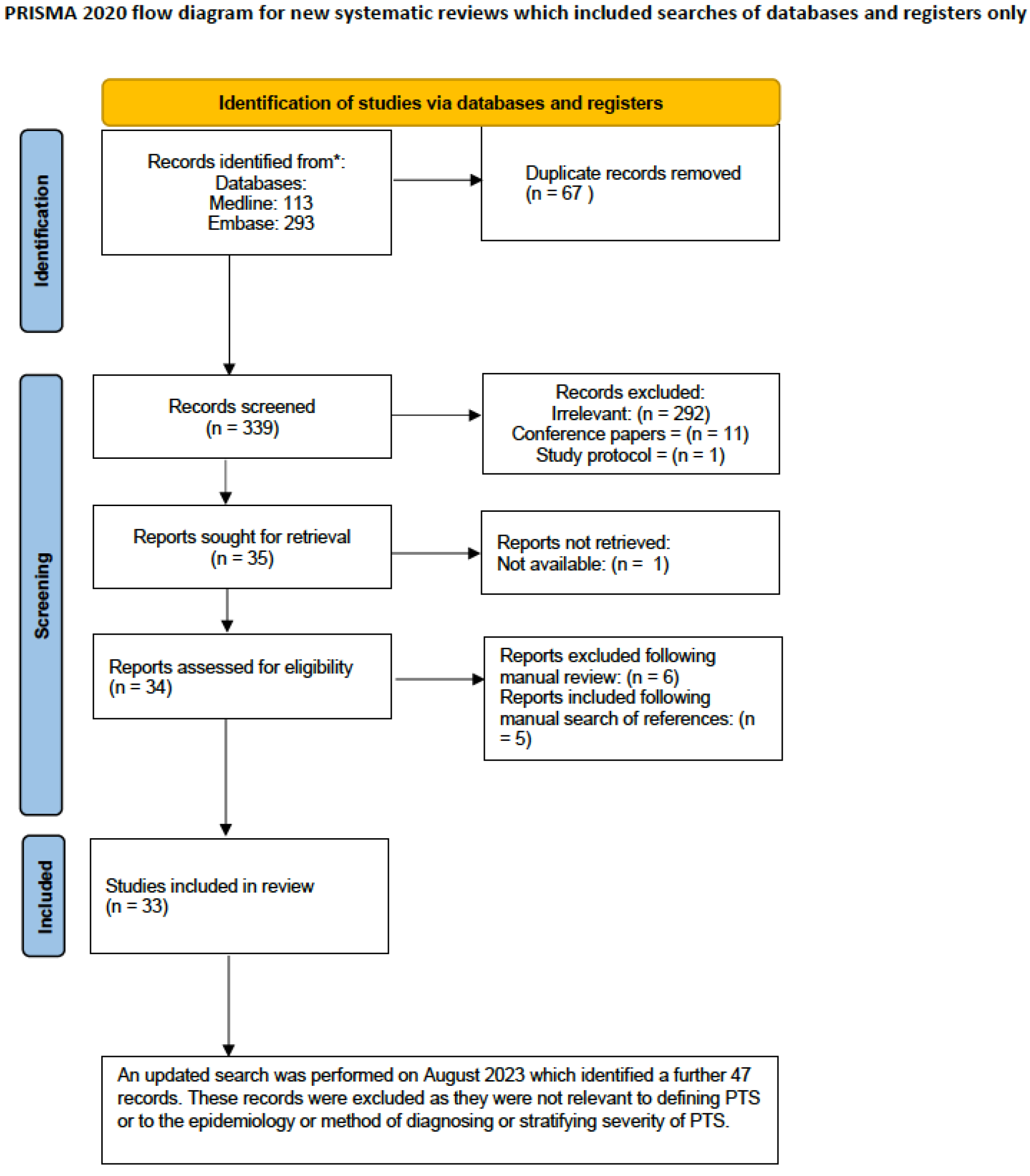
3.1. Search Strategy
3.2. Proposed Definition
- Patients should be followed up prospectively at pre-defined time intervals (every three to six months) for a minimum period of two years in order to establish PTS diagnosis. Diagnosis within three months of the index DVT should be avoided as the acute disease process may exhibit confounding signs and symptoms [11].
3.3. PTS Assessment and Severity Grading
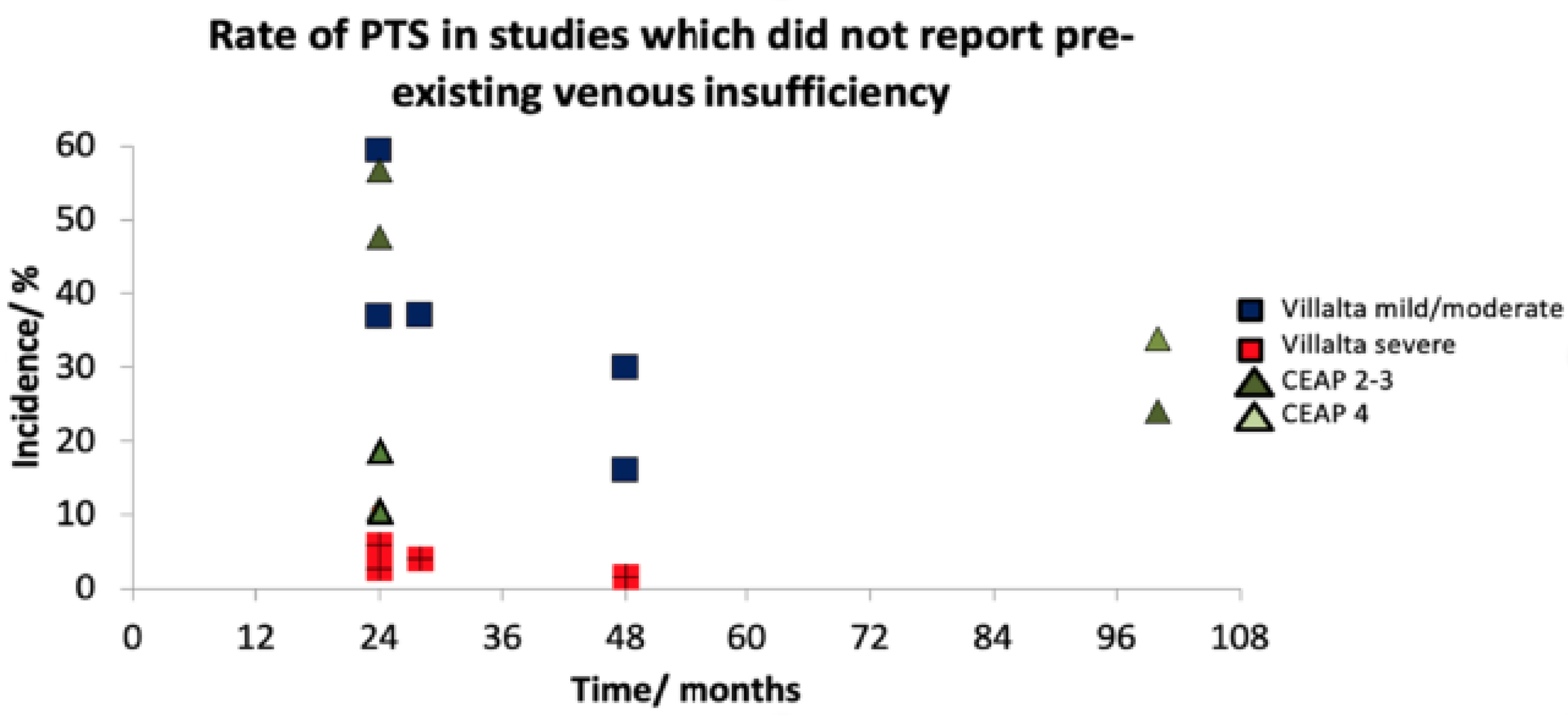
3.4. Incidence
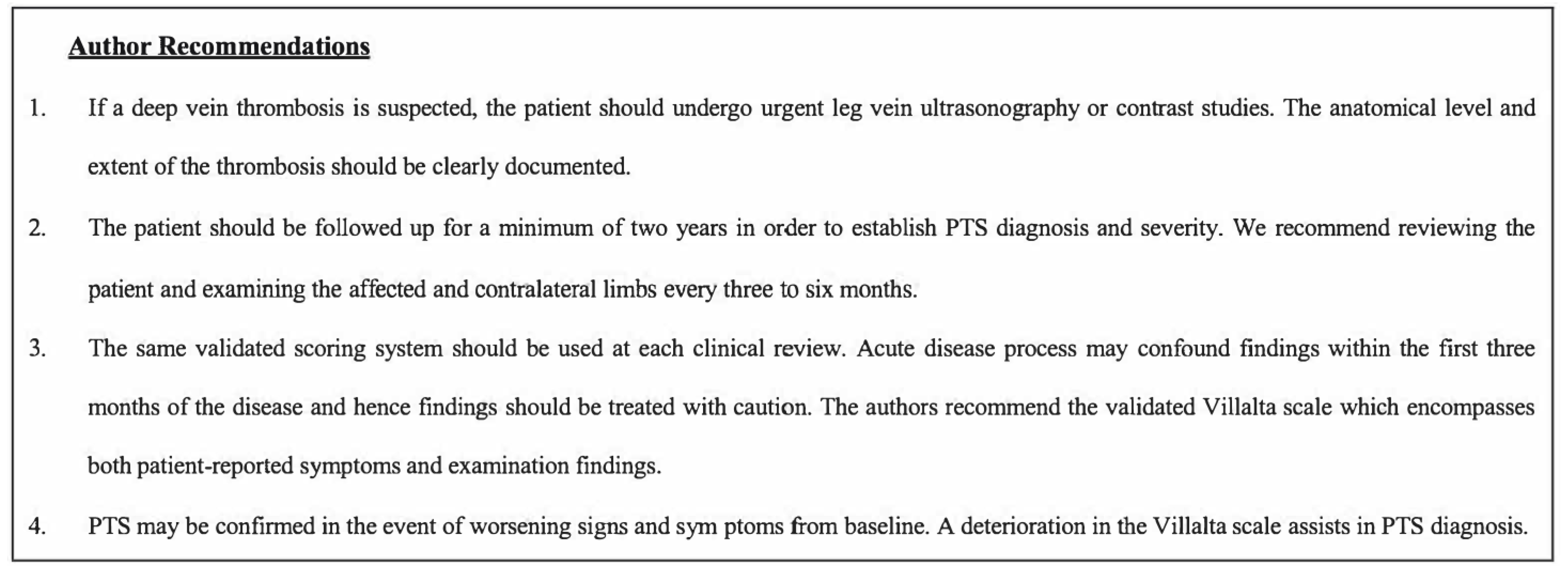
4. Discussion
- ○
- A radiologically proven index DVT.
- ○
- A minimum 24-month follow-up period with regular interval assessment.
- ○
- Assessment with a validated scoring system (ideally the PTS-specific Villalta scale).
- ○
- Documentation of baseline venous drainage with demonstration of disease progression.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahn, S.R. The post-thrombotic syndrome: The forgotten morbidity of deep venous thrombosis. J. Thromb. Thrombolysis 2006, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Ginsberg, J.S. The post-thrombotic syndrome: Current knowledge, controversies, and directions for future research. Blood Rev. 2002, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Stain, M.; Schönauer, V.; Minar, E.; Bialonczyk, C.; Hirschl, M.; Weltermann, A.; Kyrle, P.A.; Eichinger, S. The post-thrombotic syndrome: Risk factors and impact on the course of thrombotic disease. J. Thromb. Haemost. 2005, 3, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, E.; Prandoni, P. The post-thrombotic syndrome. Curr. Opin. Pulm. Med. 2000, 6, 335–342. [Google Scholar] [CrossRef][Green Version]
- Mohr, D.N.; Silverstein, M.D.; Heit, J.A.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., III. The venous stasis syndrome after deep venous thrombosis or pulmonary embolism: A population-based study. Mayo Clin. Proc. 2000, 75, 1249–1256, Elsevier. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Cogo, A.; Cuppini, S.; Villalta, S.; Carta, M.; Cattelan, A.M.; Polistena, P.; Bernardi, E.; Prins, M.H. The long-term clinical course of acute deep venous thrombosis. Ann. Intern. Med. 1996, 125, 1–7. [Google Scholar] [CrossRef]
- Brandjes, D.P.; Büller, H.R.; Heijboer, H.; Huisman, M.V.; de Rijk, M.; Jagt, H.; ten Cate, J.W. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997, 349, 759–762. [Google Scholar] [CrossRef]
- Wik, H.S.; Kahn, S.R.; Eriksson, H.; Morrison, D.; Ghanima, W.; Schulman, S.; Sandset, P.M. Post-thrombotic syndrome in patients with venous thromboembolism treated with dabigatran or warfarin: A long-term cross-sectional follow-up of RE-COVER study patients. J. Thromb. Haemost. 2021, 19, 2495–2503. [Google Scholar] [CrossRef]
- Utne, K.K.; Ghanima, W.; Foyn, S.; Kahn, S.; Sandset, P.M.; Wik, H.S. Development and validation of a tool for patient reporting of symptoms and signs of the post-thrombotic syndrome. Thromb. Haemost. 2016, 115, 361–367. [Google Scholar] [CrossRef]
- Porter, J.M.; Moneta, G.L.; Chronic, A.I.; Disease, V. Reporting standards in venous disease: An update. J. Vasc. Surg. 1995, 21, 635–645. [Google Scholar] [CrossRef]
- Kahn, S.R.; Partsch, H.; Vedantham, S.; Prandoni, P.; Kearon, C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Defin. Post-Thromb. Syndr. Leg. Use Clin. Investig. A Recomm. Stand. J. Thromb. Haemost. 2009, 7, 879–883. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.D.; Khan, K.M.; Maffulli, N.; Cook, J.L.; Wark, J.D. Studies of surgical outcome after patellar tendinopathy: Clinical significance of methodological deficiencies and guidelines for future studies. Scand. J. Med. Sci. Sports: Rev. Artic. 2000, 10, 2–11. [Google Scholar] [CrossRef]
- Beebe, H.G.; Bergan, J.J.; Bergqvist, D.; Eklof, B.; Eriksson, I.; Goldman, M.P.; Greenfield, L.J.; Hobson, R.W.; Juhan, C.; Kistner, R.L.; et al. Classification and grading of chronic venous disease in the lower limbs. A Consens. Statement. Eur. J. Vasc. Endovasc. Surg. 1996, 30, 5–11. [Google Scholar]
- Eklöf, B.; Rutherford, R.B.; Bergan, J.J.; Carpentier, P.H.; Gloviczki, P.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L.; Myers, K.; Padberg, F.T.; et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J. Vasc. Surg. 2004, 40, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.V.; Lawrence, D. Hemodynamic and clinical assessment after therapy for acute deep vein thrombosis. A Prospect. Study. Am. J. Surg. 1985, 150, 54–63. [Google Scholar]
- Porter, J.M.; Rutherford, R.B.; Clagett, G.P.; Cranley Jr, J.J.; O’Donnell, T.F.; Raju, S.; Zierler, R.E.; Browse, N.; Nicolaides, A. Reporting standards in venous disease. J. Vasc. Surg. 1988, 8, 172–181. [Google Scholar] [CrossRef]
- Villalta, S.B.; Bagatella, P.; Piccioli, A.; Lensing, A.; Prins, M.H.; Prandoni, P. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis 1994, 24 (Suppl. S1), 158a. [Google Scholar]
- NG158, N. Venous Thromboembolic Diseases: Diagnosis, Management and Thrombophilia Testing; National Institute of Health and Care Excellence (NICE): London, UK, 2020. [Google Scholar]
- Haenen, J.H.; Janssen, M.C.; Wollersheim, H.; Van’t Hof, M.A.; De Rooij, M.J.; van Langen, H.; Skotnicki, S.H.; Thien, T. The development of postthrombotic syndrome in relationship to venous reflux and calf muscle pump dysfunction at 2 years after the onset of deep venous thrombosis. J. Vasc. Surg. 2002, 35, 1184–1189. [Google Scholar] [CrossRef]
- Kahn, S.R.; Shrier, I.; Shapiro, S.; Houweling, A.H.; Hirsch, A.M.; Reid, R.D.; Kearon, C.; Rabhi, K.; Rodger, M.A.; Kovacs, M.J.; et al. Six-month exercise training program to treat post-thrombotic syndrome: A randomized controlled two-centre trial. CMAJ 2011, 183, 37–44. [Google Scholar] [CrossRef]
- Deehan, D.J.; Siddique, M.; Weir, D.J.; Pinder, I.M.; Lingard, E.M. Postphlebitic syndrome after total knee arthroplasty: 405 patients examined 2-10 years after surgery. Acta Orthop. Scand. 2001, 72, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Schindler, O.S.; Dalziel, R. Post-thrombotic syndrome after total hip or knee arthroplasty: Incidence in patients with asymptomatic deep venous thrombosis. J. Orthop. Surg. 2005, 13, 113–119. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.A.; McAlinden, M.G.; O’Connell, B.M.; Mollan, R.A. Postphlebitic syndrome after hip arthroplasty: 43 patients followed at least 5 years. Acta Orthop. Scand. 1994, 65, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, J.S.; Gent, M.; Turkstra, F.; Buller, H.R.; MacKinnon, B.; Magier, D.; Hirsh, J. Postthrombotic syndrome after hip or knee arthroplasty: A cross-sectional study. Arch. Intern. Med. 2000, 160, 669–672. [Google Scholar] [CrossRef]
- Mant, M.J.; Eurich, D.T.; Russell, D.B.; Majumdar, S.R. Post-thrombotic syndrome after total hip arthroplasty is uncommon. Acta Orthop. 2008, 79, 794–799. [Google Scholar] [CrossRef]
- Delluc, A.; Gouedard, C.; De Saint Martin, L.; Garcia, C.; Roguedas, A.M.; Bressollette, L.; Misery, L.; Mottier, D.; Le Gal, G. Incidence, risk factors and skin manifestations of post-thrombotic syndrome: A four-year follow-up of patients included in the EDITH study. Rev. Médecine Interne 2010, 31, 729–734. [Google Scholar] [CrossRef]
- Hach-Wunderle, V.; Bauersachs, R.; Gerlach, H.E.; Eberle, S.; Schellong, S.; Riess, H.; Carnarius, H.; Rabe, E. Post-thrombotic syndrome 3 years after deep venous thrombosis in the Thrombosis and Pulmonary Embolism in Out-Patients (TULIPA) PLUS Registry. J. Vasc. Surg. Venous Lymphat. Disord. 2013, 1, 5–12. [Google Scholar] [CrossRef]
- Tick, L.W.; Doggen, C.J.; Rosendaal, F.R.; Faber, W.R.; Bousema, M.T.; Mackaay, A.J.; van Balen, P.; Kramer, M.H. Predictors of the post-thrombotic syndrome with non-invasive venous examinations in patients 6 weeks after a first episode of deep vein thrombosis. J. Thromb. Haemost. 2010, 8, 2685–2692. [Google Scholar] [CrossRef]
- Lonner, J.H.; Frank, J.; McGuire, K.; Lotke, P.A. Postthrombotic syndrome after asymptomatic deep vein thrombosis following total knee and hip arthroplasty. Am. J. Orthop. 2006, 35, 469–472. [Google Scholar]
- Kurtoglu, M.; Koksoy, C.; Hasan, E.; Akcalı, Y.; Karabay, O.; Filizcan, U.; TROMBOTEK Study Group. Long-term efficacy and safety of once-daily enoxaparin plus warfarin for the outpatient ambulatory treatment of lower-limb deep vein thrombosis in the TROMBOTEK trial. J. Vasc. Surg. 2010, 52, 1262–1270. [Google Scholar] [CrossRef][Green Version]
- Ziegler, S.; Schillinger, M.; Maca, T.H.; Minar, E. Post-thrombotic syndrome after primary event of deep venous thrombosis 10 to 20 years ago. Thromb. Res. 2001, 101, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.R.; Shapiro, S.; Wells, P.S.; Rodger, M.A.; Kovacs, M.J.; Anderson, D.R.; Tagalakis, V.; Houweling, A.H.; Ducruet, T.; Holcroft, C.; et al. Compression stockings to prevent post-thrombotic syndrome: A randomised placebo-controlled trial. Lancet 2014, 383, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Partsch, H.; Kaulich, M.; Mayer, W. Immediate mobilisation in acute vein thrombosis reduces postthrombotic syndrome. Int. Angiol. 2004, 23, 206. [Google Scholar]
- Kahn, S.R.; Kearon, C.; Julian, J.A.; Mackinnon, B.; Kovacs, M.J.; Wells, P.; Crowther, M.A.; Anderson, D.R.; Van Nguyen, P.; Demers, C.; et al. Predictors of the post-thrombotic syndrome during long-term treatment of proximal deep vein thrombosis. J. Thromb. Haemost. 2005, 3, 718–723. [Google Scholar] [CrossRef]
- Saarinen, J.P.; Domonyi, K.; Zeitlin, R.; Salenius, J.P. Postthrombotic syndrome after isolated calf deep venous thrombosis: The role of popliteal reflux. J. Vasc. Surg. 2002, 36, 959–964. [Google Scholar] [CrossRef]
- Galanaud, J.P.; Holcroft, C.A.; Rodger, M.A.; Kovacs, M.J.; Betancourt, M.T.; Wells, P.S.; Anderson, D.R.; Chagnon, I.; Le Gal, G.; Solymoss, S.; et al. Comparison of the Villalta post-thrombotic syndrome score in the ipsilateral vs. contralateral leg after a first unprovoked deep vein thrombosis. J. Thromb. Haemost. 2012, 10, 1036–1042. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Prins, M.H.; Frulla, M.; Marchiori, A.; Bernardi, E.; Tormene, D.; Mosena, L.; Pagnan, A.; Girolami, A. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: A randomized, controlled trial. Ann. Intern. Med. 2004, 141, 249–256. [Google Scholar] [CrossRef]
- Monreal, M.; Martorell, A.; Callejas, J.M.; Valls, R.; Llamazares, J.F.; Lafoz, E.; Arias, A. Venographic assessment of deep vein thrombosis and risk of developing post-thrombotic syndrome: A prospective study. J. Intern. Med. 1993, 233, 233–238. [Google Scholar] [CrossRef]
- Fitzgerald, S.J.; McAndrew, C.M.; Kraay, M.J.; Goldberg, V.M. Incidence of postthrombotic syndrome in patients undergoing primary total hip arthroplasty for osteoarthritis. Clin. Orthop. Relat. Res. 2011, 469, 530–534. [Google Scholar] [CrossRef][Green Version]
- Sang, H.; Li, X.; Qian, A.; Meng, Q. Outcome of endovascular treatment in postthrombotic syndrome. Ann. Vasc. Surg. 2014, 28, 1493–1500. [Google Scholar] [CrossRef]
- Ginsberg, J.S.; Hirsh, J.; Julian, J.; Vander LaandeVries, M.; Magier, D.; MacKinnon, B.; Gent, M. Prevention and treatment of postphlebitic syndrome: Results of a 3-part study. Arch. Intern. Med. 2001, 161, 2105–2109. [Google Scholar] [CrossRef]
- McAndrew, C.M.; Fitzgerald, S.J.; Kraay, M.J.; Goldberg, V.M. Incidence of postthrombotic syndrome in patients undergoing primary total knee arthroplasty for osteoarthritis. Clin. Orthop. Relat. Res. 2010, 468, 178–181. [Google Scholar] [CrossRef][Green Version]
- Tick, L.W.; Kramer, M.H.; Rosendaal, F.R.; Faber, W.R.; Doggen, C.J. Risk factors for post-thrombotic syndrome in patients with a first deep venous thrombosis. J. Thromb. Haemost. 2008, 6, 2075–2081. [Google Scholar] [CrossRef]
- Necas, M. Duplex ultrasound in the assessment of lower extremity venous insufficiency. Australas. J. Ultrasound Med. 2010, 13, 37. [Google Scholar] [CrossRef]
- Kolbach, D.N.; Neumann, H.A.; Prins, M.H. Definition of the post-thrombotic syndrome, differences between existing classifications. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 404–414. [Google Scholar] [CrossRef]
- Ng, S.; Rodger, M.A.; Ghanima, W.; Kovacs, M.J.; Shivakumar, S.; Kahn, S.R.; Sandset, P.M.; Kearon, C.; Mallick, R.; Delluc, A. External validation of the patient reported Villalta scale for the diagnosis of post-thrombotic syndrome. Thromb. Haemost. 2022, 122, 1379–1383. [Google Scholar]
- Renton, S.; Brooks, M.; Jenkins, M.; Boyle, J.; Allen, L.; Smith, L.; Morgan, R.; Bell, R.; Mouton, R. Provision of Services for People with Vascular Disease. Vascular Society. 2021. Available online: https://www.vascularsociety.org.uk/_userfiles/pages/files/Resources/FINAL%20POVS.pdf (accessed on 4 July 2023).

| Villalta (Mild: 5–9, Moderate: 10–14, Severe: 15/Ulcer) | CEAP (Mild to Moderate: Class 2–3, Severe: Class 4–6) | Widmer | Browse (PTS = 4 or More) | Brandjes (Mild to Moderate ≤ 3, Severe ≥ 4) | Ginsberg | |
|---|---|---|---|---|---|---|
| Subjective | ||||||
| Symptomatic | Yes/No | |||||
| Pain | 0–3 | √ | ||||
| Cramps | 0–3 | |||||
| Heaviness | 0–3 | 1 | ||||
| Pruritus | 0–3 | |||||
| Paraesthesia | 0–3 | |||||
| Swelling | 1 | |||||
| Activities | 1 | |||||
| Pain (calf)—Spontaneous | 1 | |||||
| Pain (calf)—Stand/walk | 1 | √ | ||||
| Pain (thigh)—Spontaneous | 1 | |||||
| Pain (thigh)—Stand/walk | 1 | √ | ||||
| Objective | ||||||
| Telangiectasia/flare | 0–3 | Class 1 | 2 | 1 | ||
| Varicose Veins | Class 2 | Class 1 | 1 | 1 | ||
| Oedema | 0–3 | Class 3 | Class 1–3 | 2 | √ | |
| Eczema | 0–3 | Class 4 | Class 3 | 1 | ||
| Hyperpigmentation | 0–3 | Class 4 | Class 2 | 2 | 1 | |
| Induration | 0–3 | 4 | 1 | |||
| Healed ulcer | Class 5 | Class 2 | ||||
| Ulcer | Severe | Class 6 | Class 3 | 5 | 4 | |
| Pain on compression | 0–3 | |||||
| >1 cm calf circumference | 1 | |||||
| >1 cm ankle circumference | 1 | |||||
| Sample Size | Follow-Up (Months) | Score | Severity | n (%) | |
|---|---|---|---|---|---|
| Schindler and Dalziel (2005) [23] | 42 | 24 | CEAP | Mild Moderate | 3 (7%) 4 (9.5%) |
| Delluc et al. (2010) [27] | 95 | 48 | Villalta | Mild/Moderate Severe | 27 (28%) 0 |
| Hach-Wunderle et al. (2013) [28] | 135 | 36 | Villalta | Mild/Moderate Severe | 31 (23%) 2 (1.5%) |
| Prandoni et al. (2004) [38] | 166 | 24 | Villalta | Mild/Moderate Severe | 60 (36%) 5 (3%) |
| Prandoni et al. (2004) [38] | 126 * | 48 | Villalta | Mild/Moderate Severe | 40 (31%) 4 (3%) |
| Sample Size | Follow-Up (Months) | Score | Severity | n (%) | |
|---|---|---|---|---|---|
| Haenen et al. (2002) [20] | 86 | 12–24 | CEAP | Mild Moderate | 41 (47.6%) 16 (18.6%) |
| Mant et al. (2008) [26] | 25 | 24–48 | Villalta | Any | 4 (16%) |
| Kahn et al. (2014) [33] | 803 | 6–24 | Villalta | Mild/Moderate Severe | 297 (36.9%) 47 (5.9%) |
| Partsch et al. (2004) [34] | 37 | 24 | Villalta CEAP | Mild/Moderate Mild Moderate Severe | 22 (59.4%) 21 (56.8%) 4 (10.8%) 1 (2.7%) |
| Kahn et al. (2005) [35] | 145 | 13–43 | Villalta | Mild/Moderate Severe | 54 (37%) 6 (4%) |
| Saarinen et al. (2002) [36] | 50 | 72–120 | CEAP | Mild Moderate | 12 (24%) 17 (34%) |
| Galanaud et al. (2012) [37] | 137 | 6–48 | Villalta | Mild/Moderate Severe | 100 (29.9%) 6 (1.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangwani, J.; Roberts, V.; Shannak, O.; Divall, P.; Srinivasan, A.; Dias, J. Epidemiology and Diagnosis of Post-Thrombotic Syndrome: Qualitative Synthesis with a Systematic Review. J. Clin. Med. 2023, 12, 5896. https://doi.org/10.3390/jcm12185896
Mangwani J, Roberts V, Shannak O, Divall P, Srinivasan A, Dias J. Epidemiology and Diagnosis of Post-Thrombotic Syndrome: Qualitative Synthesis with a Systematic Review. Journal of Clinical Medicine. 2023; 12(18):5896. https://doi.org/10.3390/jcm12185896
Chicago/Turabian StyleMangwani, Jitendra, Veronica Roberts, Odei Shannak, Pip Divall, Ananth Srinivasan, and Joseph Dias. 2023. "Epidemiology and Diagnosis of Post-Thrombotic Syndrome: Qualitative Synthesis with a Systematic Review" Journal of Clinical Medicine 12, no. 18: 5896. https://doi.org/10.3390/jcm12185896
APA StyleMangwani, J., Roberts, V., Shannak, O., Divall, P., Srinivasan, A., & Dias, J. (2023). Epidemiology and Diagnosis of Post-Thrombotic Syndrome: Qualitative Synthesis with a Systematic Review. Journal of Clinical Medicine, 12(18), 5896. https://doi.org/10.3390/jcm12185896






