Bolstering Cognitive and Locomotor Function in Post-Stroke Dementia Using Human–Robotic Interactive Gait Training
Abstract
1. Introduction
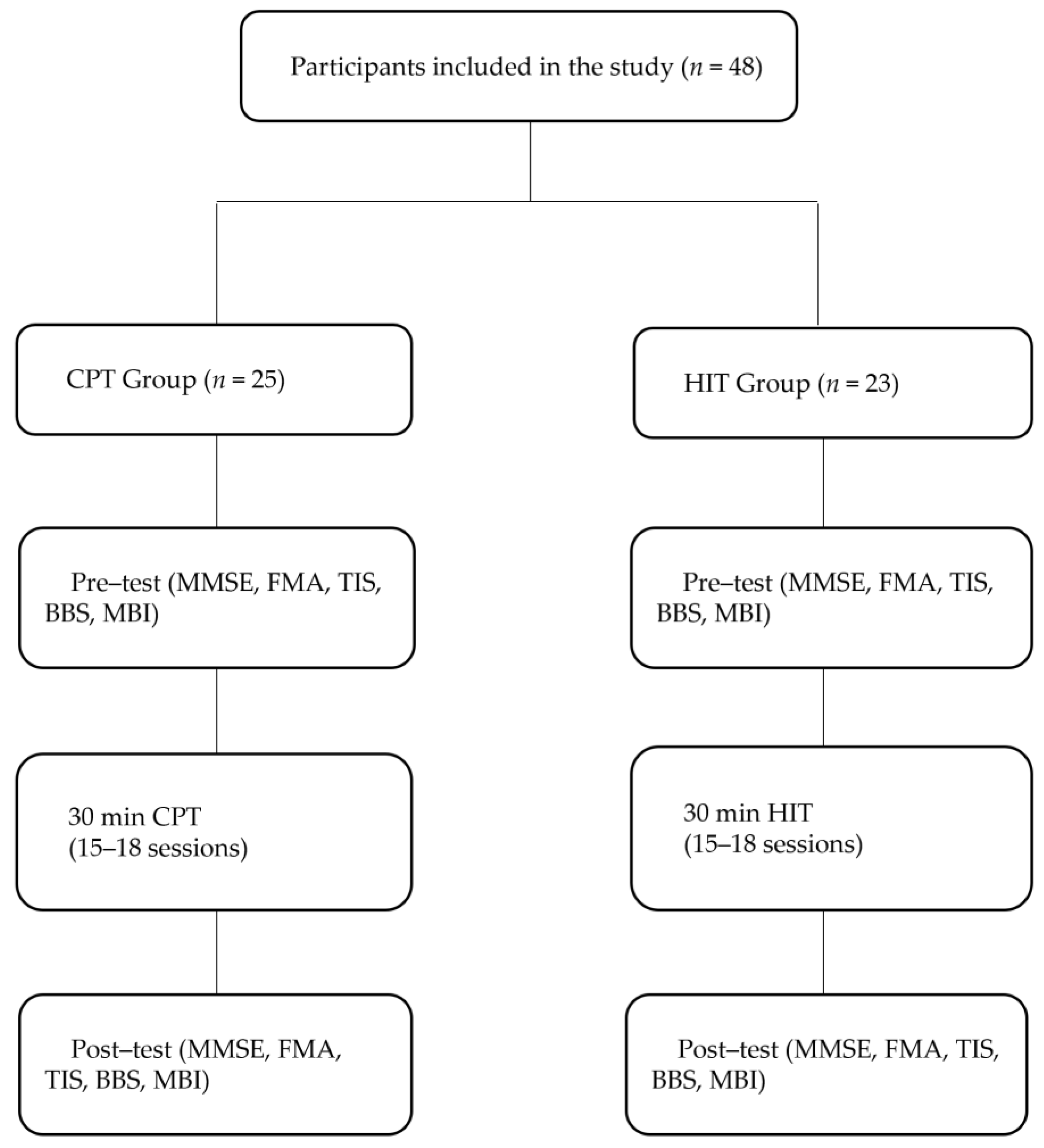
2. Materials and Methods
2.1. Participants
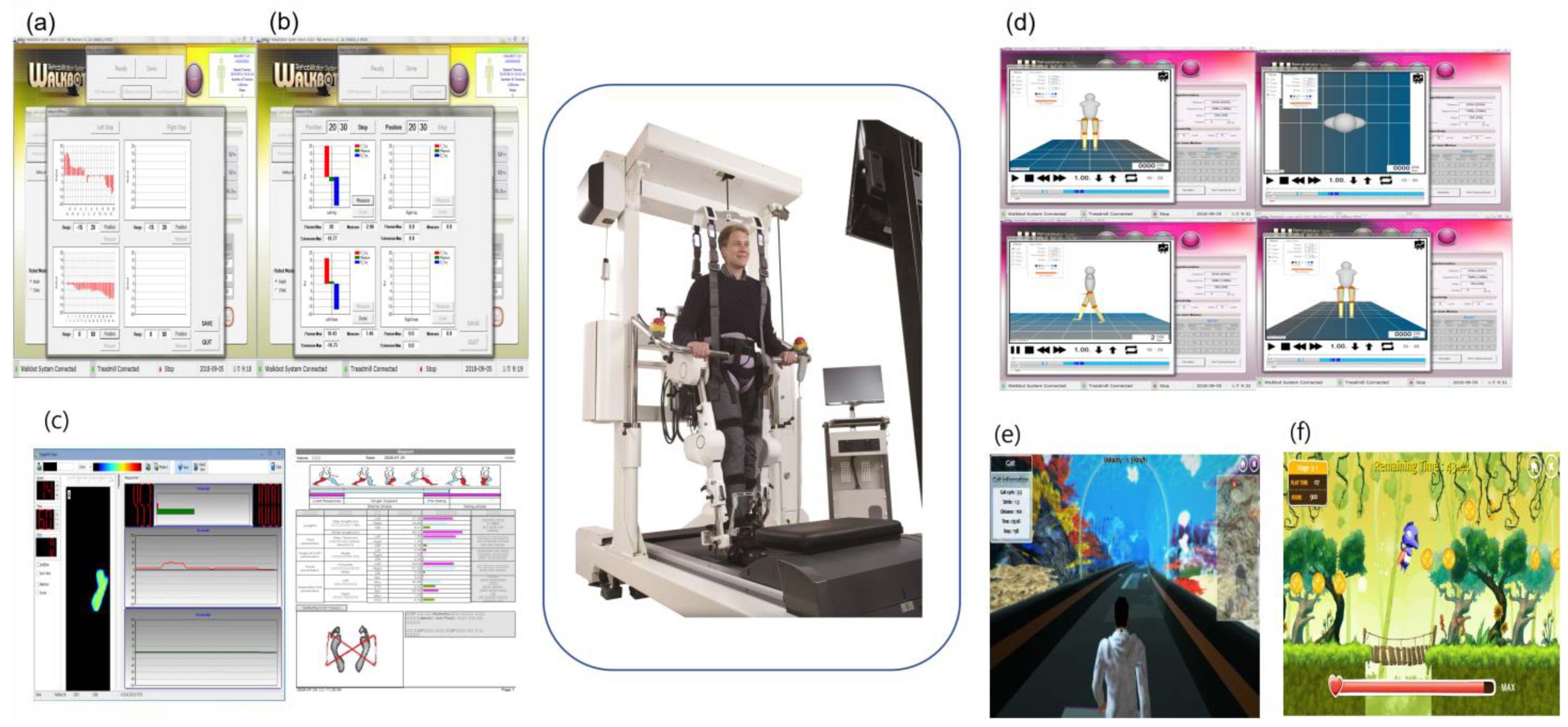
2.2. Study Design
2.3. Clinical Outcome Measurements
2.3.1. Mini-Mental State Examination
2.3.2. Fugl–Meyer Assessment
2.3.3. Trunk Impairment Scale
2.3.4. Berg Balance Scale
2.3.5. Modified Barthel Index
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Participants
3.2. Clinical Outcome Measurements
3.2.1. Mini-Mental State Examination
3.2.2. Fugl–Meyer Assessment
3.2.3. Trunk Impairment Scale
3.2.4. Berg Balance Scale
3.2.5. Modified Barthel Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.-C.; Chen, Y.-M. Post-stroke dementia: Epidemiology, mechanisms and management. Int. J. Gerontol. 2017, 11, 210–214. [Google Scholar] [CrossRef]
- Korczyn, A.D.; Vakhapova, V.; Grinberg, L.T. Vascular dementia. J. Neurol. Sci. 2012, 322, 2–10. [Google Scholar] [CrossRef] [PubMed]
- T O’Brien, J.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Sibolt, G.; Curtze, S.; Jokinen, H.; Pohjasvaara, T.; Kaste, M.; Karhunen, P.J.; Erkinjuntti, T.; Melkas, S.; Oksala, N.K. Post-stroke dementia and permanent institutionalization. J. Neurol. Sci. 2021, 421, 117307. [Google Scholar] [CrossRef]
- Teasell, R.; Salbach, N.M.; Foley, N.; Mountain, A.; Cameron, J.I.; Jong, A.D.; Acerra, N.E.; Bastasi, D.; Carter, S.L.; Fung, J. Canadian stroke best practice recommendations: Rehabilitation, recovery, and community participation following stroke. Part one: Rehabilitation and recovery following stroke; Update 2019. Int. J. Stroke 2020, 15, 763–788. [Google Scholar] [CrossRef]
- Minati, L.; Frasca, M.; Yoshimura, N.; Koike, Y. Versatile locomotion control of a hexapod robot using a hierarchical network of nonlinear oscillator circuits. IEEE Access 2018, 6, 8042–8065. [Google Scholar] [CrossRef]
- Grillner, S. Biological pattern generation: The cellular and computational logic of networks in motion. Neuron 2006, 52, 751–766. [Google Scholar] [CrossRef]
- Pohjasvaara, T.; Erkinjuntti, T.; Ylikoski, R.; Hietanen, M.; Vataja, R.; Kaste, M. Clinical determinants of poststroke dementia. Stroke 1998, 29, 75–81. [Google Scholar] [CrossRef]
- Whitney, D.G.; Dutt-Mazumder, A.; Peterson, M.D.; Krishnan, C. Fall risk in stroke survivors: Effects of stroke plus dementia and reduced motor functional capacity. J. Neurol. Sci. 2019, 401, 95–100. [Google Scholar] [CrossRef]
- Duncan, P.W.; Sullivan, K.J.; Behrman, A.L.; Azen, S.P.; Wu, S.S.; Nadeau, S.E.; Dobkin, B.H.; Rose, D.K.; Tilson, J.K.; Cen, S. Body-weight–supported treadmill rehabilitation after stroke. N. Engl. J. Med. 2011, 364, 2026–2036. [Google Scholar] [PubMed]
- Schwartz, I.; Meiner, Z. The influence of locomotor treatment using robotic body-weight-supported treadmill training on rehabilitation outcome of patients suffering from neurological disorders. Harefuah 2013, 152, 166–171, 182, 181. [Google Scholar] [PubMed]
- Calabrò, R.S.; De Luca, R.; Leo, A.; Balletta, T.; Marra, A.; Bramanti, P. Lokomat training in vascular dementia: Motor improvement and beyond! Aging Clin. Exp. Res. 2015, 27, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.G.; Lee, W.H.; Lee, S.H.; Yi, Y.; Kim, K.D.; Oh, B.-M. Robotic-assisted gait training combined with transcranial direct current stimulation in chronic stroke patients: A pilot double-blind, randomized controlled trial. Restor. Neurol. Neurosci. 2017, 35, 527–536. [Google Scholar]
- Park, C.; Oh-Park, M.; Bialek, A.; Friel, K.; Edwards, D.; You, J.S.H. Abnormal synergistic gait mitigation in acute stroke using an innovative ankle–knee–hip interlimb humanoid robot: A preliminary randomized controlled trial. Sci. Rep. 2021, 11, 22823. [Google Scholar] [CrossRef]
- Jeong, S.A.; Park, C.; Oh, S.J.; You, J.S.H. Multiple relationships between cognition-motor impairment and activity-based clinical outcome measures in 218 hemiplegic stroke patients. NeuroRehabilitation 2021, 49, 553–563. [Google Scholar] [CrossRef]
- Park, C.; Oh-Park, M.; Dohle, C.; Bialek, A.; Friel, K.; Edwards, D.; Krebs, H.I.; You, J.S.H. Effects of innovative hip-knee-ankle interlimb coordinated robot training on ambulation, cardiopulmonary function, depression, and fall confidence in acute hemiplegia. NeuroRehabilitation 2020, 46, 577–587. [Google Scholar]
- Kim, H.Y.; Shin, J.-H.; Yang, S.P.; Shin, M.A.; Lee, S.H. Robot-assisted gait training for balance and lower extremity function in patients with infratentorial stroke: A single-blinded randomized controlled trial. J. Neuroeng. Rehabil. 2019, 16, 99. [Google Scholar]
- Park, I.J.; Park, J.-H.; Seong, H.Y.; You, J.S.H.; Kim, S.J.; Min, J.H.; Ko, H.Y.; Shin, Y.-I. Comparative effects of different assistance force during robot-assisted gait training on locomotor functions in patients with subacute stroke: An assessor-blind, randomized controlled trial. Am. J. Phys. Med. Rehabil. 2019, 98, 58–64. [Google Scholar]
- Park, J.-H.; Shin, Y.-I.; You, J.S.H.; Park, M.S. Comparative effects of robotic-assisted gait training combined with conventional physical therapy on paretic hip joint stiffness and kinematics between subacute and chronic hemiparetic stroke. NeuroRehabilitation 2018, 42, 181–190. [Google Scholar]
- Kim, S.-Y.; Yang, L.; Park, I.J.; Kim, E.J.; Park, M.S.; You, S.H.; Kim, Y.-H.; Ko, H.-Y.; Shin, Y.-I. Effects of innovative WALKBOT robotic-assisted locomotor training on balance and gait recovery in hemiparetic stroke: A prospective, randomized, experimenter blinded case control study with a four-week follow-up. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 636–642. [Google Scholar]
- Vahia, V. N. Diagnostic and statistical manual of mental disorders 5: A quick glance. Am. Psychiatr. Assoc. 2013, 55, 220. [Google Scholar]
- Vertesi, A.; Lever, J.A.; Molloy, D.W.; Sanderson, B.; Tuttle, I.; Pokoradi, L.; Principi, E. Standardized Mini-Mental State Examination. Use and interpretation. Can. Fam. Physician 2001, 47, 2018–2023. [Google Scholar]
- Carota, A.; Bogousslavsky, J. Mood disorders after stroke. Manif. Stroke 2012, 30, 70–74. [Google Scholar]
- Atigossou, O.L.G.; Ouédraogo, F.; Honado, A.S.; Alagnidé, E.; Kpadonou, T.G.; Batcho, C.S. Association between post-stroke psychological disorders, activity limitations and health-related quality of life in chronic stroke survivors in Benin. Disabil. Rehabil. 2023, 45, 2087–2094. [Google Scholar] [PubMed]
- Hafsteinsdóttir, T.; Algra, A.; Kappelle, L.; Grypdonck, M. Neurodevelopmental treatment after stroke: A comparative study. J. Neurol. Neurosurg. Psychiatry 2005, 76, 788–792. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Sullivan, S.B.; Schmitz, T.J. Improving Functional Outcomes in Physical Rehabilitation; FA Davis: Philadelphia, PA, USA, 2016. [Google Scholar]
- Graham, J.V.; Eustace, C.; Brock, K.; Swain, E.; Irwin-Carruthers, S. The Bobath concept in contemporary clinical practice. Top. Stroke Rehabil. 2009, 16, 57–68. [Google Scholar]
- Kang, Y.; Na, D.L.; Hahn, S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Molloy, D.W.; Alemayehu, E.; Roberts, R. Reliability of a standardized mini-mental state examination compared with the traditional mini-mental state examination. Am. J. Psychiatry 1991, 148, 102–105. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [Google Scholar]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.-H.; Di Bella, P.; Johnson, G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [PubMed]
- Verheyden, G.; Nieuwboer, A.; Mertin, J.; Preger, R.; Kiekens, C.; De Weerdt, W. The Trunk Impairment Scale: A new tool to measure motor impairment of the trunk after stroke. Clin. Rehabil. 2004, 18, 326–334. [Google Scholar] [PubMed]
- Verheyden, G.; Kersten, P. Investigating the internal validity of the Trunk Impairment Scale (TIS) using Rasch analysis: The TIS 2.0. Disabil. Rehabil. 2010, 32, 2127–2137. [Google Scholar] [CrossRef]
- Berg, T. Berg balance scale. Arch. Phys. Med. Rehabil. 2009, 73, 2–5. [Google Scholar]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [PubMed]
- Leung, S.O.; Chan, C.C.; Shah, S. Development of a Chinese version of the Modified Barthel Index—Validity and reliability. Clin. Rehabil. 2007, 21, 912–922. [Google Scholar]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar]
- Ten Brinke, L.F.; Bolandzadeh, N.; Nagamatsu, L.S.; Hsu, C.L.; Davis, J.C.; Miran-Khan, K.; Liu-Ambrose, T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Sports Med. 2015, 49, 248–254. [Google Scholar] [PubMed]
- Kandola, A.; Hendrikse, J.; Lucassen, P.J.; Yücel, M. Aerobic exercise as a tool to improve hippocampal plasticity and function in humans: Practical implications for mental health treatment. Front. Hum. Neurosci. 2016, 10, 373. [Google Scholar]
- Killeen, T. Advanced Gait Analysis: Insights into Human Locomotor Control; Universität Zürich: Zürich, Switzerland, 2017. [Google Scholar]
- Huxhold, O.; Li, S.-C.; Schmiedek, F.; Lindenberger, U. Dual-tasking postural control: Aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res. Bull. 2006, 69, 294–305. [Google Scholar]
- Sigrist, R.; Rauter, G.; Riener, R.; Wolf, P. Augmented visual, auditory, haptic, and multimodal feedback in motor learning: A review. Psychon. Bull. Rev. 2013, 20, 21–53. [Google Scholar] [CrossRef]
- Kim, S.-J.; Krebs, H.I. Effects of implicit visual feedback distortion on human gait. Exp. Brain Res. 2012, 218, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, G.; Shin, J.-H.; You, J.H. Neuroplastic effects of end-effector robotic gait training for hemiparetic stroke: A randomised controlled trial. Sci. Rep. 2020, 10, 12461. [Google Scholar] [CrossRef] [PubMed]
- Yokota, C.; Yamamoto, Y.; Kamada, M.; Nakai, M.; Nishimura, K.; Ando, D.; Sato, T.; Koga, M.; Ihara, M.; Toyoda, K. Acute stroke rehabilitation for gait training with cyborg type robot Hybrid Assistive Limb: A pilot study. J. Neurol. Sci. 2019, 404, 11–15. [Google Scholar] [CrossRef] [PubMed]
- You, S.H.; Jang, S.H.; Kim, Y.-H.; Hallett, M.; Ahn, S.H.; Kwon, Y.-H.; Kim, J.H.; Lee, M.Y. Virtual reality–induced cortical reorganization and associated locomotor recovery in chronic stroke: An experimenter-blind randomized study. Stroke 2005, 36, 1166–1171. [Google Scholar] [CrossRef]
- Eggenberger, P.; Theill, N.; Holenstein, S.; Schumacher, V.; de Bruin, E.D. Multicomponent physical exercise with simultaneous cognitive training to enhance dual-task walking of older adults: A secondary analysis of a 6-month randomized controlled trial with 1-year follow-up. Clin. Interv. Aging 2015, 10, 1711–1732. [Google Scholar] [CrossRef]
- You, J.H.; Shetty, A.; Jones, T.; Shields, K.; Belay, Y.; Brown, D. Effects of dual-task cognitive-gait intervention on memory and gait dynamics in older adults with a history of falls: A preliminary investigation. NeuroRehabilitation 2009, 24, 193–198. [Google Scholar] [CrossRef]
- Van Tran, Q.; Kim, S.; Lee, K.; Kang, S.; Ryu, J. Force/torque sensorless impedance control for indirect driven robot-aided gait rehabilitation system. In Proceedings of the 2015 IEEE International Conference on Advanced Intelligent Mechatronics (AIM), Busan, Korea, 7–11 July 2015; pp. 652–657. [Google Scholar]
- Winter, D.A. Biomechanics and Motor Control of Human Movement; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]

| CPT 1 Group (n = 25) | HIT 2 Group (n = 23) | p-Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 68.16 ± 12.76 | 68.35 ± 11.09 | 0.948 |
| Height (cm) | 164.60 ± 6.71 | 164.83 ± 7.80 | 0.902 |
| Weight (kg) | 60.08 ± 10.05 | 60.92 ± 7.60 | 0.724 |
| Onset time (months) | |||
| 0–3 (%) | 15 (60) | 13 (57) | 0.799 |
| 4–6 (%) | 7 (28) | 6 (26) | |
| 7–12 (%) | 3 (12) | 4 (17) | |
| Sex | |||
| Male | 16 (64) | 13 (57) | 0.497 |
| Female | 9 (36) | 10 (43) | |
| Type of stroke | |||
| Hemorrhage (%) | 12 (48) | 15 (65) | 0.330 |
| Infarction (%) | 13 (52) | 8 (35) | |
| Affected side | |||
| Left (%) | 10 (40) | 10 (43) | 0.809 |
| Right (%) | 15 (60) | 13 (57) | |
| Location of lesion | |||
| ACA (%) | 2 | 2 | 0.446 |
| MCA (%) | 17 | 15 | |
| PCA (%) | 4 | 6 | |
| VB (%) | 1 | 2 | |
| Etc. (%) | 1 | 0 | |
| Clinical characteristics | |||
| MMSE 3 | 18.00 | 20.00 | 0.358 |
| FMA 4 | 36.00 | 49.00 | 0.156 |
| TIS 5 | 5.00 | 6.00 | 0.557 |
| BBS 6 | 5.00 | 6.00 | 0.587 |
| MBI 7 | 31.00 | 36.00 | 0.154 |
| Box’s M | 85.206 |
|---|---|
| F | 0.868 |
| df1 | 66 |
| df2 | 3804.394 |
| Sig. | 0.769 |
| CPT 1 | HIT 2 | |||||
|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | Pre-Test | Post-Test | χ2 | p-Value | |
| MMSE 3 | 18.00 | 19.00 | 20.00 | 22.00 | 16.07 | 0.007 * |
| FMA 4 | 36.00 | 40.00 | 49.00 | 50.00 | 16.55 | 0.005 * |
| TIS 5 | 5.00 | 6.00 | 6.00 | 10.00 | 14.27 | 0.014 * |
| BBS 6 | 5.00 | 7.00 | 6.00 | 9.00 | 8.83 | 0.116 |
| MBI 7 | 31.00 | 40.00 | 36.00 | 54.00 | 9.66 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Park, C.; Yoon, B.; You, J.H. Bolstering Cognitive and Locomotor Function in Post-Stroke Dementia Using Human–Robotic Interactive Gait Training. J. Clin. Med. 2023, 12, 5661. https://doi.org/10.3390/jcm12175661
Kim Y, Park C, Yoon B, You JH. Bolstering Cognitive and Locomotor Function in Post-Stroke Dementia Using Human–Robotic Interactive Gait Training. Journal of Clinical Medicine. 2023; 12(17):5661. https://doi.org/10.3390/jcm12175661
Chicago/Turabian StyleKim, Yunhwan, Chanhee Park, Buhyun Yoon, and Joshua (Sung) H. You. 2023. "Bolstering Cognitive and Locomotor Function in Post-Stroke Dementia Using Human–Robotic Interactive Gait Training" Journal of Clinical Medicine 12, no. 17: 5661. https://doi.org/10.3390/jcm12175661
APA StyleKim, Y., Park, C., Yoon, B., & You, J. H. (2023). Bolstering Cognitive and Locomotor Function in Post-Stroke Dementia Using Human–Robotic Interactive Gait Training. Journal of Clinical Medicine, 12(17), 5661. https://doi.org/10.3390/jcm12175661






_H._You.png)

