Analysis of Physical–Cognitive Tasks Including Feedback-Based Technology for Alzheimer’s Disorder in a Randomized Experimental Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Variables
2.3. Outcome Measures
- –
- Attention: This was assessed with the Oddball test (first) and ANT-Elderly (second) test [28]. The tasks of both tests required the patient to respond to an objective stimulus. In the Oddball test, the patient had to react by pressing a button before the appearance of red and white chessboards. This task involved 200 tries, of which 50 required a response. In the ANT-Elderly test, 5 arrows (imperative stimulus) pointing to the right or left were displayed. They did not necessarily have to point to the same side. The arrows had the following characteristics: a width of 7.37°, a height of 0.86°, and a duration of 350 ms. The patients had to click the right or left mouse button depending on the central arrow, which was presented as a target stimulus. As a previous stimulus, a cue (clue) could appear to indicate when the arrows would appear or where on the screen they would appear. The cues had the following characteristics (asterisk): a width of 0.86°, a height of 0.86°, and a duration of 150 ms. The time between the cue and the arrows was 850 ms, and the time between the arrows and the next cue ranged from 150 to 1000 ms. The appearance or non-appearance of cues was programmed into the tool to objectively assess the different attentional networks. The Attention Network test was separated into two parts (72 trials/a 5 min break to avoid fatigue/72 trials). The rest of the data on this tool can be found in [23].
- –
- Memory: This was assessed with the Mini-Mental State Examination (MMSE). At the end of the test, the patients received a score that determined the presence or absence of a cognitive decline. The Spanish version, which is called the Cognitive Mini-Examination (MEC), consists of 30 items that are grouped into five parts: orientation, memorization, retrospective memory, concentration and calculation, and language and construction. The sum the parts leads to the total score. The cut-off point for dementia was set at 24. Values closer to 30 corresponded to optimal cognitive abilities.
- –
- Balance: This outcome was measured with the application of the Berg scale [29]. The scale allowed the quantification of balance in different positions, resulting in a global score that guided the balance function in general, which could be used to predict the possibility of the use of a wheelchair, other technical aids, or total independence for the development of the gait. The patients executed 14 different items, with each of them being valued between 0 and 4 points. The total value was provided by the sum of the items. The interpretation of balance could be good (41–56 points), compromised (21–40 points), and affected (0–20 points).
- –
- Gait: This was evaluated through the Timed Up and Go test [30]. This test measured how long it took for a subject to get up from a chair, walk three meters, and go back to the starting position. A patient’s gait was considered normal gait if they took less than 10 s to perform the test; they were considered to have a slight risk of falling if the test time was between 11 and 20 s and a high risk of falling if it took more than 20 s to perform the test.
- –
- Functionality and risk of falls [31]: This outcome was measured through the application of the Tinetti scale, an instrument that evaluates balance, gait, and falls. There were 16 items in total, and the total score was 28 points. The scale had two dimensions: balance (9 items), with a score between 0 and 16, and gait (7 items), with a score between 0 and 12 points. The interpretation of the functionality and risk of falls was as follows: high risk (<19 points); risk (19–23 points); low or slight risk (24–28 points).
2.4. Treatment Using Feedback-Based Technology
2.5. Laboratory Conditions
2.6. Intervention Protocol and Phases of the Research
- –
- Phase I: On the first day, the legal guardians read and signed the informed consent form. Subsequently, the users, in the presence of their companions, were brought together for a preliminary assessment and anamnesis that included questions on moderating variables (e.g., gender, dominance, drugs, etc.). Three days later, the patients who met the inclusion criteria were assessed with computerized tests and functional assessment scales/tests to determine their physical–cognitive capacities.
- –
- Phase II: This phase involved the collection of the initial assessments by each evaluator and, later, the insertion of these data into a database (i.e., ratings of each of the two observers and the average of the two).
- –
- Phase III: This phase involved treatment by using technology (games) in the intervention group. The total number of 30 min sessions was 16, with a frequency of 2 times per week [32]. Each session consisted of 3 series of “Penguin Slide”, 1 series of “Step Plus”, and 3 series more of “Penguin Slide”. The scores obtained in the sessions were recorded on a sheet to encourage the patients and the caregivers. The infrastructure of this study made it possible to test two users at the same to improve their motivation and adherence. The control group continued with their conventional treatment (independent sessions of mobility exercises and cognitive stimulation in their Alzheimer’s association).
- –
- Phase IV: This phase involved the appointment of the patients for the final assessment and performance of the tests so that the physiotherapists and neuropsychologists could determine their physical–cognitive capacities.
- –
- Phase V: This phase involved the total assessments of each observer and the average scores, as well as the insertion of the data into the database.
- –
- Phase VI: In this phase, the database was completed, the data were blinded to the observers, and statistical analyses were performed.
- –
- Phase VII: Once the data collection was finished, the control group received the same treatment as the intervention group.
2.7. Data Collection Procedures
2.8. Ethical Considerations of the Study
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANT | Attention Network Test |
| Arth | Arthrosis |
| BS | Basic Studies |
| CG | Control Group |
| D | Donepezil |
| DM | Diabetes |
| EG | Experimental Group |
| GDS | Geriatric Deterioration Scale |
| IT | Informational Technology |
| LH | Left-handed |
| M | Man |
| Me | Memantine |
| MEC | Cognitive Mini-Examination |
| MMSE | Mini-Mental State Examination |
| MS | Mild stage |
| O | Osteoporosis |
| PD | Pulmonary Disease |
| PE | Primary Education |
| Pk | Parkinson |
| R | Rivastigmine |
| RH | Right-handed |
| SE | Secondary Education |
| Sk | Stoke |
| SS | Severe stage |
| W | Woman |
References
- Rodriguez-Laso, A.; McLaughlin, S.J.; Urdaneta, E.; Yanguas, J. Defining and Estimating Healthy Aging in Spain: A Cross-Sectional Study. Gerontologist 2018, 58, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing Populations: The Challenges Ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lu, H.; Metmer, H.; Li, W.X.Y.; Lu, J. Evaluating Functional Connectivity of Executive Control Network and Frontoparietal Network in Alzheimer’s Disease. Brain Res. 2018, 1678, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Yogev-Seligmann, G.; Hausdorff, J.M.; Giladi, N. The Role of Executive Function and Attention in Gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef]
- Schumacher, J.; Cromarty, R.; Gallagher, P.; Firbank, M.J.; Thomas, A.J.; Kaiser, M.; Blamire, A.M.; O’Brien, J.T.; Peraza, L.R.; Taylor, J.P. Structural Correlates of Attention Dysfunction in Lewy Body Dementia and Alzheimer’s Disease: An Ex-Gaussian Analysis. J. Neurol. 2019, 266, 1716–1726. [Google Scholar] [CrossRef]
- Zhao, Q.; Sang, X.; Metmer, H.; Swati, Z.n.N.K.; Lu, J. Functional Segregation of Executive Control Network and Frontoparietal Network in Alzheimer’s Disease. Cortex 2019, 120, 36–48. [Google Scholar] [CrossRef]
- Arai, H.; Satake, S.; Kozaki, K. Cognitive Frailty in Geriatrics. Clin. Geriatr. Med. 2018, 34, 667–675. [Google Scholar] [CrossRef]
- Walston, J.; Buta, B.; Xue, Q.-L. Frailty Screening and Interventions. Clin. Geriatr. Med. 2018, 34, 25–38. [Google Scholar] [CrossRef]
- Fan, J.; Posner, M. Human Attentional Networks. Psychiatr. Prax. 2004, 31, 210–214. [Google Scholar] [CrossRef]
- Sarrias-Arrabal, E.; Izquierdo-Ayuso, G.; Vázquez-Marrufo, M. Attentional Networks in Neurodegenerative Diseases: Anatomical and Functional Evidence from the Attention Network Test. Neurologia 2023, 38, 206–217. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Minguillón, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s Disease Prevention: From Risk Factors to Early Intervention. Alzheimers Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Hersi, M.; Irvine, B.; Gupta, P.; Gomes, J.; Birkett, N.; Krewski, D. Risk Factors Associated with the Onset and Progression of Alzheimer’s Disease: A Systematic Review of the Evidence. Neurotoxicology 2017, 61, 143–187. [Google Scholar] [CrossRef] [PubMed]
- Dunsky, A. The Effect of Balance and Coordination Exercises on Quality of Life in Older Adults: A Mini-Review. Front. Aging Neurosci. 2019, 11, 318. [Google Scholar] [CrossRef]
- Ali, N.; Tian, H.; Thabane, L.; Ma, J.; Wu, H.; Zhong, Q.; Gao, Y.; Sun, C.; Zhu, Y.; Wang, T. The Effects of Dual-Task Training on Cognitive and Physical Functions in Older Adults with Cognitive Impairment; A Systematic Review and Meta-Analysis. J. Prev. Alzheimers Dis. 2022, 9, 359–370. [Google Scholar] [CrossRef]
- Fritz, N.E.; Cheek, F.M.; Nichols-Larsen, D.S. Motor-Cognitive Dual-Task Training in Persons with Neurologic Disorders. J. Neurol. Phys. Ther. 2015, 39, 142–153. [Google Scholar] [CrossRef]
- Chamorro-Moriana, G.; Moreno, A.; Sevillano, J. Technology-Based Feedback and Its Efficacy in Improving Gait Parameters in Patients with Abnormal Gait: A Systematic Review. Sensors 2018, 18, 142. [Google Scholar] [CrossRef]
- Iruthayarajah, J.; McIntyre, A.; Cotoi, A.; Macaluso, S.; Teasell, R. The Use of Virtual Reality for Balance among Individuals with Chronic Stroke: A Systematic Review and Meta-Analysis. Top. Stroke Rehabil. 2017, 24, 68–79. [Google Scholar] [CrossRef]
- Hill, N.T.M.; Mowszowski, L.; Naismith, S.L.; Chadwick, V.L.; Valenzuela, M.; Lampit, A. Computerized Cognitive Training in Older Adults with Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Am. J. Psychiatry 2017, 174, 329–340. [Google Scholar] [CrossRef]
- Choi, S.D.; Guo, L.; Kang, D.; Xiong, S. Exergame Technology and Interactive Interventions for Elderly Fall Prevention: A Systematic Literature Review. Appl. Ergon. 2017, 65, 570–581. [Google Scholar] [CrossRef]
- Chao, Y.-Y.; Scherer, Y.K.; Montgomery, C.A. Effects of Using Nintendo WiiTM Exergames in Older Adults. J. Aging Health 2015, 27, 379–402. [Google Scholar] [CrossRef]
- Pieruccini-Faria, F.; Lord, S.R.; Toson, B.; Kemmler, W.; Schoene, D. Mental Flexibility Influences the Association between Poor Balance and Falls in Older People—A Secondary Analysis. Front. Aging Neurosci. 2019, 11, 133. [Google Scholar] [CrossRef]
- Benitez-Lugo, M.-L.; Suárez-Serrano, C.; Galvao-Carmona, A.; Vazquez-Marrufo, M.; Chamorro-Moriana, G. Effectiveness of Feedback-Based Technology on Physical and Cognitive Abilities in the Elderly. Front. Aging Neurosci. 2022, 14, 1050518. [Google Scholar] [CrossRef]
- The World Medical Association, Inc. Declaration of helsinki ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hrobjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. Declaración SPIRIT 2013: Definición de Los Elementos Estándares Del Protocolo de Un Ensayo Clínico * [SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials]. Rev. Panam. Salud Pública 2015, 38, 506–514. [Google Scholar] [PubMed]
- Sousa, M.; Navas, Z.; Laborde, M.; Alfaro, B.; Carrascosa, U. Niveles de Evidencia Clínica y Grados de Recomendación Levels of Scientific Evidence and Degrees of Recommendation. Repos Salud 2017, 29, 1–14. [Google Scholar]
- Cobos-Carbó, A.; Augustovski, F. Declaración CONSORT 2010: Actualización de La Lista de Comprobación Para Informar Ensayos Clínicos Aleatorizados de Grupos Paralelos. Med. Clin. 2011, 137, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Marrufo, M.; Luisa Benitez, M.; Rodriguez-Gomez, G.; Galvao-Carmona, A.; Fernandez-Del Olmo, A.; Vaquero-Casares, E. Attentional Neural Networks Impairment in Healthy Aging. Rev. Neurol. 2011, 52, 20–26. [Google Scholar]
- Downs, S. The Berg Balance Scale. J. Physiother. 2015, 61, 46. [Google Scholar] [CrossRef]
- Browne, W.; Nair, B.R. The Timed up and Go Test. Med. J. Aust. 2019, 210, 13. [Google Scholar] [CrossRef]
- Park, S.-H. Tools for Assessing Fall Risk in the Elderly: A Systematic Review and Meta-Analysis. Aging Clin. Exp. Res. 2018, 30, 1–16. [Google Scholar] [CrossRef]
- Lai, C.-H.; Peng, C.-W.; Chen, Y.-L.; Huang, C.-P.; Hsiao, Y.-L.; Chen, S.-C. Effects of Interactive Video-Game Based System Exercise on the Balance of the Elderly. Gait Posture 2013, 37, 511–515. [Google Scholar] [CrossRef]
- Padala, K.P.; Padala, P.R.; Malloy, T.R.; Geske, J.A.; Dubbert, P.M.; Dennis, R.A.; Garner, K.K.; Bopp, M.M.; Burke, W.J.; Sullivan, D.H. Wii-Fit for Improving Gait and Balance in an Assisted Living Facility: A Pilot Study. J. Aging Res. 2012, 2012, 597573. [Google Scholar] [CrossRef] [PubMed]
- Agmon, M.; Perry, C.K.; Phelan, E.; Demiris, G.; Nguyen, H.Q. A Pilot Study of Wii Fit Exergames to Improve Balance in Older Adults. J. Geriatr. Phys. Ther. 2011, 34, 161–167. [Google Scholar] [CrossRef]
- Maci, T.; Le Pira, F.; Quattrocchi, G.; Di Nuovo, S.; Perciavalle, V.; Zappia, M. Physical and Cognitive Stimulation in Alzheimer Disease. The GAIA Project. Am. J. Alzheimers Dis. Other Dement. 2012, 27, 107–113. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Maki, Y.; Takahashi, K. Rehabilitation for Dementia Using Enjoyable Video-Sports Games. Int. Psychogeriatr. 2011, 23, 674–676. [Google Scholar] [CrossRef]
- Fernandez-Duque, D.; Black, S.E. Attentional Networks in Normal Aging and Alzheimer’s Disease. Neuropsychology 2006, 20, 133–143. [Google Scholar] [CrossRef]
- Festa-Martino, E.; Ott, B.R.; Heindel, W.C. Interactions between Phasic Alerting and Spatial Orienting: Effects of Normal Aging and Alzheimer’s Disease. Neuropsychology 2004, 18, 258–268. [Google Scholar] [CrossRef]
- Pichierri, G.; Wolf, P.; Murer, K.; de Bruin, E.D. Cognitive and Cognitive-Motor Interventions Affecting Physical Functioning: A Systematic Review. BMC Geriatr. 2011, 11, 29. [Google Scholar] [CrossRef]
- Scherder, E.; Eggermont, L.; Visscher, C.; Scheltens, P.; Swaab, D. Understanding Higher Level Gait Disturbances in Mild Dementia in Order to Improve Rehabilitation: ‘Last in–First Out’. Neurosci. Biobehav. Rev. 2011, 35, 699–714. [Google Scholar] [CrossRef]
- Ginis, P.; Nieuwboer, A.; Dorfman, M.; Ferrari, A.; Gazit, E.; Canning, C.G.; Rocchi, L.; Chiari, L.; Hausdorff, J.M.; Mirelman, A. Feasibility and Effects of Home-Based Smartphone-Delivered Automated Feedback Training for Gait in People with Parkinson’s Disease: A Pilot Randomized Controlled Trial. Park. Relat. Disord. 2016, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Mak, M.K.Y. Balance and Gait Training with Augmented Feedback Improves Balance Confidence in People with Parkinson’s Disease. Neurorehabil. Neural Repair 2014, 28, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Won, S.H.; Kim, J.C.; Oh, D.-W. Effects of a Novel Walking Training Program with Postural Correction and Visual Feedback on Walking Function in Patients with Post-Stroke Hemiparesis. J. Phys. Ther. Sci. 2015, 27, 2581–2583. [Google Scholar] [CrossRef] [PubMed]
- Shaw, F.E. Multifactorial Intervention after a Fall in Older People with Cognitive Impairment and Dementia Presenting to the Accident and Emergency Department: Randomised Controlled Trial. BMJ 2003, 326, 73. [Google Scholar] [CrossRef]
- Speechley, M. Unintentional Falls in Older Adults: A Methodological Historical Review. Can. J. Aging 2011, 30, 21–32. [Google Scholar] [CrossRef]


| Day 1 | Day 3 | Day 8 | Day 62 | Day 65 |
|---|---|---|---|---|
| Report about the study, signing of consent forms (legal guardian), and anamnesis. | Initial evaluation for each study (application of the tests). | Starting of treatment in the experimental group (first session). | Last treatment session in the experimental group. | Final evaluation for each study (re-test). |
| About 60 min. CG and EG. | About 60 min. CG and EG. | 16 sessions in total (twice per week); 30 min. per session. EG. | EG | CG and EG. |
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | EG | CG | CG | CG | EG | EG | CG | EG | CG | EG | CG | CG | EG | CG | EG |
| Genre | M | W | W | M | W | W | M | W | W | W | W | M | W | W | W |
| Subgroup | SS | SS | SS | MS | SS | MS | MS | MS | MS | SS | SS | MS | SS | SS | MS |
| Dominance | RH | RH | RH | RH | RH | RH | RH | RH | RH | RH | RH | RH | RH | RH | RH |
| Level of education | BS | BS | BS | SS | SS | PE | BS | PE | PE | BS | BS | PE | BS | BS | PE |
| Sleeping hours | 11 | 9 | 11 | 9 | 8 | 9 | 9 | 8.5 | 9 | 7 | 9 | 9 | 8 | 10 | 9 |
| Weight (kg) | 54 | 73.5 | 71 | 70 | 80.5 | 79 | 80 | 57 | 69.6 | 62.4 | 83.2 | 103.4 | 60.7 | 69 | 56.6 |
| Age | 62 | 79 | 82 | 83 | 81 | 80 | 79 | 79 | 77 | 79 | 66 | 82 | 82 | 76 | 74 |
| Other diseases | Pk DM | O | Pk | Sk DM | Arth | Sk DM | PD DM | Arth DM | Pk | Arth | Arth DM | Pk | O | Arth | Sk |
| Drug treatment for Alzheimer’s | Me | Me | D | R | D | R | R | R | R | D | Me | R | Me | Me | R |
| Dependent Variables (Scales and Test) | Statistical Tests | p |
|---|---|---|
| Age | Mann–Whitney U test | 0.463 |
| GDS | Student’s s-test | 0.216 |
| MEC | Student’s s-test | 0.267 |
| Berg scale | Mann–Whitney U test | 0.397 |
| Tinetti scale | Mann–Whitney U test | 0.072 |
| Timed up and Go Test | Mann–Whitney U test | 0.867 |
| Oddball(Accuracy) | Mann–Whitney U test | 0.613 |
| Oddball (Reaction time) | Mann–Whitney U test | 0.189 |
| ANT (Alert) | Welch’s t-test | 0.059 |
| ANT (Orienting) | Welch’s t-test | 0.158 |
| ANT (Executive) | Welch’s t-test | 0.970 |
| Dependent Variables (Scales, etc.) | Groups | Mean-SD or Median-Interquartile Range (Indicated by *) | Contrast Test (p < 0.05) | Interaction between Groups (p < 0.05) | Effect Size |
|---|---|---|---|---|---|
| GDS (test) | CG | 4.25 ± 1.03 | |||
| EG | 3.50 ± 1.04 | 0.347 | 0.68 | ||
| GDS (re-test) | CG | 4.38 ± 1.30 | 0.598 | ||
| EG | 3.83 ± 1.32 | 0.172 | |||
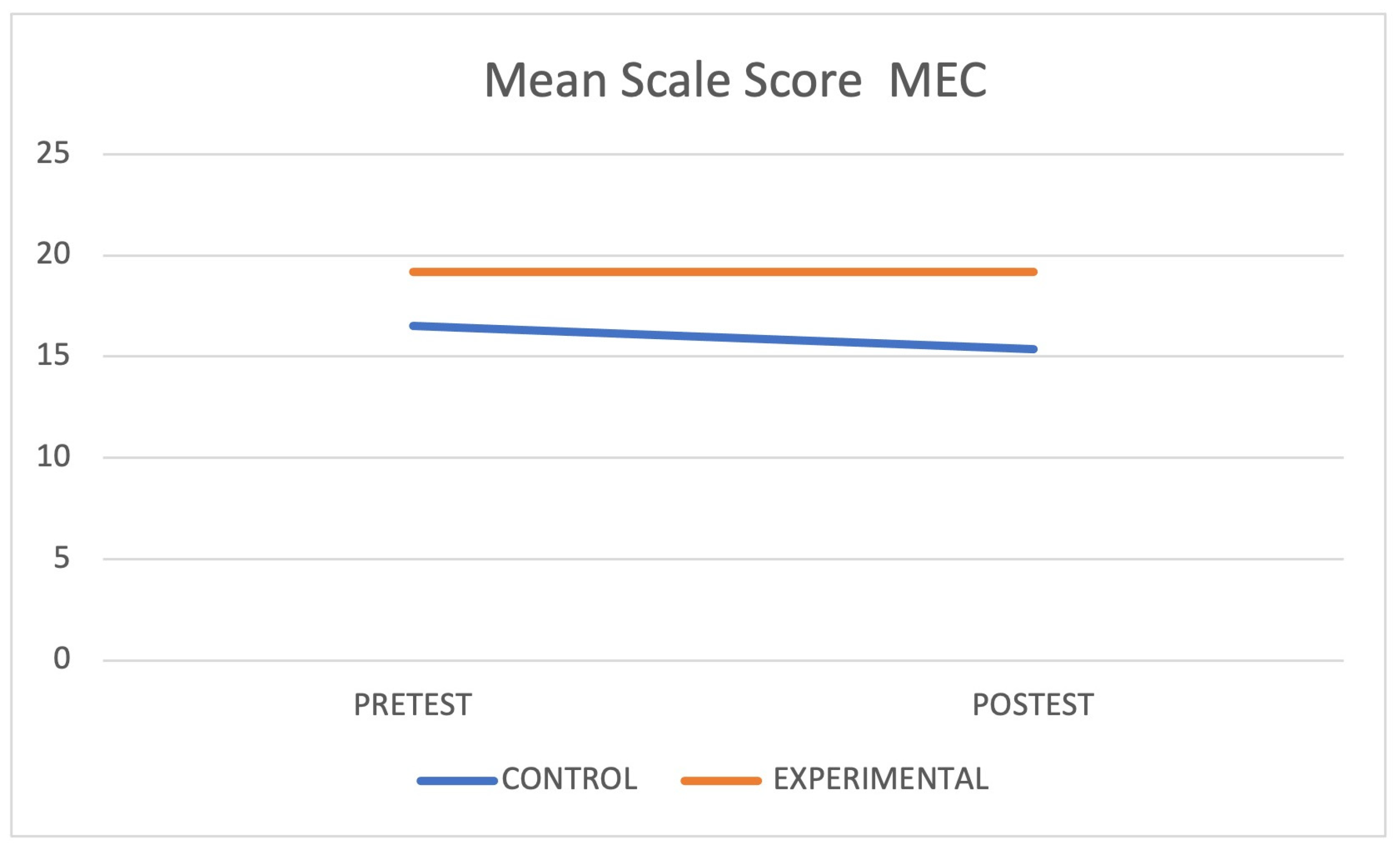
| MEC (test) | CG | 16.50 ± 5.29 | |||
| EG | 19.17 ± 4.70 | 0.029 | 0.14 | ||
| MEC (re-test) | CG | 15.38 ± 5.31 | 0.026 | ||
| EG | 19.17 ± 7.02 | 0.675 | |||
| Berg Scale (test) | CG | 43 ± 8.5 | |||
| EG | 47.14 ± 6.09 | 0.105 | 0.76 | ||
| Berg Scale (re-test) | CG | 34 ± 10.43 | 0.070 | ||
| EG | 49.47 ± 5.99 | 0.248 | |||
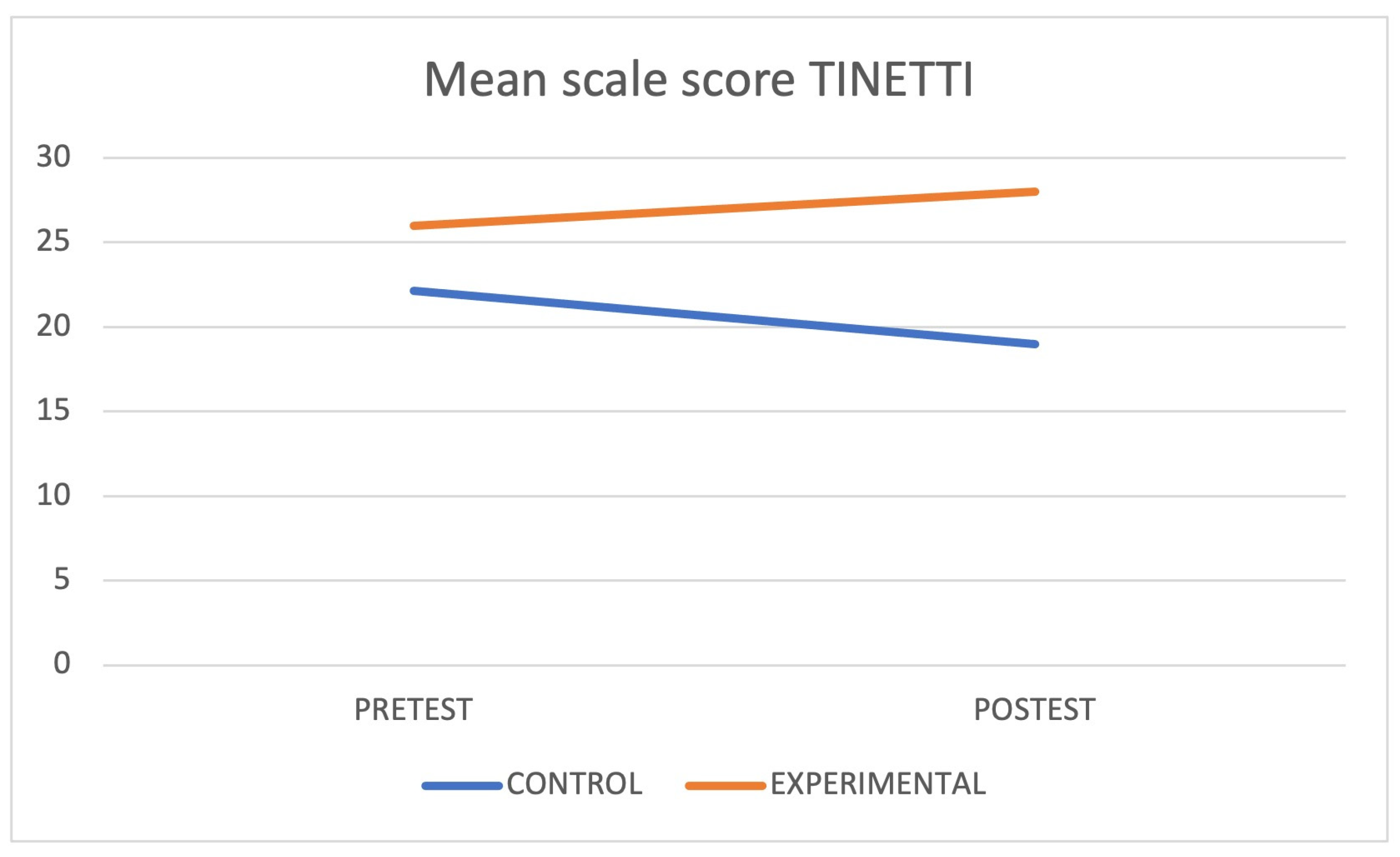
| Tinetti (test) | CG | 22.13 ± 2.74 | |||
| EG | 26 ± 4 * | 0.029 | 0.68 | ||
| Tinetti (re-test) | CG | 19 ± 3.92 | 0.010 | ||
| EG | 28 ± 2 * | 0.068 | |||
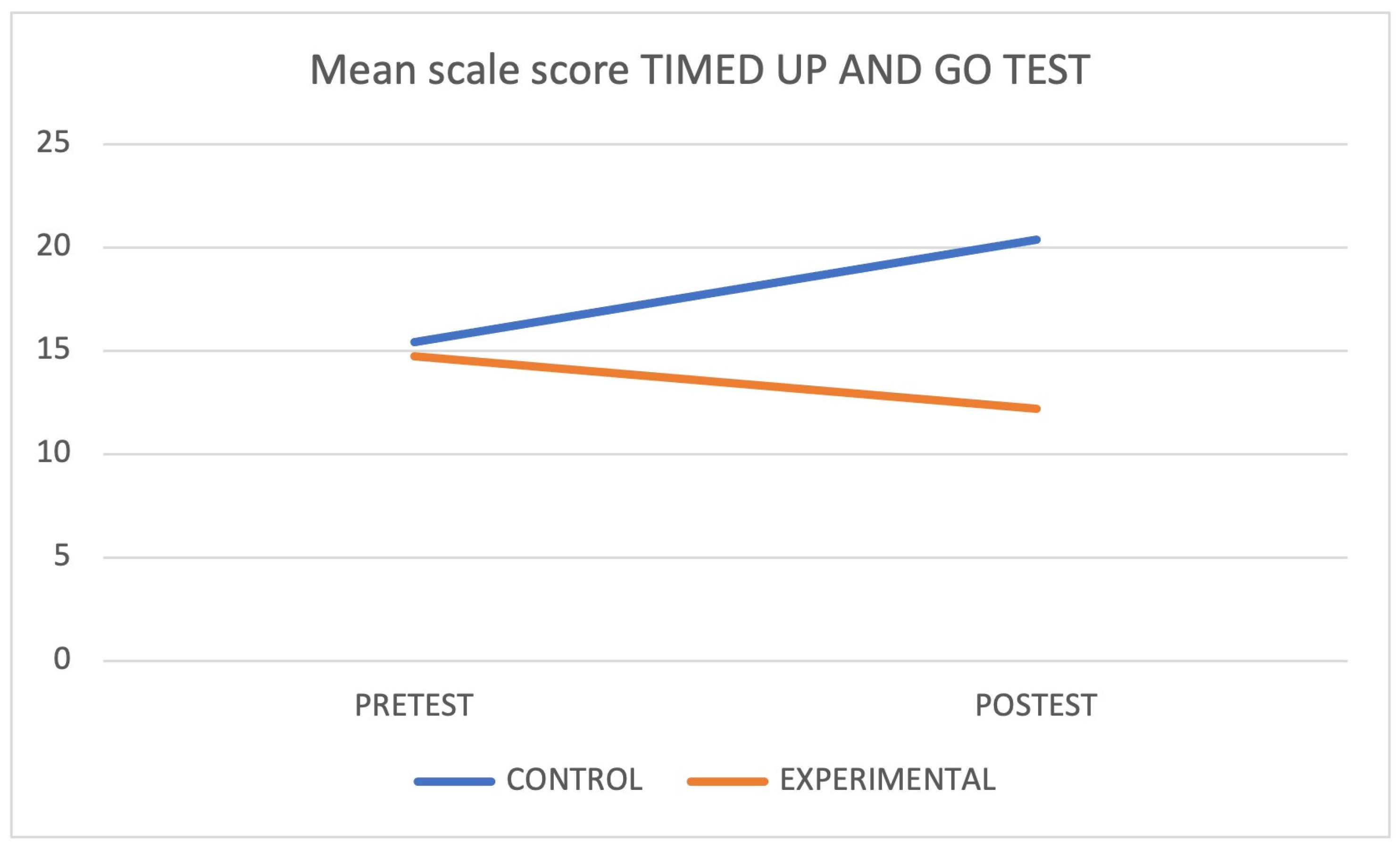
| Timed Up and Go (test) | CG | 15.42 ± 17.76 * | |||
| EG | 14.73 ± 2.94 | 0.141 | 0.65 | ||
| Timed Up and Go (re-test) | CG | 20.39 ± 6.38 | 0.401 | ||
| EG | 12.69 ± 2.73 | 0.020 | |||
| Oddball—Accuracy (test) | CG | 83.25 ± 20.87 * | |||
| EG | 85.75 ± 7.85 | 0.159 | 0.60 | ||
| Oddball—Accuracy (re-test) | CG | 84.50 ± 11.15 | 0.401 | ||
| EG | 84.75 ± 8.51 | 0.837 | |||
| Oddball—Reaction Time (test) | CG | 401.25 ± 149 * | |||
| EG | 436.50 ± 111 * | 0.242 | 0.41 | ||
| Oddball—Reaction Time (re-test) | CG | 395 ± 89.75 * | 0.401 | ||
| EG | 402.32 ± 80.09 | 0.499 | |||
| ANT—alert (test) | CG | −85.60 ± 192.87 | |||
| EG | 49.66 ± 116.49 | 0.161 | 0.59 | ||
| ANT—alert (re-test) | CG | −88.20 ± 67.67 | 0.726 | ||
| EG | −55.66 ± 61.33 | 0.118 | |||
| ANT—orienting (test) | CG | 90.60 ± 196.07 | |||
| EG | −3.5 ± 72.85 | 0.231 | 0.48 | ||
| ANT—orienting (re-test) | CG | 116.60 ± 121.80 | 0.459 | ||
| EG | −22.16 ± 136.16 | 0.777 | |||
| ANT—executive (test) | CG | 85.20 ± 54.78 | |||
| EG | 61.66 ± 36.36 | 0.04 | 1.19 | ||
| ANT—executive (re-test) | CG | 106.80 ± 151.22 | 0.374 | ||
| EG | −60.66 ± 120.93 | 0.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benitez-Lugo, M.-L.; Vazquez-Marrufo, M.; Pinero-Pinto, E.; Chamorro-Moriana, G.; Perez-Cabezas, V.; Suarez-Serrano, C. Analysis of Physical–Cognitive Tasks Including Feedback-Based Technology for Alzheimer’s Disorder in a Randomized Experimental Pilot Study. J. Clin. Med. 2023, 12, 5484. https://doi.org/10.3390/jcm12175484
Benitez-Lugo M-L, Vazquez-Marrufo M, Pinero-Pinto E, Chamorro-Moriana G, Perez-Cabezas V, Suarez-Serrano C. Analysis of Physical–Cognitive Tasks Including Feedback-Based Technology for Alzheimer’s Disorder in a Randomized Experimental Pilot Study. Journal of Clinical Medicine. 2023; 12(17):5484. https://doi.org/10.3390/jcm12175484
Chicago/Turabian StyleBenitez-Lugo, Maria-Luisa, Manuel Vazquez-Marrufo, Elena Pinero-Pinto, Gema Chamorro-Moriana, Veronica Perez-Cabezas, and Carmen Suarez-Serrano. 2023. "Analysis of Physical–Cognitive Tasks Including Feedback-Based Technology for Alzheimer’s Disorder in a Randomized Experimental Pilot Study" Journal of Clinical Medicine 12, no. 17: 5484. https://doi.org/10.3390/jcm12175484
APA StyleBenitez-Lugo, M.-L., Vazquez-Marrufo, M., Pinero-Pinto, E., Chamorro-Moriana, G., Perez-Cabezas, V., & Suarez-Serrano, C. (2023). Analysis of Physical–Cognitive Tasks Including Feedback-Based Technology for Alzheimer’s Disorder in a Randomized Experimental Pilot Study. Journal of Clinical Medicine, 12(17), 5484. https://doi.org/10.3390/jcm12175484








