The Heart and Seizures: Friends or Enemies?
Abstract
1. Introduction
2. Methods
2.1. Search of the Literature
2.2. Study Selection
2.3. Inclusion Criteria
2.4. Qualitative Data Extraction
3. Clinical Cardiovascular Events Connected to Seizures
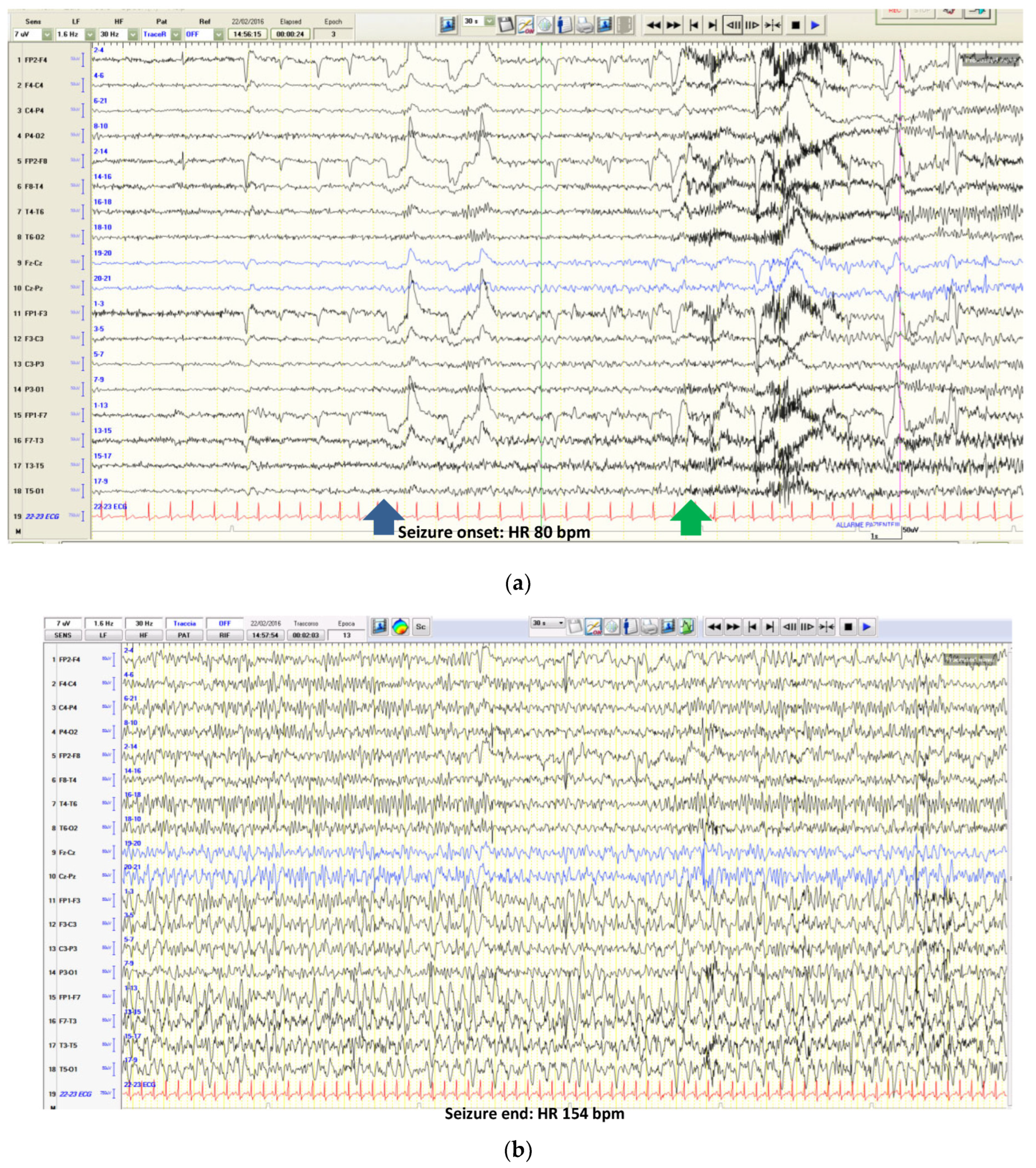
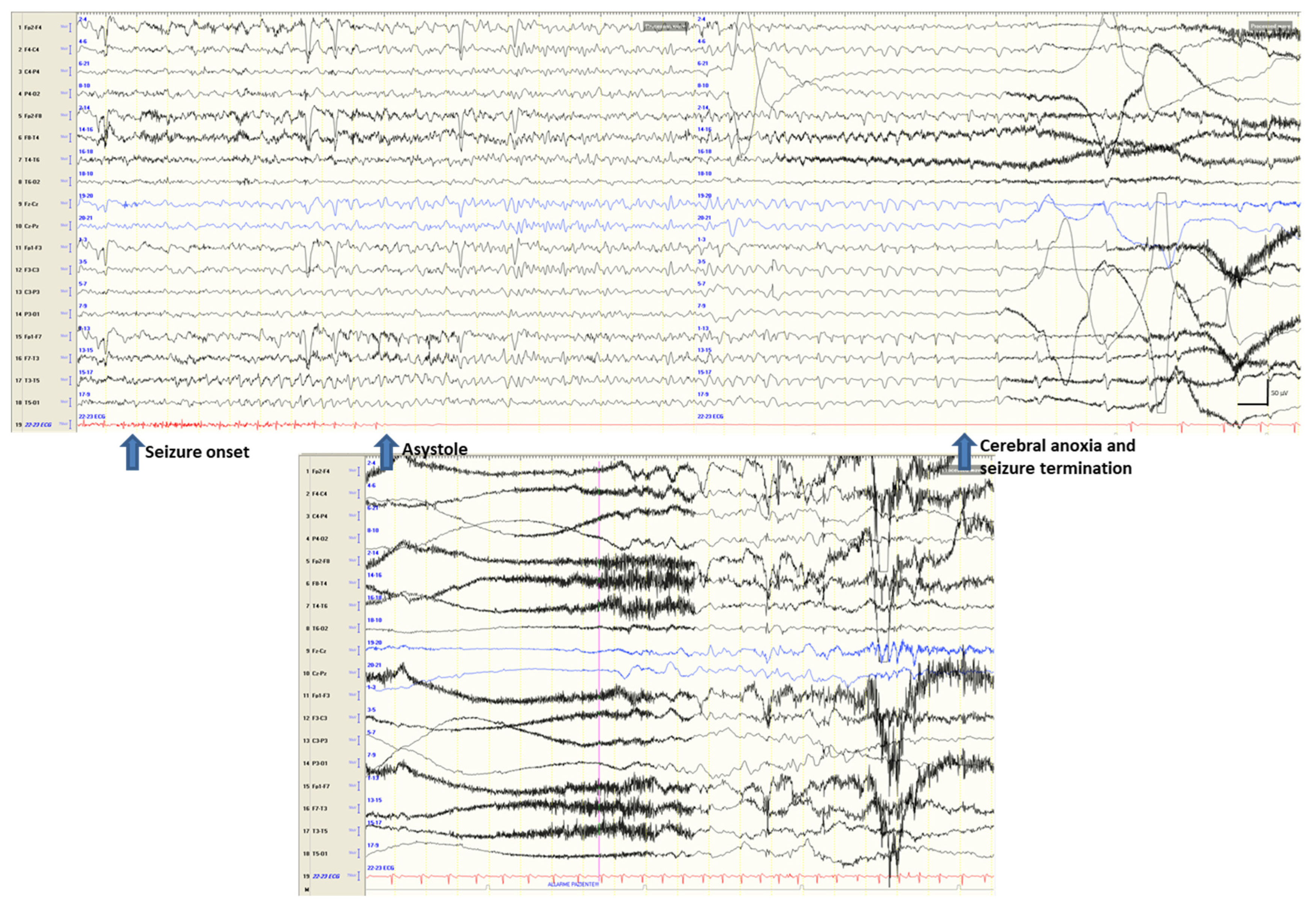
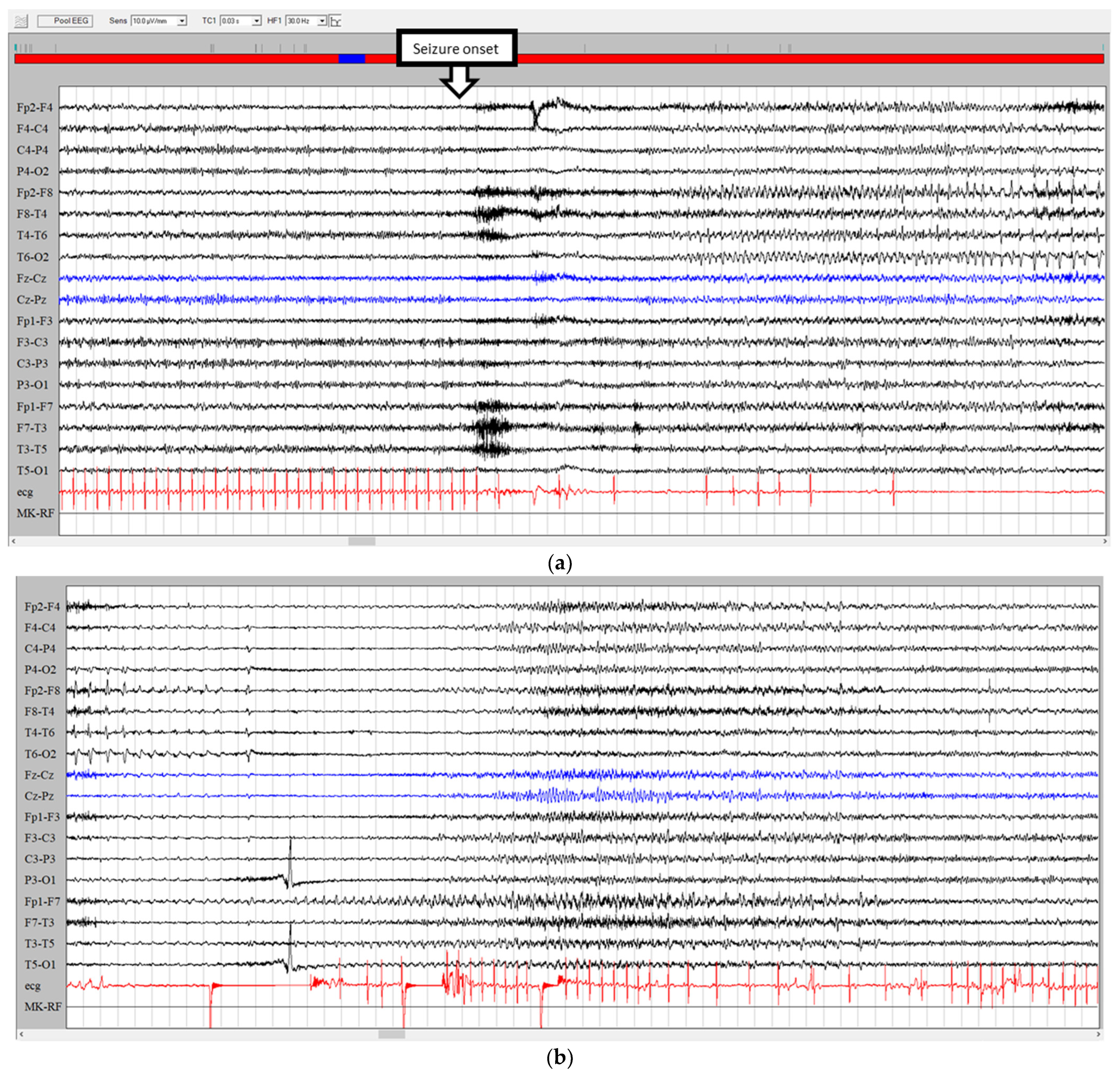
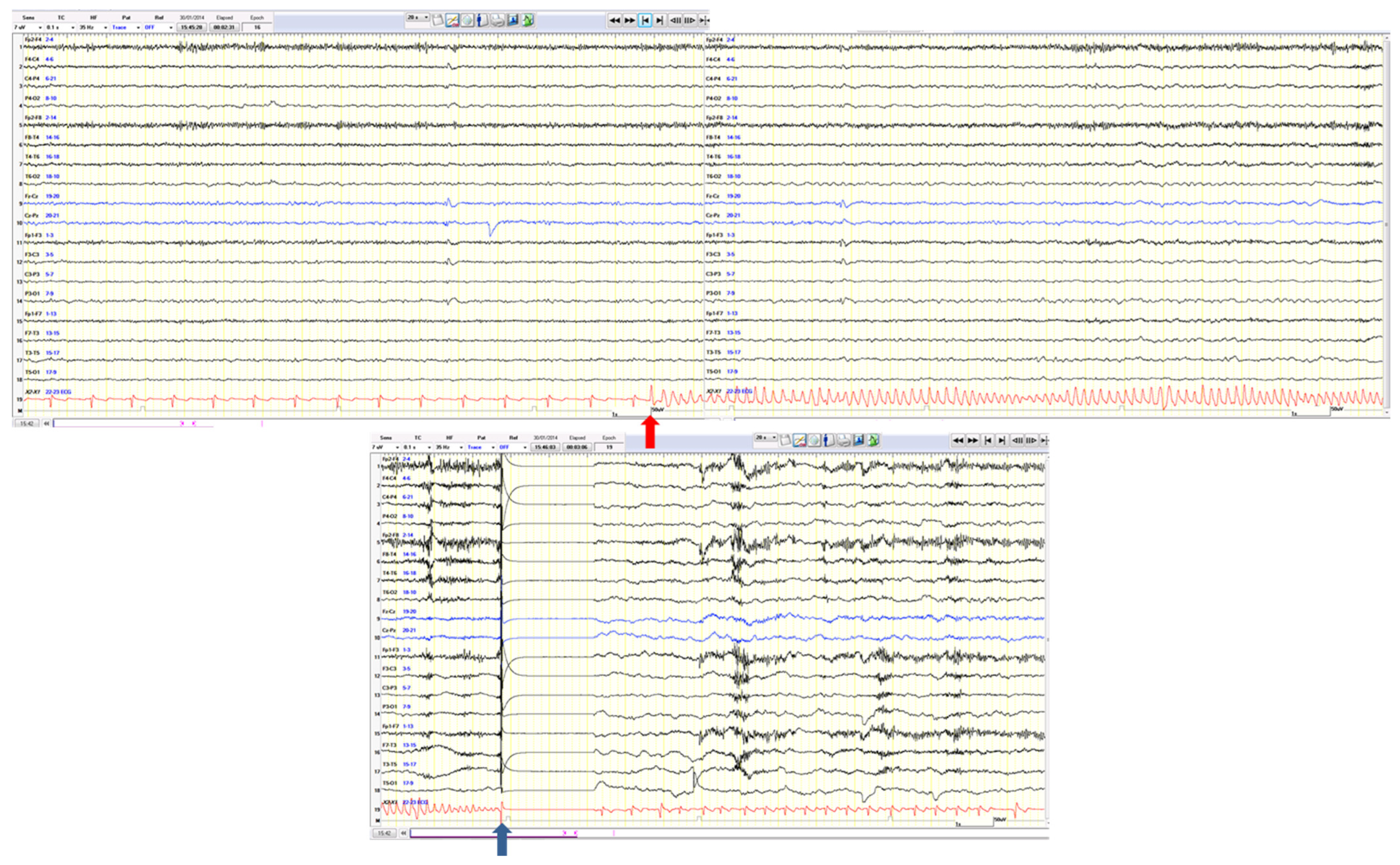
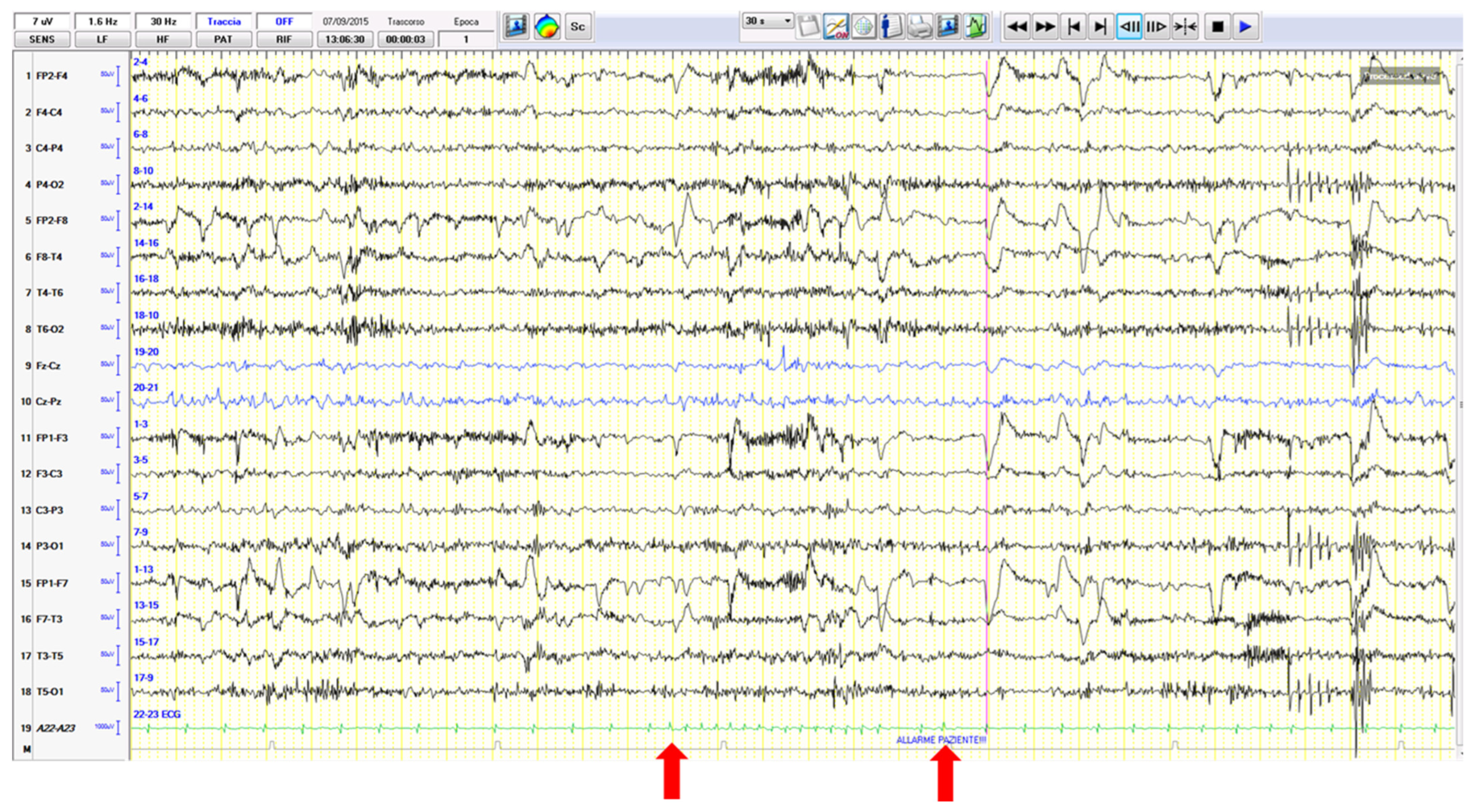
3.1. Ictal Arrhythmias
3.2. Other Ictal or Post-Ictal Arrhythmias
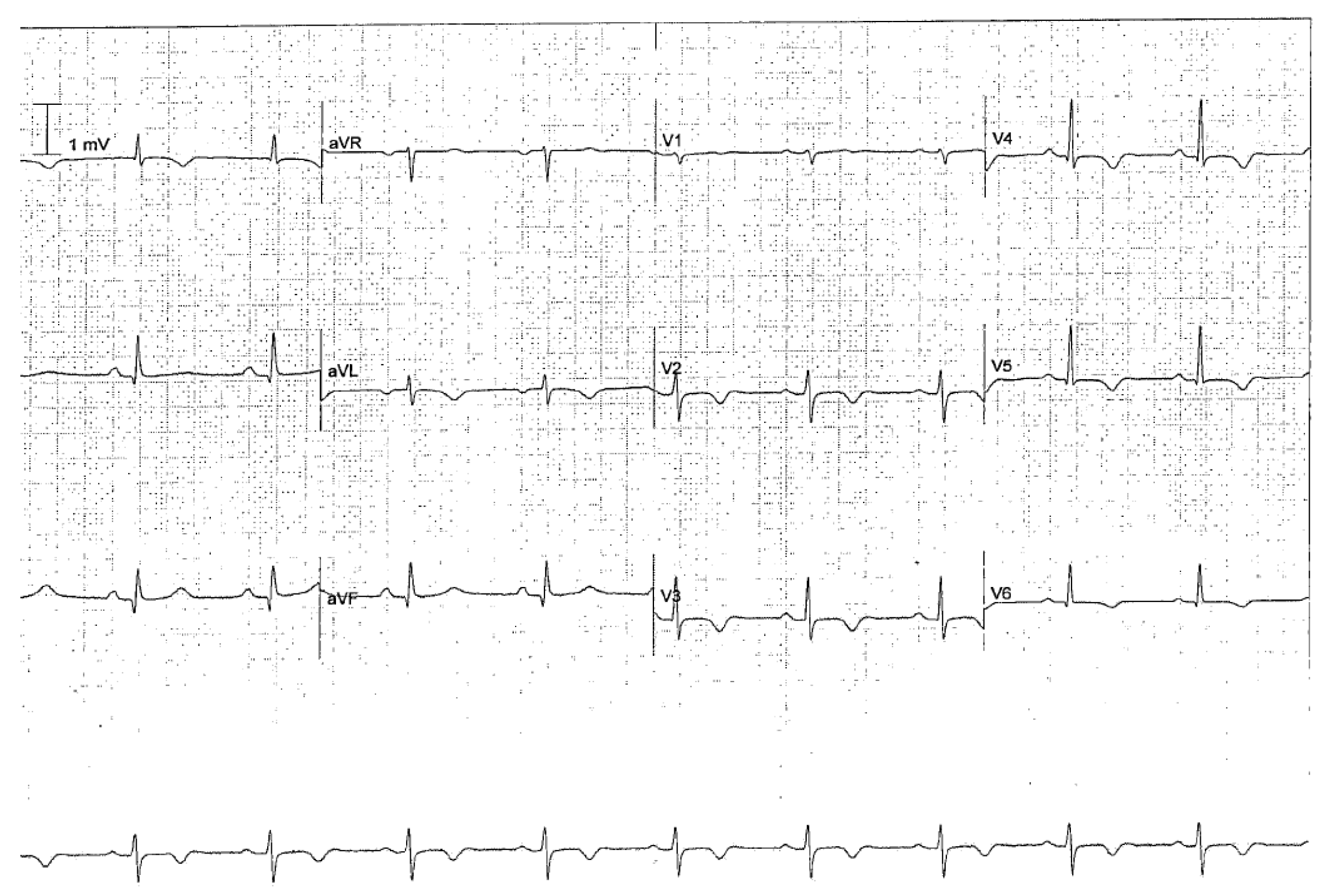
3.3. Other Seizure-Related Cardiovascular Effects
3.4. Differential Diagnoses
4. Pathophysiology
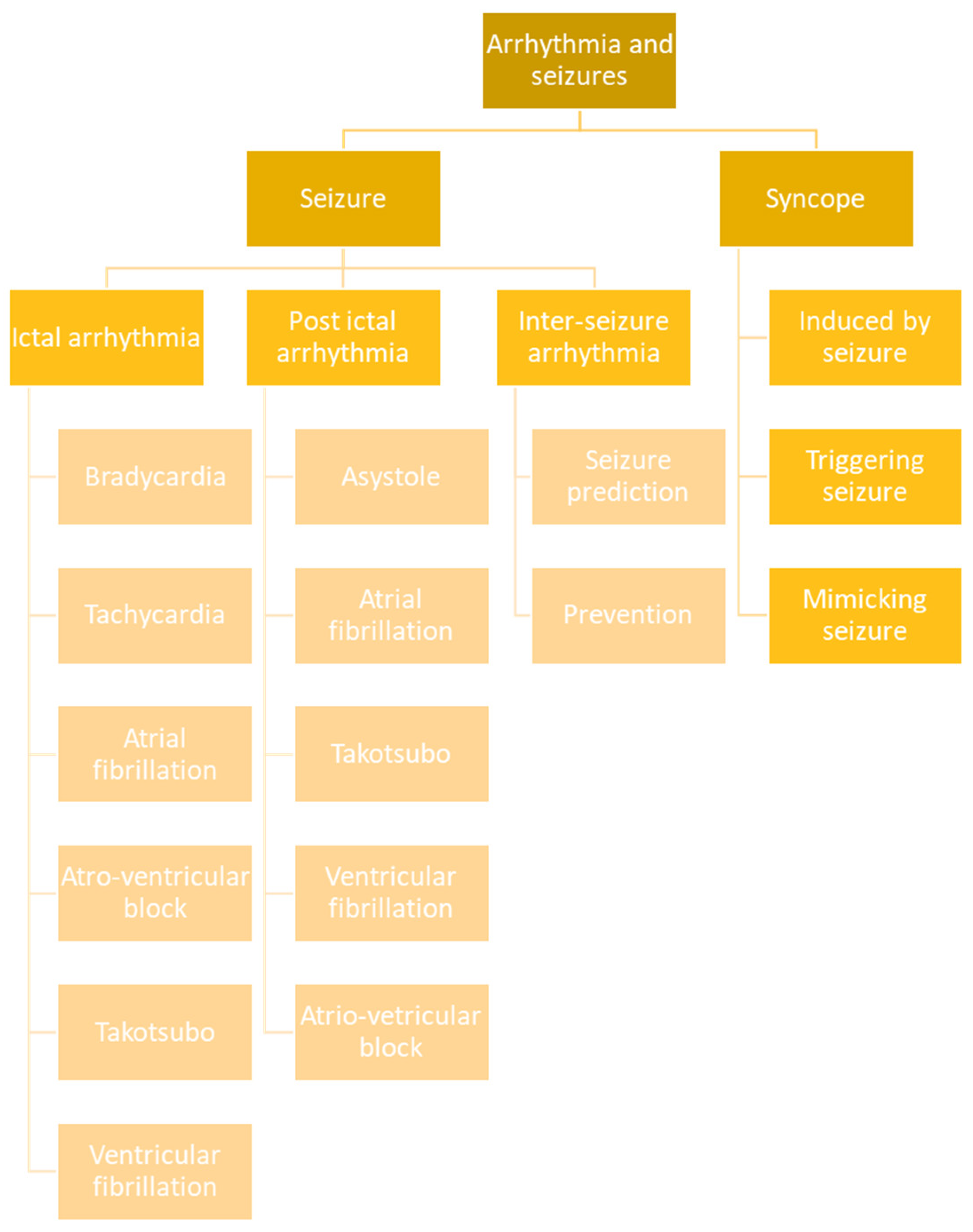
5. Classification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fong, M.W.K.; Norris, S.; Percy, J.; Hirsch, L.J.; Herlopian, A. Hemisphere-Dependent Ictal Tachycardia Versus Ictal Bradycardia in a Critically Ill Patient. J. Clin. Neurophysiol. 2022, 39, e15–e18. [Google Scholar] [CrossRef]
- Eggleston, K.S.; Olin, B.D.; Fisher, R.S. Ictal tachycardia: The head–heart connection. Seizure 2014, 23, 496–505. [Google Scholar] [CrossRef]
- Tinuper, P.; Bisulli, F.; Cerullo, A.; Carcangiu, R.; Marini, C.; Pierangeli, G.; Cortelli, P. Ictal bradycardia in partial epileptic seizures: Autonomic investigation in three cases and literature review. Brain 2001, 124, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Leutmezer, F.; Schernthaner, C.; Lurger, S.; Pötzelberger, K.; Baumgartner, C. Electrocardiographic Changes at the Onset of Epileptic Seizures. Epilepsia 2003, 44, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Rocamora, R.; Kurthen, M.; Lickfett, L.; Von Oertzen, J.; Elger, C.E. Cardiac Asystole in Epilepsy: Clinical and Neurophysiologic Features. Epilepsia 2003, 44, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.W.; Ghearing, G.R.; Benarroch, E.E.; Cascino, G.D. The Ictal Bradycardia Syndrome: Localization and Lateralization. Epilepsia 2006, 47, 737–744. [Google Scholar] [CrossRef]
- Schuele, S.U.; Bermeo, A.C.; Alexopoulos, A.V.; Locatelli, E.R.; Burgess, R.C.; Dinner, D.S.; Foldvary-Schaefer, N. Video-electrographic and clinical features in patients with ictal asystole. Neurology 2007, 69, 434–441. [Google Scholar] [CrossRef]
- Rugg-Gunn, F.J.; Duncan, J.S.; Smith, S.J.M. Epileptic cardiac asystole. J. Neurol. Neurosurg. Psychiatry 2000, 68, 100–126. [Google Scholar] [CrossRef][Green Version]
- Kawai, M.; Goldsmith, I.L.; Verma, A. Differential effects of left and right hemispheric seizure onset on heart rate. Neurology 2006, 66, 1279–1280. [Google Scholar] [CrossRef]
- Catenoix, H.; Mauguière, F.; Guénot, M.; Isnard, J.; Ryvlin, P. Recording the insula during ictal asystole. Int. J. Cardiol. 2013, 169, e28–e30. [Google Scholar] [CrossRef]
- Hagiwara, K.; Okadome, T.; Mukaino, T.; Uehara, T.; Tanaka, H.; Kamada, T.; Miyoshi, A.; Akamatsu, N.; Ohara, S.; Shigeto, H. Ictal asystole as a manifestation of pure insular epilepsy. Seizure 2021, 91, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Sowden, N.; Booth, C.; Kaye, G. Syncope, Epilepsy and Ictal Asystole: A Case Series and Narrative Review. Heart Lung Circ. 2022, 31, 25–31. [Google Scholar] [CrossRef]
- Monté, C.P.; Monté, C.J.; Boon, P.; Arends, J. Epileptic seizures associated with syncope: Ictal bradycardia and ictal asystole. Epilepsy Behav. 2019, 90, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Tényi, D.; Gyimesi, C.; Kupó, P.; Horváth, R.; Bóné, B.; Barsi, P.; Kovács, N.; Simor, T.; Siegler, Z.; Környei, L.; et al. Ictal asystole: A systematic review. Epilepsia 2017, 58, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lawn, N.; Prentice, D.; Chan, J. Anti-NMDA receptor encephalitis associated with ictal asystole. J. Clin. Neurosci. 2011, 18, 1716–1718. [Google Scholar] [CrossRef] [PubMed]
- Millichap, J.J.; Goldstein, J.L.; Laux, L.C.; Nordli, D.R., Jr.; Stack, C.V.; Wainwright, M.S. Anti-NMDA receptor encephalitis associated with ictal asystole, Ictal asystole and anti-N-methyl-D-aspartate receptor antibody encephalitis. Pediatrics 2011, 127, e781–e786. [Google Scholar] [CrossRef]
- Nguyen-Michel, H.; Adam, C.; Dinkelacker, V.; Pichit, P.; Boudali, Y.; Dupont, S.; Baulac, M.; Navarro, V. Characterization of seizure-induced syncopes: EEG, ECG and clinical features. Epilepsia 2014, 55, 146–155. [Google Scholar] [CrossRef]
- Bestawros, M.; Darbar, D.; Arain, A.; Abou-Khalil, B.; Plummer, D.; Dupont, W.D.; Raj, S.R. Ictal asystole and ictal syncope: Insights into clinical management. Circ. Arrhythm. Electrophysiol. 2015, 8, 159–164. [Google Scholar] [CrossRef]
- Rubboli, G.; Bisulli, F.; Michelucci, R.; Meletti, S.; Ribani, M.A.; Cortelli, P.; Naldi, I.; Riguzzi, P.; Tassinari, C.A.; Tinuper, P. Sudden falls due to seizure-induced cardiac asystole in drug-resistant focal epilepsy. Neurology 2008, 70, 1933–1935. [Google Scholar] [CrossRef]
- Battaglia, A.; Guerrini, R.; Gastaut, H. Epileptic seizures induced by syncopal attacks. J. Epilepsy 1989, 2, 137–145. [Google Scholar] [CrossRef]
- Horrocks, I.A.; Nechay, A.; Stephenson, J.B.P.; Zuberi, S.M. Anoxic-epileptic seizures: Observational study of epileptic seizures induced by syncopes. Arch. Dis. Child. 2005, 90, 1283–1287. [Google Scholar] [PubMed]
- Bergey, G.K.; Krumholz, A.; Fleming, C.P. Complex partial seizure provocation by vasovagal syncope: Video-EEG and intracranial electrode documentation. Epilepsia 1997, 38, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Michelucci, R. Fit and faint or faint and fit? Clin. Neurophysiol. 2021, 132, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Basili, L.M.; Morano, A.; Fattouch, J.; Fanella, M.; Albini, M.; Avorio, F.; CerulliIrelli, E.; Manfredi, M.; Urani, C.; Strano, S.; et al. Ictal atrial fibrillation during focal seizures: A case report and literature review. Clin. Comment. Epileptic Disord. 2019, 21, 295–301. [Google Scholar]
- Allana, S.S.; Ahmed, H.N.; Shah, K.; Kelly, A.F. Ictal bradycardia and atrioventricular block: A cardiac manifestation of epilepsy. Oxf. Med. Case Rep. 2014, 19, 33–35. [Google Scholar] [CrossRef]
- Myung, H.S.; Young Sung, W. A case of near-sudden unexpected death in epilepsy due to ventricular fibrillation. Open Access Emerg. Med. 2019, 19, 161–166. [Google Scholar]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Finsterer, J.; Bersano, A. Seizure-triggered Takotsubo syndrome rarely causes SUDEP. Seizure 2015, 31, 84–87. [Google Scholar] [CrossRef]
- Shmuely, S.; van der Lende, M.; Lamberts, R.J.; Sander, J.W.; Thijs, R.D. The heart of epilepsy: Current views and future concepts. Seizure 2017, 44, 176–183. [Google Scholar] [CrossRef]
- Leung, H.; Schindler, K.; Kwan, P.; Elger, C. Asystole induced by electrical stimulation of the left cingulate gyrus. Epileptic Disord. 2007, 9, 77–81. [Google Scholar]
- Pasini, E.; Riguzzi, P.; Michelucci, R. Iatrogenic ictal asystole. J. Neurol. Sci. 2022, 434, 120183. [Google Scholar] [CrossRef] [PubMed]
- Dono, F.; Evangelista, F.; Consoli, S.; Venditti, R.; Russo, M.; De Angelis, M.V.; Faustino, M.; Di Iorio, A.; Vollono, C.; Anzellotti, F.; et al. Heart rate variability modifications in adult patients with early versus late-onset temporal lobe epilepsy: A comparative observational study. Neurophysiol. Clin. 2023, 53, 102852. [Google Scholar] [CrossRef] [PubMed]
- Ryvlin, P.; Nashef, L.; Lhatoo, S.D.; Bateman, L.M.; Bird, J.; Bleasel, A.; Boon, P.; Crespel, A.; Dworetzky, B.A.; Høgenhaven, H. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): A retrospective study. Lancet Neurol. 2013, 12, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Benditt, D.G.; van Dijk, G.; Thijs, R.D. Ictal asystole: Life-threatening vagal storm or a benign seizure self-termination mechanism? Circ. Arrhythm. Electrophysiol. 2015, 8, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Van der Lende, M.; Surges, R.; Sander, J.W.; Thijs, R.D. Cardiac arrhythmias during or after epileptic seizures. J. Neurol. Neurosurg. Psychiatry 2016, 87, 69–74. [Google Scholar]
- Singh, V.; Ryan, J.M.; Auerbach, D.S. It is premature for a unified hypothesis of sudden unexpected death in epilepsy: A great amount of research is still needed to understand the multisystem cascade. Epilepsia 2023, 64, 2006–2010. [Google Scholar] [CrossRef]
- Paulussen, A.D.C.; Gilissen, R.A.H.J.; Armstrong, M.; Doevendans, P.A.; Verhasselt, P.; Smeets, H.J.M.; Shulze-Bahr, E.; Haverkamp, W.; Breithardt, G.; Cohen, N.; et al. Genetic variations of KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J. Mol. Med. 2004, 82, 182–188. [Google Scholar] [CrossRef]
| International Takotsubo Diagnostic Criteria | |
|---|---|
| 1 | Patients show transient a left ventricular dysfunction (hypokinesia, akinesia, or dyskinesia) presenting as apical ballooning or midventricular, basal, or focal wall motion abnormalities. Right ventricular involvement can be present. Besides these regional wall motion patterns, transitions between all types can exist. The regional wall motion abnormality usually extends beyond a single epicardial vascular distribution; however, rare cases can exist where the regional wall motion abnormality is present in the subtended myocardial territory of a single coronary artery (focal TTS). b |
| 2 | An emotional, physical, or combined trigger can precede the Takotsubo syndrome event, but this is not obligatory. |
| 3 | Neurologic disorders (e.g., subarachnoid hemorrhage, stroke/transient ischemic attack, or seizures) as well as pheochromocytoma may serve as triggers for Takotsubo syndrome. |
| 4 | New ECG abnormalities are present (ST-segment elevation, ST-segment depression, T-wave inversion, and QTc prolongation); however, rare cases exist without any ECG changes. |
| 5 | Levels of cardiac biomarkers (troponin and creatine kinase) are moderately elevated in most cases; significant elevation of brain natriuretic peptide is common. |
| 6 | Significant coronary artery disease is not a contradiction in Takotsubo syndrome. |
| 7 | Patients have no evidence of infectious myocarditis. b |
| 8 | Postmenopausal women are predominantly affected |
| SYNCOPE | SEIZURE | |
|---|---|---|
| TRIGGERS | Frequent | Rare |
| PRECEDING SYMPTOMS | Nausea, visual blurring, epigastric sensation, heat, headache, or tinnitus | Sensorial, psychic, somatosensory ‘auras’ ormotor phenomena |
| POSITION | Usually while standing or sitting. Supine very rare | Any |
| LOSS OF CONSCIOUSNESS | ‘Fading away’ in young patients or abrupt loss in elderly persons | Abrupt loss |
| FALL | Slow or flaccid | Fast or tonic |
| SKIN COLOR | Pale | Sometimes perilabial cyanosis |
| EYE DEVIATION | Transient upward or lateral deviation | Sustained lateral deviation |
| INCONTINENCE | Common | Common |
| TONGUE BITE | Uncommon; localization: on the tip of the tongue | Common; localization: on the side of the tongue |
| CONVULSIONS | Lasts a few seconds and is arrhythmic, multifocal, or generalized | May last a few minutes and is rhythmic and generalized |
| DURATION | 3–30 s | Depends on the type of seizure: up to 5 min for GTCS and shorter for others |
| POST-ICTAL PERIOD | Somnolence, fatigue, or headache | Confusion, somnolence, or headache |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasini, E.; Michelucci, R. The Heart and Seizures: Friends or Enemies? J. Clin. Med. 2023, 12, 5805. https://doi.org/10.3390/jcm12185805
Pasini E, Michelucci R. The Heart and Seizures: Friends or Enemies? Journal of Clinical Medicine. 2023; 12(18):5805. https://doi.org/10.3390/jcm12185805
Chicago/Turabian StylePasini, Elena, and Roberto Michelucci. 2023. "The Heart and Seizures: Friends or Enemies?" Journal of Clinical Medicine 12, no. 18: 5805. https://doi.org/10.3390/jcm12185805
APA StylePasini, E., & Michelucci, R. (2023). The Heart and Seizures: Friends or Enemies? Journal of Clinical Medicine, 12(18), 5805. https://doi.org/10.3390/jcm12185805






