The Correlation of Aortic Neck Angle and Length in Abdominal Aortic Aneurysm with Severe Neck Angulation for Prediction of Intraoperative Neck Complications and Postoperative Outcomes after Endovascular Aneurysm Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
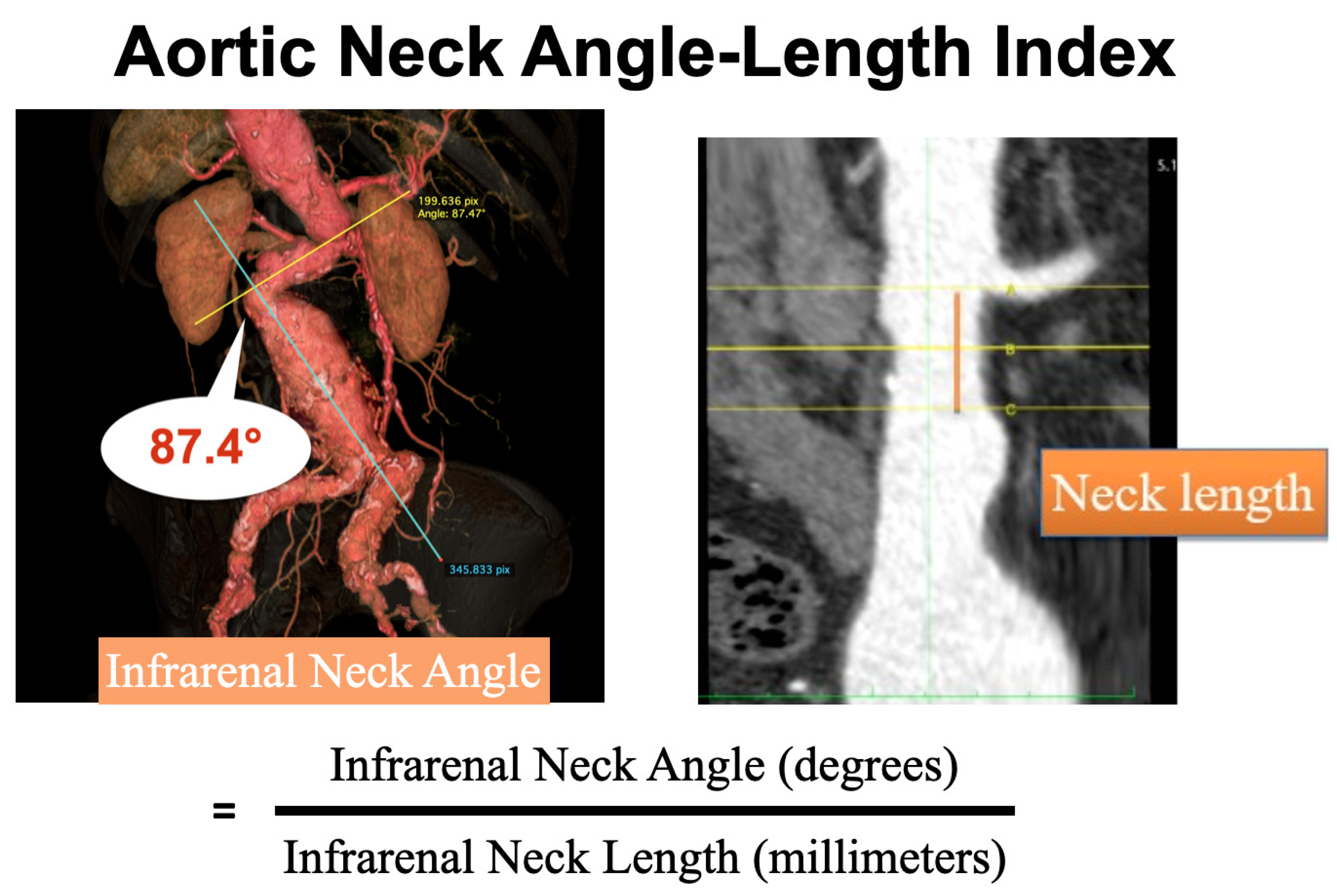
2.2. Preoperative CT Scan Measurement
2.3. Definitions and Outcomes Measurement
2.4. Statistical Analysis
3. Results
3.1. Intraobserver and Interobserver Reliability
3.2. Aortic Neck Angle-Length Index
3.3. AAA Morphology
3.4. Intraoperative Outcomes
3.5. Operative Details
3.6. Early Postoperative Outcomes
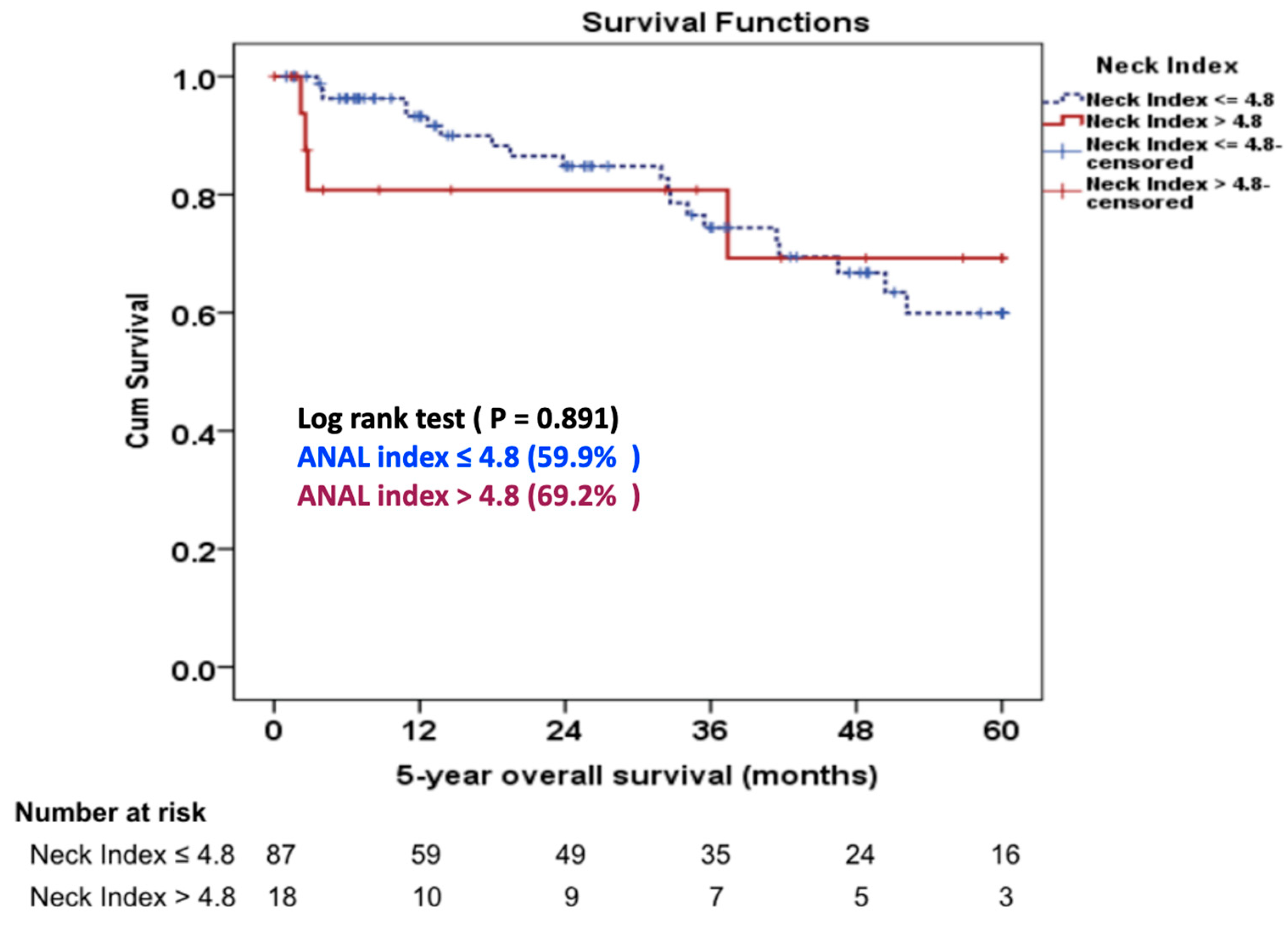
3.7. Late Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marone, E.M.; Freyrie, A.; Ruotolo, C.; Michelagnoli, S.; Antonello, M.; Speziale, F.; Veroux, P.; Gargiulo, M.; Gaggiano, A. Expert Opinion on Hostile Neck Definition in Endovascular Treatment of Abdominal Aortic Aneurysms (a Delphi Consensus). Ann. Vasc. Surg. 2020, 62, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Rockley, M.; Hadziomerovic, A.; van Walraven, C.; Bose, P.; Scallan, O.; Jetty, P. A new “angle” on aortic neck angulation measurement. J. Vasc. Surg. 2019, 70, 756–761.e1. [Google Scholar] [CrossRef] [PubMed]
- Hobo, R.; Kievit, J.; Leurs, L.J.; Buth, J. Influence of severe infrarenal aortic neck angulation on complications at the proximal neck following endovascular AAA repair: A EUROSTAR study. J. Endovasc. Ther. 2007, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- AbuRahma, A.F.; Campbell, J.; Stone, P.A.; Nanjundappa, A.; Jain, A.; Dean, L.S.; Habib, J.; Keiffer, T.; Emmett, M. The correlation of aortic neck length to early and late outcomes in endovascular aneurysm repair patients. J. Vasc. Surg. 2009, 50, 738–748. [Google Scholar] [CrossRef]
- Leurs, L.J.; Kievit, J.; Dagnelie, P.C.; Nelemans, P.J.; Buth, J. Influence of infrarenal neck length on outcome of endovascular abdominal aortic aneurysm repair. J. Endovasc. Ther. 2006, 13, 640–648. [Google Scholar] [CrossRef]
- Bastos Goncalves, F.; de Vries, J.P.; van Keulen, J.W.; Dekker, H.; Moll, F.L.; Van Herwaarden, J.A.; Verhagen, H.J.M. Severe proximal aneurysm neck angulation: Early results using the Endurant stentgraft system. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 193–200. [Google Scholar] [CrossRef]
- Oliveira, N.F.; Bastos Goncalves, F.M.; de Vries, J.P.; Ultee, K.H.J.; Werson, D.A.B.; Hoeks, S.E.; Moll, F.; Van Herwaarden, J.A.; Verhagen, H.J.M. Mid-Term Results of EVAR in Severe Proximal Aneurysm Neck Angulation. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 19–27. [Google Scholar] [CrossRef]
- Oliveira, N.F.; Gonçalves, F.B.; Hoeks, S.E.; van Rijn, M.J.; Ultee, K.; Pinto, J.P.; Raa, S.T.; van Herwaarden, J.A.; de Vries, J.-P.P.; Verhagen, H.J. Long-term outcomes of standard endovascular aneurysm repair in patients with severe neck angulation. J. Vasc. Surg. 2018, 68, 1725–1735. [Google Scholar] [CrossRef]
- van Keulen, J.W.; Moll, F.L.; Tolenaar, J.L.; Verhagen, H.J.; van Herwaarden, J.A. Validation of a new standardized method to measure proximal aneurysm neck angulation. J. Vasc. Surg. 2010, 51, 821–828. [Google Scholar] [CrossRef]
- Sweet, M.P.; Fillinger, M.F.; Morrison, T.M.; Abel, D. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. J. Vasc. Surg. 2011, 54, 931–937. [Google Scholar] [CrossRef]
- Aburahma, A.F.; Campbell, J.E.; Mousa, A.Y.; Hass, S.M.; Stone, P.A.; Jain, A.; Nanjundappa, A.; Dean, L.S.; Keiffer, T.; Habib, J. Clinical outcomes for hostile versus favorable aortic neck anatomy in endovascular aortic aneurysm repair using modular devices. J. Vasc. Surg. 2011, 54, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Blankensteijn, J.D.; Harris, P.L.; White, G.H.; Zarins, C.K.; Bernhard, V.M.; Matsumura, J.S.; May, J.; Veith, F.J.; Fillinger, M.F.; et al. Reporting standards for endovascular aortic aneurysm repair. J. Vasc. Surg. 2002, 35, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Wurpts, I.C.; Geiser, C. Is adding more indicators to a latent class analysis beneficial or detrimental? Results of a Monte-Carlo study. Front. Psychol. 2014, 5, 920. [Google Scholar] [CrossRef] [PubMed]
- Scrucca, L.; Fop, M.; Murphy, T.B.; Raftery, A.E. mclust 5: Clustering, Classification and Density Estimation Using Gaussian Finite Mixture Models. R J. 2016, 8, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Daniel Spurk, A.H.; Wang, M.; Valero, D.; Kauffeld, S. Latent profile analysis: A review and “how to” guide of its application within vocational behavior research. J. Vocat. Behav. 2020, 120, 130445. [Google Scholar]
- Kaewchoothong, Y.A.N.; Assawalertsakul, T.; Nuntadusit, C.; Chatpun, S. Computational Study of Abdominal Aortic Aneurysms with Severely Angulated Neck Based on Transient Hemodynamics Using an Idealized Model. Appl. Sci. 2022, 12, 2113. [Google Scholar] [CrossRef]
- De Bock, S.; Iannaccone, F.; De Beule, M.; Vermassen, F.; Segers, P.; Verhegghe, B. What if you stretch the IFU? A mechanical insight into stent graft Instructions For Use in angulated proximal aneurysm necks. Med. Eng. Phys. 2014, 36, 1567–1576. [Google Scholar] [CrossRef]
- Arbănași, E.M.; Mureșan, A.V.; Coșarcă, C.M.; Arbănași, E.M.; Niculescu, R.; Voidăzan, S.T.; Ivănescu, A.D.; Hălmaciu, I.; Filep, R.C.; Mărginean, L.; et al. Computed Tomography Angiography Markers and Intraluminal Thrombus Morphology as Predictors of Abdominal Aortic Aneurysm Rupture. Int. J. Environ. Res. Public. Health 2022, 19, 15961. [Google Scholar] [CrossRef]
- You, J.H.; Lee, C.W.; Huh, U.; Lee, C.S.; Ryu, D. Comparative Study of Aortic Wall Stress According to Geometric Parameters in Abdominal Aortic Aneurysms. Appl. Sci. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Georgiadis, G.S.; Antoniou, S.A.; Kuhan, G.; Murray, D. A meta-analysis of outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile and friendly neck anatomy. J. Vasc. Surg. 2013, 57, 527–538. [Google Scholar] [CrossRef]
- Broos, P.P.; Stokmans, R.A.; van Sterkenburg, S.M.; Torsello, G.; Vermassen, F.; Cuypers, P.W.; van Sambeek, M.R.; Teijink, J.A. Performance of the Endurant stent graft in challenging anatomy. J. Vasc. Surg. 2015, 62, 312–318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cox, D.E.; Jacobs, D.L.; Motaganahalli, R.L.; Wittgen, C.M.; Peterson, G.J. Outcomes of endovascular AAA repair in patients with hostile neck anatomy using adjunctive balloon-expandable stents. Vasc. Endovasc. Surg. 2006, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.M.; Osman, K.; Antoniou, G.A.; McWilliams, R.G.; Brennan, J.A.; Fisher, R.K. Outcomes of persistent intraoperative type Ia endoleak after standard endovascular aneurysm repair. J. Vasc. Surg. 2015, 61, 1185–1191. [Google Scholar] [CrossRef]
- Bastos Gonçalves, F.; Verhagen, H.J.; Vasanthananthan, K.; Zandvoort, H.J.; Moll, F.L.; van Herwaarden, J.A. Spontaneous delayed sealing in selected patients with a primary type-Ia endoleak after endovascular aneurysm repair. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Torsello, G.; Troisi, N.; Donas, K.P.; Austermann, M. Evaluation of the Endurant stent graft under instructions for use vs off-label conditions for endovascular aortic aneurysm repair. J. Vasc. Surg. 2011, 54, 300–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catanese, V.; Sangiorgi, G.; Sotgiu, G.; Saderi, L.; Settembrini, A.; Donelli, C.; Martelli, E. Clinical and anatomical variables associated in the literature to limb graft occlusion after endovascular aneurysm repair compared to the experience of a tertiary referral center. Minerva Chir. 2020, 75, 51–59. (In English) [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Baseline Characteristics | Group 1 ANAL Index ≤ 4.8 (n = 95) | Group 2 ANAL Index > 4.8 (n = 20) | p Value |
|---|---|---|---|
| Age, mean ± SD years | 77.1 ± 6.4 | 75.7 ± 7.2 | 0.360 |
| Male gender, no. (%) | 67 (70.5%) | 16 (80%) | 0.559 |
| Cardiac disease, no. (%) | 31 (32.6%) | 4 (20%) | 0.396 |
| COPD, no. (%) | 14 (14.7%) | 1 (5%) | 0.463 |
| Hypertension, no. (%) | 76 (80%) | 15 (75%) | 0.762 |
| Dyslipidemia, no. (%) | 43 (45.3%) | 8 (40%) | 0.885 |
| Chronic kidney disease, no. (%) | 23 (24.2%) | 2 (10%) | 0.235 |
| Diabetes mellitus, no. (%) | 14 (14.7%) | 1 (5%) | 0.463 |
| Cerebrovascular disease, no. (%) | 13 (13.7%) | 1 (5%) | 0.458 |
| ASA class II, no. (%) | 22 (23.2%) | 6 (30%) | 0.674 |
| ASA class III, no. (%) | 71 (74.7%) | 14 (70%) | 0.674 |
| Aneurysm Morphology | Group 1 ANAL Index ≤ 4.8 (n = 95) | Group 2 ANAL Index > 4.8 (n = 20) | p Value |
|---|---|---|---|
| AAA morphology, fusiform (%) | 95 (100%) | 20 (100%) | 1.000 |
| AAA length (mm), mean ± SD | 127.7 ± 24.8 | 134.2 ± 24.6 | 0.285 |
| AAA max. diameter (mm), mean ± SD | 64.5 ± 14.6 | 66.6 ± 12.0 | 0.550 |
| Suprarenal neck angle (°), median (min, max) | 43.6 (0, 133.7) | 40.5 (13.5, 91.3) | 0.912 |
| Infrarenal neck angle (°), mean ± SD | 82.9 ± 17.1 | 89.7 ± 15.9 | 0.108 |
| Neck length (mm), mean ± SD | 34.1 ± 13.8 | 14.0 ± 3.7 | <0.001 |
| Neck calcification, no. (%) | 4 (4.2%) | 0 (0%) | 1.000 |
| Neck thrombus, no. (%) | 6 (6.3%) | 2 (10%) | 0.626 |
| Neck morphology, no. (%) | 0.751 | ||
| Cylindrical | 74 (77.9%) | 14 (70%) | |
| Conical | 14 (14.7%) | 4 (20%) | |
| Reverse conical | 7 (7.4%) | 2 (10%) |
| Variable | Group 1 ANAL Index ≤ 4.8 (n = 95) | Group 2 ANAL Index > 4.8 (n = 20) | p Value |
|---|---|---|---|
| Stent graft product, no. (%) | |||
| Endurant | 52 (54.7%) | 11 (55%) | 0.986 |
| Zenith | 39 (41.1%) | 8 (40%) | 0.986 |
| Treovance | 4 (4.2%) | 1 (5%) | 0.986 |
| Intraoperative neck complications no. (%) | 20 (21.1%) | 11 (55%) | 0.005 |
| Type IA endoleak | 10 (10.5%) | 8 (40%) | 0.003 |
| Endograft migration Type IA endoleak and endograft migration | 4 (4.2%) 4 (4.2%) | 1 (5%) 2 (10%) | 1.000 0.279 |
| Renal artery coverage | 2 (2.1%) | 0 (0%) | 1.000 |
| Adjunctive neck procedures, no. (%) | 18 (18.9%) | 12 (60%) | <0.001 |
| Aortic cuff | 7 (7.4%) | 5 (25%) | 0.034 |
| Palmaz stent | 9 (9.5%) | 4 (20%) | 0.237 |
| Aortic cuff and Palmaz stent | 2 (2.1%) | 3 (15%) | 0.036 |
| Operative details | |||
| Procedure time, mean ± SD (min.) | 178.1 ± 63.5 | 206.0 ± 75.2 | 0.087 |
| Fluoroscopic time (min.), median (min, max) | 34.6 (15, 110) | 40.1 (19, 97) | 0.248 |
| Volume of contrast usage (mL), median (min, max) | 122 (28, 300) | 142.5 (54, 470) | 0.162 |
| Blood loss (mL), median (min, max) | 250 (50, 1500) | 352.5 (75, 2000) | 0.082 |
| Post-Operative Outcomes | Group 1 ANAL Index ≤ 4.8 (n = 95) | Group 2 ANAL Index > 4.8 (n = 20) | p Value |
|---|---|---|---|
| 30-day complication, no. (%) | 29 (30.5%) | 8 (40%) | 0.575 |
| Myocardial infarction | 0 (0%) | 1 (5%) | 0.174 |
| Congestive heart failure | 3 (3.2%) | 1 (5%) | 0.540 |
| Respiratory failure | 2 (2.1%) | 2 (10%) | 0.139 |
| Renal failure | 5 (5.3%) | 1 (5%) | 1.000 |
| Deployment-related complications, no. (%) | 6 (6.3%) | 2 (10%) | 0.626 |
| 30-day mortality, no. (%) | 1 (1.1%) | 1 (5%) | 0.319 |
| In-hospital mortality *, no. (%) | 3 (3.1%) | 2 (10%) | 0.439 |
| Length of stay, (days) Median (min, max) | 7 (2, 124) | 8 (2, 85) | 0.401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinsakchai, K.; Sirivech, T.; Moll, F.L.; Tongsai, S.; Hongku, K. The Correlation of Aortic Neck Angle and Length in Abdominal Aortic Aneurysm with Severe Neck Angulation for Prediction of Intraoperative Neck Complications and Postoperative Outcomes after Endovascular Aneurysm Repair. J. Clin. Med. 2023, 12, 5797. https://doi.org/10.3390/jcm12185797
Chinsakchai K, Sirivech T, Moll FL, Tongsai S, Hongku K. The Correlation of Aortic Neck Angle and Length in Abdominal Aortic Aneurysm with Severe Neck Angulation for Prediction of Intraoperative Neck Complications and Postoperative Outcomes after Endovascular Aneurysm Repair. Journal of Clinical Medicine. 2023; 12(18):5797. https://doi.org/10.3390/jcm12185797
Chicago/Turabian StyleChinsakchai, Khamin, Thana Sirivech, Frans L. Moll, Sasima Tongsai, and Kiattisak Hongku. 2023. "The Correlation of Aortic Neck Angle and Length in Abdominal Aortic Aneurysm with Severe Neck Angulation for Prediction of Intraoperative Neck Complications and Postoperative Outcomes after Endovascular Aneurysm Repair" Journal of Clinical Medicine 12, no. 18: 5797. https://doi.org/10.3390/jcm12185797
APA StyleChinsakchai, K., Sirivech, T., Moll, F. L., Tongsai, S., & Hongku, K. (2023). The Correlation of Aortic Neck Angle and Length in Abdominal Aortic Aneurysm with Severe Neck Angulation for Prediction of Intraoperative Neck Complications and Postoperative Outcomes after Endovascular Aneurysm Repair. Journal of Clinical Medicine, 12(18), 5797. https://doi.org/10.3390/jcm12185797





