Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation
Abstract
:1. Introduction
2. Failure of Anticoagulation Therapy to Prevent Ischemic Stroke
3. Identification of Mechanisms Underlying Stroke despite Oral Anticoagulation
- (1)
- Ischemic stroke not related to AF, i.e., other stroke subtypes;
- (2)
- Insufficient OAC;
- (3)
- Cardioembolic stroke despite adequate OAC.
3.1. Ischemic Stroke Not Related to Atrial Fibrillation
3.2. Insufficient OAC
3.3. Cardioembolic Stroke despite Adequate OAC
3.4. Diagnostic Workflow
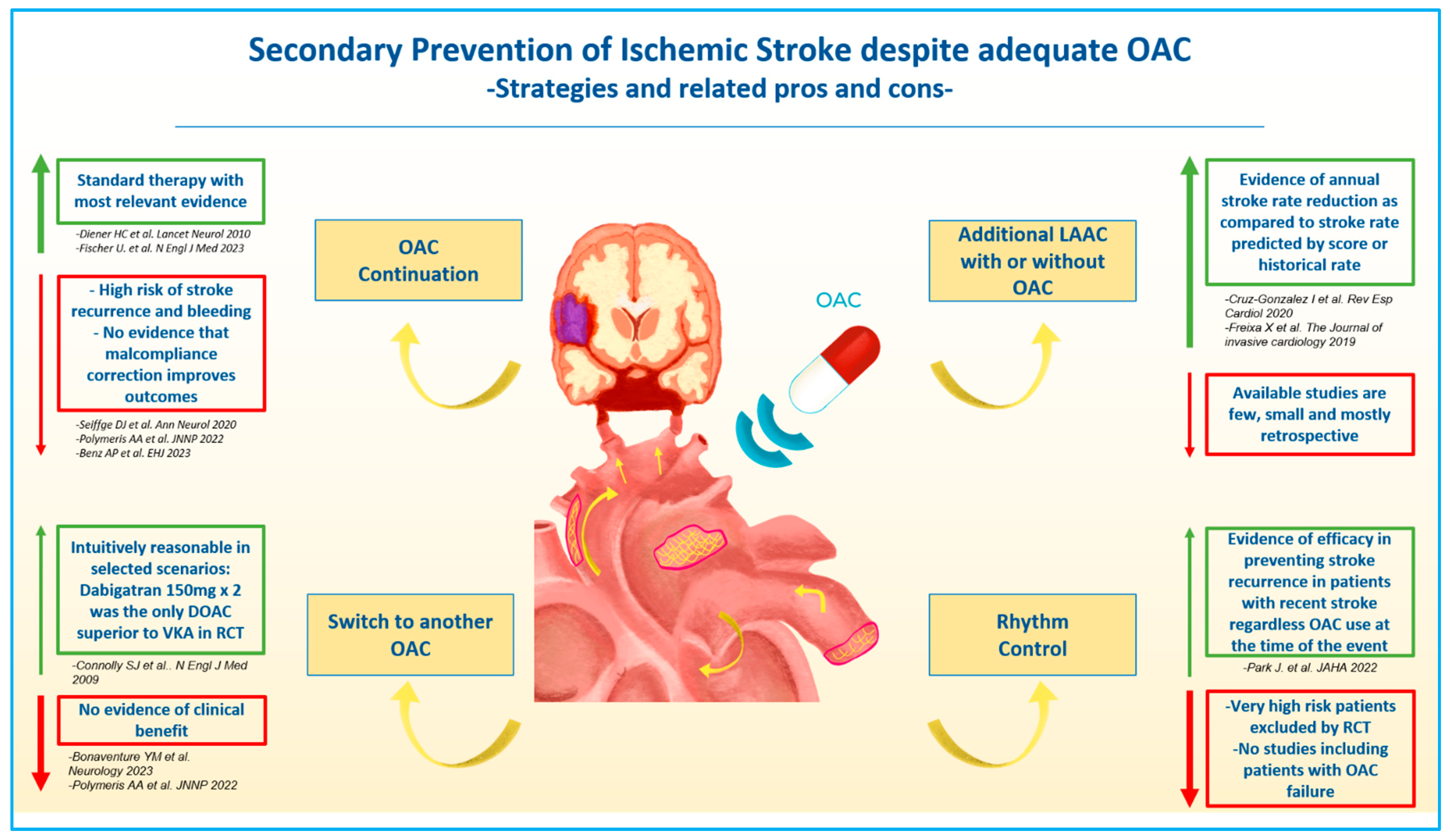
4. Secondary Prevention of Ischemic Stroke despite Oral Anticoagulation
4.1. OAC Continuation
4.2. Addition of Antiplatelet Therapy
4.3. Left Atrial Appendage Closure
4.4. Rhythm Control
5. Future Perspectives
6. Conclusions
Funding
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Spinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; Bellolio, M.F.; McBane, R.D.; Shah, N.D.; Noseworthy, P.A. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003725. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Henninger, N.; Giles, J.A.; Leon Guerrero, C.; Mistry, E.; Liberman, A.L.; Asad, D.; Liu, A.; Nagy, M.; Kaushal, A.; et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: The IAC study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Meinel, T.R.; Branca, M.; De Marchis, G.M.; Nedeltchev, K.; Kahles, T.; Bonati, L.; Arnold, M.; Heldner, M.R.; Jung, S.; Carrera, E.; et al. Prior Anticoagulation in Patients with Ischemic Stroke and Atrial Fibrillation. Ann. Neurol. 2021, 89, 42–53. [Google Scholar] [CrossRef]
- Xian, Y.; O’Brien, E.C.; Liang, L.; Xu, H.; Schwamm, L.H.; Fonarow, G.C.; Bhatt, D.L.; Smith, E.E.; Olson, D.M.; Maisch, L.; et al. Association of Preceding Antithrombotic Treatment With Acute Ischemic Stroke Severity and In-Hospital Outcomes Among Patients With Atrial Fibrillation. Jama 2017, 317, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Seiffge, D.J.; De Marchis, G.M.; Koga, M.; Paciaroni, M.; Wilson, D.; Cappellari, M.; Macha Md, K.; Tsivgoulis, G.; Ambler, G.; Arihiro, S.; et al. Ischemic Stroke despite Oral Anticoagulant Therapy in Patients with Atrial Fibrillation. Ann. Neurol. 2020, 87, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Koga, M.; Lee, K.J.; Kim, B.J.; Park, E.L.; Lee, J.; Mizoguchi, T.; Yoshimura, S.; Cha, J.K.; Lee, B.C.; et al. Atrial Fibrillation-Associated Ischemic Stroke Patients With Prior Anticoagulation Have Higher Risk for Recurrent Stroke. Stroke 2020, 51, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Benz, A.P.; Hohnloser, S.H.; Eikelboom, J.W.; Carnicelli, A.P.; Giugliano, R.P.; Granger, C.B.; Harrington, J.; Hijazi, Z.; Morrow, D.A.; Patel, M.R.; et al. Outcomes of patients with atrial fibrillation and ischemic stroke while on oral anticoagulation. Eur. Heart J. 2023, 44, 1807–1814. [Google Scholar] [CrossRef]
- Ip, Y.M.B.; Lau, K.K.; Ko, H.; Lau, L.; Yao, A.; Wong, G.L.; Yip, T.C.; Leng, X.; Chan, H.; Chan, H.; et al. Association of Alternative Anticoagulation Strategies and Outcomes in Patients With Ischemic Stroke While Taking a Direct Oral Anticoagulant. Neurology 2023, 101, e358–e369. [Google Scholar] [CrossRef]
- Polymeris, A.A.; Meinel, T.R.; Oehler, H.; Holscher, K.; Zietz, A.; Scheitz, J.F.; Nolte, C.H.; Stretz, C.; Yaghi, S.; Stoll, S.; et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J. Neurol. Neurosurg. Psychiatry 2022, 93, 588–598. [Google Scholar] [CrossRef]
- Best, J.G.; Cardus, B.; Klijn, C.J.M.; Lip, G.; Seiffge, D.J.; Smith, E.E.; Werring, D.J. Antithrombotic dilemmas in stroke medicine: New data, unsolved challenges. J. Neurol. Neurosurg. Psychiatry 2022, 93, 939–951. [Google Scholar] [CrossRef]
- Stretz, C.; Wu, T.Y.; Wilson, D.; Seiffge, D.J.; Smith, E.E.; Gurol, M.E.; Yaghi, S. Ischaemic stroke in anticoagulated patients with atrial fibrillation. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Reichman, M.E.; Wernecke, M.; Zhang, R.; Southworth, M.R.; Levenson, M.; Sheu, T.C.; Mott, K.; Goulding, M.R.; Houstoun, M.; et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015, 131, 157–164. [Google Scholar] [CrossRef]
- Fernandes, L.; Sargento-Freitas, J.; Milner, J.; Silva, A.; Novo, A.; Goncalves, T.; Marinho, A.V.; Mariano Pego, G.; Cunha, L.; Antonio, N. Ischemic stroke in patients previously anticoagulated for non-valvular atrial fibrillation: Why does it happen? Rev. Port. Cardiol. (Engl. Ed.) 2019, 38, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Paciaroni, M.; Agnelli, G.; Caso, V.; Silvestrelli, G.; Seiffge, D.J.; Engelter, S.; De Marchis, G.M.; Polymeris, A.; Zedde, M.L.; Yaghi, S.; et al. Causes and Risk Factors of Cerebral Ischemic Events in Patients With Atrial Fibrillation Treated With Non-Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention. Stroke 2019, 50, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Liberman, A.L.; Henninger, N.; Grory, B.M.; Nouh, A.; Scher, E.; Giles, J.; Liu, A.; Nagy, M.; Kaushal, A.; et al. Factors associated with therapeutic anticoagulation status in patients with ischemic stroke and atrial fibrillation. J. Stroke Cerebrovasc. Dis. 2020, 29, 104888. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Rota, E.; Testa, L.; Di Brigida, G.; Agosti, S.; Rovere, M.E.; Risso, R.; Morelli, N. The management of patients with acute ischemic stroke while on direct oral anticoagulants (DOACs): Data from an Italian cohort and a proposed algorithm. J. Thromb. Thrombolysis 2020, 50, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Ovbiagele, B.; Kim, J.S. Evaluation of cryptogenic stroke with advanced diagnostic techniques. Stroke 2014, 45, 1186–1194. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.H.; Lee, M.J.; Park, Y.G.; Ahn, M.J.; Bang, O.Y. Clues to occult cancer in patients with ischemic stroke. PLoS ONE 2012, 7, e44959. [Google Scholar] [CrossRef]
- Seiffge, D.J.; Kagi, G.; Michel, P.; Fischer, U.; Bejot, Y.; Wegener, S.; Zedde, M.; Turc, G.; Cordonnier, C.; Sandor, P.S.; et al. Rivaroxaban plasma levels in acute ischemic stroke and intracerebral hemorrhage. Ann. Neurol. 2018, 83, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Song, C.; Gray, W.A.; Furie, K.L.; Elkind, M.S.; Kamel, H. Left Atrial Appendage Function and Stroke Risk. Stroke 2015, 46, 3554–3559. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Connolly, S.J.; Ezekowitz, M.D.; Wallentin, L.; Reilly, P.A.; Yang, S.; Xavier, D.; Di Pasquale, G.; Yusuf, S. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: A subgroup analysis of the RE-LY trial. Lancet Neurol. 2010, 9, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Koga, M.; Strbian, D.; Branca, M.; Abend, S.; Trelle, S.; Paciaroni, M.; Thomalla, G.; Michel, P.; Nedeltchev, K.; et al. Early versus Later Anticoagulation for Stroke with Atrial Fibrillation. N. Engl. J. Med. 2023, 388, 2411–2421. [Google Scholar] [CrossRef]
- Cruz-Gonzalez, I.; Gonzalez-Ferreiro, R.; Freixa, X.; Gafoor, S.; Shakir, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; et al. Left atrial appendage occlusion for stroke despite oral anticoagulation (resistant stroke). Results from the Amplatzer Cardiac Plug registry. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 28–34. [Google Scholar] [CrossRef]
- Freixa, X.; Cruz-Gonzalez, I.; Regueiro, A.; Nombela-Franco, L.; Estevez-Loureiro, R.; Ruiz-Salmeron, R.; Bethencourt, A.; Gutierrez-Garcia, H.; Fernandez-Diaz, J.A.; Moreno-Samos, J.C.; et al. Left Atrial Appendage Occlusion as Adjunctive Therapy to Anticoagulation for Stroke Recurrence. J. Invasive Cardiol. 2019, 31, 212–216. [Google Scholar]
- Park, J.; Shim, J.; Lee, J.M.; Park, J.K.; Heo, J.; Chang, Y.; Song, T.J.; Kim, D.H.; Lee, H.A.; Yu, H.T.; et al. Risks and Benefits of Early Rhythm Control in Patients With Acute Strokes and Atrial Fibrillation: A Multicenter, Prospective, Randomized Study (the RAFAS Trial). J. Am. Heart Assoc. 2022, 11, e023391. [Google Scholar] [CrossRef] [PubMed]
- Oldgren, J.; Asberg, S.; Hijazi, Z.; Wester, P.; Bertilsson, M.; Norrving, B.; National, T.C. Early Versus Delayed Non-Vitamin K Antagonist Oral Anticoagulant Therapy After Acute Ischemic Stroke in Atrial Fibrillation (TIMING): A Registry-Based Randomized Controlled Noninferiority Study. Circulation 2022, 146, 1056–1066. [Google Scholar] [CrossRef]
- Dentali, F.; Douketis, J.D.; Lim, W.; Crowther, M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: A meta-analysis of randomized trials. Arch. Intern. Med. 2007, 167, 117–124. [Google Scholar] [CrossRef]
- Benz, A.P.; Johansson, I.; Dewilde, W.J.M.; Lopes, R.D.; Mehran, R.; Sartori, S.; Sarafoff, N.; Yasuda, S.; McIntyre, W.F.; Healey, J.S.; et al. Antiplatelet therapy in patients with atrial fibrillation: A systematic review and meta-analysis of randomized trials. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 648–659. [Google Scholar] [CrossRef]
- Cresti, A.; Garcia-Fernandez, M.A.; Sievert, H.; Mazzone, P.; Baratta, P.; Solari, M.; Geyer, A.; De Sensi, F.; Limbruno, U. Prevalence of extra-appendage thrombosis in non-valvular atrial fibrillation and atrial flutter in patients undergoing cardioversion: A large transoesophageal echo study. EuroIntervention 2019, 15, e225–e230. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Korsholm, K.; Rodes-Cabau, J.; Saw, J.; Berti, S.; Alkhouli, M.A. Left atrial appendage occlusion. EuroIntervention 2023, 18, e1038–e1065. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Holmes, D.R.; Cavalcante, J.L.; Freeman, J.V.; Goldsweig, A.M.; Kavinsky, C.J.; Moussa, I.D.; Munger, T.M.; Price, M.J.; Reisman, M.; et al. SCAI/HRS expert consensus statement on transcatheter left atrial appendage closure. Heart Rhythm. 2023, 20, e1–e16. [Google Scholar] [CrossRef]
- Galloo, X.; Carmeliet, T.; Prihadi, E.A.; Lochy, S.; Scott, B.; Verheye, S.; Schoors, D.; Vermeersch, P. Left atrial appendage occlusion in recurrent ischaemic stroke, a multicentre experience. Acta Clin. Belg. 2022, 77, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Masjuan, J.; Salido, L.; DeFelipe, A.; Hernandez-Antolin, R.; Fernandez-Golfin, C.; Cruz-Culebras, A.; Matute, C.; Vera, R.; Perez-Torre, P.; Zamorano, J.L. Oral anticoagulation and left atrial appendage closure: A new strategy for recurrent cardioembolic stroke. Eur. J. Neurol. 2019, 26, 816–820. [Google Scholar] [CrossRef]
- Pracon, R.; Zielinski, K.; Bangalore, S.; Konka, M.; Kruk, M.; Kepka, C.; Trochimiuk, P.; Debski, M.; Przyluski, J.; Kaczmarska, E.; et al. Residual stroke risk after left atrial appendage closure in patients with prior oral anticoagulation failure. Int. J. Cardiol. 2022, 354, 17–21. [Google Scholar] [CrossRef]
- Maarse, M.; Seiffge, D.; Fierro, N.; Tondo, C.; Pracon, R.; De Backer, O.; Nielsen-Kudsk, J.; Estevez-Loureiro, R.; Benito-Gonzalez, T.; Nombela-Franco, L.; et al. Left Atrial Appendage Occlusion versus standard of care in patients with atrial fibrillation and prior thromboembolic event despite anticoagulation therapy: A propensity score matched comparison-ESOC 2022 in Lyon. Eur. Heart J. 2022, 43, ehac544.632. [Google Scholar] [CrossRef]
- Galea, R.; Aminian, A.; Meneveau, N.; De Marco, F.; Heg, D.; Anselme, F.; Grani, C.; Huber, A.T.; Teiger, E.; Iriart, X.; et al. Impact of Preprocedural Computed Tomography on Left Atrial Appendage Closure Success: A Swiss-Apero Trial Subanalysis. JACC Cardiovasc. Interv. 2023, 16, 1332–1343. [Google Scholar] [CrossRef]
- Galea, R.; Räber, L.; Fuerholz, M.; Häner, J.D.; Siontis, G.C.; Brugger, N.; Moschovitis, A.; Heg, D.; Fischer, U.; Meier, B.; et al. Impact of echocardiographic guidance on safety and efficacy of left atrial appendage closure: An observational study. JACC Cardiovasc. Interv. 2021, 14, 1815–1826. [Google Scholar] [CrossRef]
- Galea, R.; De Marco, F.; Meneveau, N.; Aminian, A.; Anselme, F.; Grani, C.; Huber, A.T.; Teiger, E.; Iriart, X.; Babongo Bosombo, F.; et al. Amulet or Watchman Device for Percutaneous Left Atrial Appendage Closure: Primary Results of the SWISS-APERO Randomized Clinical Trial. Circulation 2021, 145, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Galea, R.; Mahmoudi, K.; Grani, C.; Elhadad, S.; Huber, A.T.; Heg, D.; Siontis, G.C.M.; Brugger, N.; Sebag, F.; Windecker, S.; et al. Watchman FLX vs. Watchman 2.5 in a Dual-Center Left Atrial Appendage Closure Cohort: The WATCH-DUAL study. EP Eur. 2022, 24, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Sebag, F.A.; Garot, P.; Galea, R.; De Backer, O.; Lepillier, A.; De Meesteer, A.; Hildick-Smith, D.; Armero, S.; Moubarak, G.; Ducrocq, G.; et al. Left atrial appendage closure for thrombus trapping: The international, multicentre TRAPEUR registry. EuroIntervention 2021, 18, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021, 384, 2081–2091. [Google Scholar] [CrossRef]
- Paciaroni, M.; Caso, V.; Agnelli, G.; Mosconi, M.G.; Giustozzi, M.; Seiffge, D.J.; Engelter, S.T.; Lyrer, P.; Polymeris, A.A.; Kriemler, L.; et al. Recurrent Ischemic Stroke and Bleeding in Patients With Atrial Fibrillation Who Suffered an Acute Stroke While on Treatment With Nonvitamin K Antagonist Oral Anticoagulants: The RENO-EXTEND Study. Stroke 2022, 53, 2620–2627. [Google Scholar] [CrossRef]
- Camm, A.J.; Naccarelli, G.V.; Mittal, S.; Crijns, H.; Hohnloser, S.H.; Ma, C.S.; Natale, A.; Turakhia, M.P.; Kirchhof, P. The Increasing Role of Rhythm Control in Patients With Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1932–1948. [Google Scholar] [CrossRef]
- Korsholm, K.; Damgaard, D.; Valentin, J.B.; Packer, E.J.S.; Odenstedt, J.; Sinisalo, J.; Putaala, J.; Naess, H.; Al-Jazi, M.A.; Karlsson, J.E.; et al. Left atrial appendage occlusion vs novel oral anticoagulation for stroke prevention in atrial fibrillation: Rationale and design of the multicenter randomized occlusion-AF trial. Am. Heart J. 2022, 243, 28–38. [Google Scholar] [CrossRef]
| Study Design | First Author/ Year of Publication | Patients No. | Age Median | CHADS2 Score Mean | History of Stroke/TIA/SE % | Mean Follow-Up Duration (y) | Treatment Arms | Ischemic Stroke Rate in Patients under DOAC %/Patient/Year | Ischemic Stroke Rate in Patients under VKA %/Patient/Year | |
|---|---|---|---|---|---|---|---|---|---|---|
| RCT | Connolly SJ et al./2009 [5] | 18,113 | 71.5 * | 2.2 | 20 ¶ | 2 | Dabigatran (150/110 mg) | VKA (INR 2–3) | 1.34 in dabigatran 110 mg | 1.20 |
| 0.92 in dabigatran 150 mg | ||||||||||
| RCT | Patel MR et al./2011 [8] | 14,264 | 73 | 3.5 | 54.8 | 1.9 | Rivaroxaban (20/15 mg) | VKA (INR 2–3) | 1.7 µ | 2.2 µ |
| RCT | Granger CB et al./2011 [7] | 18,201 | 70 | 2.1 | 19.4 | 1.9 | Apixaban (5/2.5 mg) | VKA (INR 2–3) | 0.97 | 1.05 |
| RCT | Giugliano RP et al./2013 [6] | 21,105 | 72 | 2.8 | 28.3 ¶ | 2.8 | Edoxaban (60/30 mg) | VKA (INR 2–3) | 1.25 in edoxaban 60 mg | 1.25 |
| 1.77 in edoxaban 30 mg | ||||||||||
| First Author/ Year of Publication | Study Design | Patients No. | Age Mean | CHADS2 Score Mean | LAAC Device Implanted | Main Post-LAAC Antithrombotic Drug Regimen | Mean Follow-Up Duration (y) | Ischemic Stroke Recurrence Rate %/Patient/Year |
|---|---|---|---|---|---|---|---|---|
| Freixa X et al./2019 [36] | SC—RS | 22 | NA | NA | NA | OAC | 1.8 * | 2.5 |
| Masjuan J et al./2019 [46] | MC—PS | 19 | 72.1 | 5.3 | ACP or Amulet | OAC + Aspirin | 1.45 | 0 |
| Cruz-Gonzalez I et al./2020 [35] | MC—RS | 115 | 73.8 | 5.5 | ACP | DAPT | 1.35 | 2.6 |
| Galloo X et al./2022 [45] | MC—RS | 15 | 78.1 | 6 | Amulet or Wachman | OAC | NA | 2 events |
| Pracon R et al./2022 [47] | SC—PS | 39 | 73 * | 5 * | Amulet or Wachman | DAPT | 1 | 7.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galea, R.; Seiffge, D.; Räber, L. Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation. J. Clin. Med. 2023, 12, 5784. https://doi.org/10.3390/jcm12185784
Galea R, Seiffge D, Räber L. Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation. Journal of Clinical Medicine. 2023; 12(18):5784. https://doi.org/10.3390/jcm12185784
Chicago/Turabian StyleGalea, Roberto, David Seiffge, and Lorenz Räber. 2023. "Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation" Journal of Clinical Medicine 12, no. 18: 5784. https://doi.org/10.3390/jcm12185784
APA StyleGalea, R., Seiffge, D., & Räber, L. (2023). Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation. Journal of Clinical Medicine, 12(18), 5784. https://doi.org/10.3390/jcm12185784






