Differences in Associated Factors of Sedentary Behavior by Diabetes Mellitus Status: A Nationwide Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables
2.3. Statistical Analysis
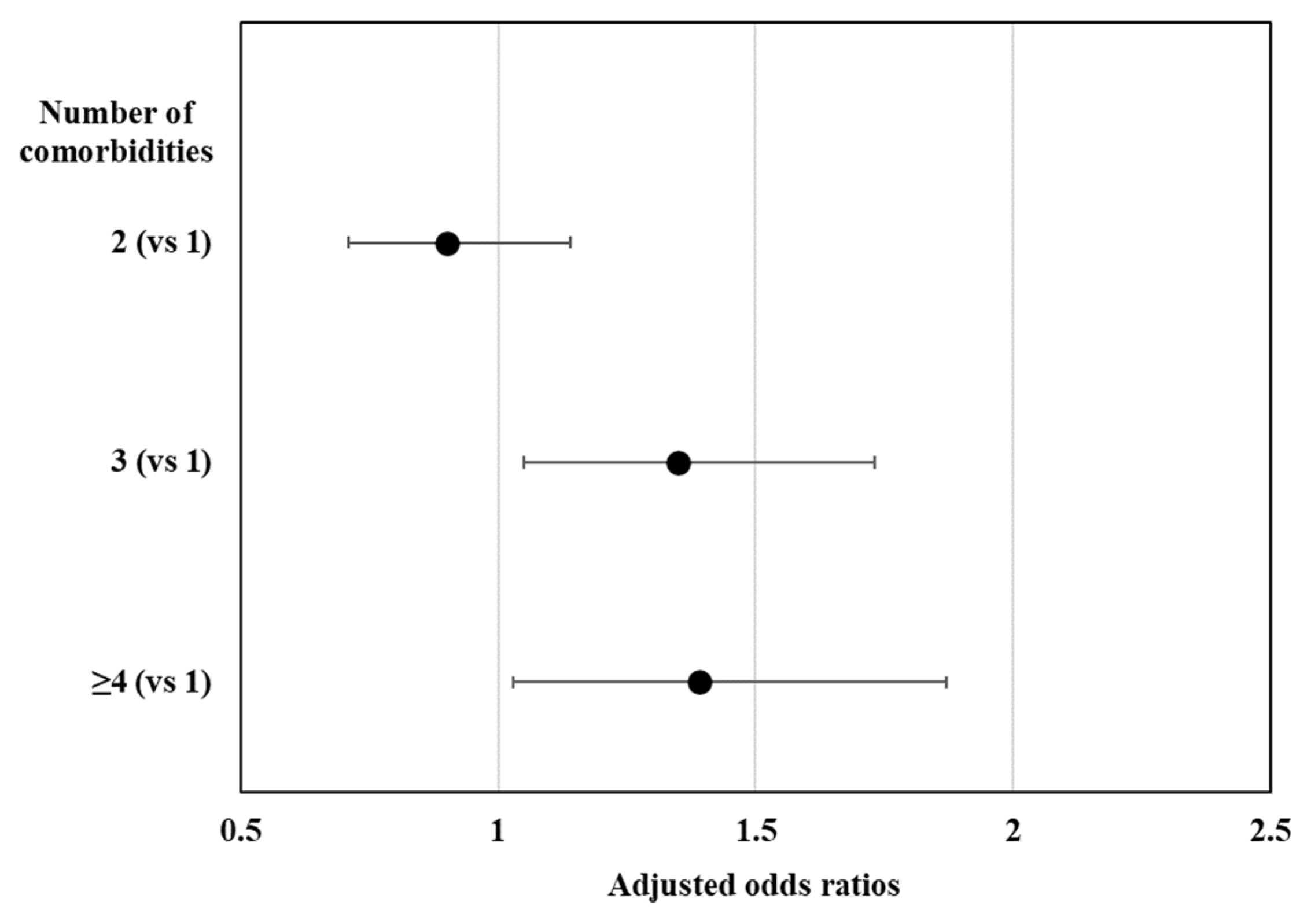
3. Results
3.1. Participant Characteristics
3.2. Factors Associated with Sedentary Time by Diabetes Mellitus Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Participants, S.T.C.P. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Jing, Y.; Chen, J.; Wu, Y.; Wan, Y. Recent trends in sedentary time: A systematic literature review. Healthcare 2021, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Sng, E. The effects of objectively measured sedentary behavior on all-cause mortality in a national sample of adults with diabetes. Prev. Med. 2016, 86, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.C.; Owen, N.; Biddle, S.J.; Dunstan, D.W. Managing sedentary behavior to reduce the risk of diabetes and cardiovascular disease. Curr. Diabetes Rep. 2014, 14, 522. [Google Scholar] [CrossRef]
- Balducci, S.; D’Errico, V.; Haxhi, J.; Sacchetti, M.; Orlando, G.; Cardelli, P.; Vitale, M.; Bollanti, L.; Conti, F.; Zanuso, S.; et al. Effect of a behavioral intervention strategy on sustained change in physical activity and sedentary behavior in patients with type 2 diabetes: The IDES_2 Randomized Clinical Trial. JAMA 2019, 321, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Rockette-Wagner, B.; Edelstein, S.; Venditti, E.M.; Reddy, D.; Bray, G.A.; Carrion-Petersen, M.L.; Dabelea, D.; Delahanty, L.M.; Florez, H.; Franks, P.W.; et al. The impact of lifestyle intervention on sedentary time in individuals at high risk of diabetes. Diabetologia 2015, 58, 1198–1202. [Google Scholar] [CrossRef]
- Power, C.; Pinto Pereira, S.M.; Law, C.; Ki, M. Obesity and risk factors for cardiovascular disease and type 2 diabetes: Investigating the role of physical activity and sedentary behaviour in mid-life in the 1958 British cohort. Atherosclerosis 2014, 233, 363–369. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, Q.; Zhang, D.; Qin, P.; Li, Q.; Tian, G.; Liu, D.; Chen, X.; Liu, L.; Liu, F.; et al. Association of total sedentary behaviour and television viewing with risk of overweight/obesity, type 2 diabetes and hypertension: A dose-response meta-analysis. Diabetes Obes. Metab. 2020, 22, 79–90. [Google Scholar] [CrossRef]
- Indelicato, L.; Dauriz, M.; Bacchi, E.; Dona, S.; Santi, L.; Negri, C.; Cacciatori, V.; Bonora, E.; Nouwen, A.; Moghetti, P. Sex differences in the association of psychological status with measures of physical activity and sedentary behaviour in adults with type 2 diabetes. Acta Diabetol. 2018, 55, 627–635. [Google Scholar] [CrossRef]
- Loprinzi, P.D. Accelerometer-determined sedentary and physical activity estimates among older adults with diabetes: Considerations by demographic and comorbidity characteristics. J. Aging Phys. Act. 2014, 22, 432–440. [Google Scholar] [CrossRef]
- Falconer, C.L.; Cooper, A.R.; Walhin, J.P.; Thompson, D.; Page, A.S.; Peters, T.J.; Montgomery, A.A.; Sharp, D.J.; Dayan, C.M.; Andrews, R.C. Sedentary time and markers of inflammation in people with newly diagnosed type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.F.; Buck, C.; Freiberger, E.; Murphy, M.; Brug, J.; Cardon, G.; O’Donoghue, G.; Pigeot, I.; Oppert, J.M. Systematic literature review of determinants of sedentary behaviour in older adults: A DEDIPAC study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- van der Berg, J.D.; Bosma, H.; Caserotti, P.; Eiriksdottir, G.; Arnardottir, N.Y.; Martin, K.R.; Brychta, R.J.; Chen, K.Y.; Sveinsson, T.; Johannsson, E.; et al. Midlife determinants associated with sedentary behavior in old age. Med. Sci. Sports Exerc. 2014, 46, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Compernolle, S.; Cerin, E.; Barnett, A.; Zhang, C.J.P.; Van Cauwenberg, J.; Van Dyck, D. The role of socio-demographic factors and physical functioning in the intra- and interpersonal variability of older adults’ sedentary time: An observational two-country study. BMC Geriatr. 2022, 22, 495. [Google Scholar] [CrossRef]
- Oh, K.; Kim, Y.; Kweon, S.; Kim, S.; Yun, S.; Park, S.; Lee, Y.K.; Kim, Y.; Park, O.; Jeong, E.K. Korea National Health and Nutrition Examination Survey, 20th anniversary: Accomplishments and future directions. Epidemiol. Health 2021, 43, e2021025. [Google Scholar] [CrossRef]
- World Health Organization. Global Physical Activity Questionnaire (GPAQ) Analysis Guide. Available online: https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/physical-activity-surveillance (accessed on 19 June 2023).
- Lee, J.; Lee, C.; Min, J.; Kang, D.W.; Kim, J.Y.; Yang, H.I.; Park, J.; Lee, M.K.; Lee, M.Y.; Park, I.; et al. Development of the Korean Global Physical Activity Questionnaire: Reliability and validity study. Glob. Health Promot. 2020, 27, 44–55. [Google Scholar] [CrossRef]
- Bull, F.C.; Maslin, T.S.; Armstrong, T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys Act Health 2009, 6, 790–804. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 19 June 2023).
- Choi, Y.A.; Lee, J.S.; Park, J.H.; Kim, Y.H. Patterns of physical activity and sedentary behavior and their associated factors among nondisabled stroke survivors. Maturitas 2022, 158, 10–15. [Google Scholar] [CrossRef]
- World Health Organization; Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating positive health behaviors and well-being to improve health outcomes: Standards of care in diabetes-2023. Diabetes Care 2023, 46, S68–S96. [Google Scholar] [CrossRef]
- AC, B.L.; Ferrari, G.; Fisberg, R.M.; Kovalskys, I.; Gomez, G.; Cortes, L.Y.; Yepez Garcia, M.C.; Herrera-Cuenca, M.; Rigotti, A.; Liria-Dominguez, M.R.; et al. Co-occurrence and clustering of sedentary behaviors, diet, sugar-sweetened beverages, and alcohol intake among adolescents and adults: The Latin American Nutrition and Health Study (ELANS). Nutrients 2021, 13, 1809. [Google Scholar] [CrossRef]
- Nooijen, C.F.J.; Moller, J.; Forsell, Y.; Ekblom, M.; Galanti, M.R.; Engstrom, K. Do unfavourable alcohol, smoking, nutrition and physical activity predict sustained leisure time sedentary behaviour? A population-based cohort study. Prev. Med. 2017, 101, 23–27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 19 June 2023).
- Ahmed, A.T.; Karter, A.J.; Warton, E.M.; Doan, J.U.; Weisner, C.M. The relationship between alcohol consumption and glycemic control among patients with diabetes: The Kaiser Permanente Northern California Diabetes Registry. J. Gen. Intern. Med. 2008, 23, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Wilmot, E.G.; Edwardson, C.L.; Achana, F.A.; Davies, M.J.; Gorely, T.; Gray, L.J.; Khunti, K.; Yates, T.; Biddle, S.J. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia 2012, 55, 2895–2905. [Google Scholar] [CrossRef]
- Sardinha, L.B.; Magalhaes, J.P.; Santos, D.A.; Judice, P.B. Sedentary patterns, physical activity, and cardiorespiratory fitness in association to glycemic control in type 2 diabetes patients. Front. Physiol. 2017, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, L.B.; Andersen, L.B.; Anderssen, S.A.; Quiterio, A.L.; Ornelas, R.; Froberg, K.; Riddoch, C.J.; Ekelund, U. Objectively measured time spent sedentary is associated with insulin resistance independent of overall and central body fat in 9- to 10-year-old Portuguese children. Diabetes Care 2008, 31, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Brown, W.J.; Steene-Johannessen, J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.E.; Lee, I.M. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850,060 participants. Br. J. Sports Med. 2019, 53, 886–894. [Google Scholar] [CrossRef]
- Paudel, S.; Ahmadi, M.; Phongsavan, P.; Hamer, M.; Stamatakis, E. Do associations of physical activity and sedentary behaviour with cardiovascular disease and mortality differ across socioeconomic groups? A prospective analysis of device-measured and self-reported UK Biobank data. Br. J. Sports Med. 2023, 57, 921–929. [Google Scholar] [CrossRef]
- Lawton, J.; Ahmad, N.; Hanna, L.; Douglas, M.; Hallowell, N. ‘I can’t do any serious exercise’: Barriers to physical activity amongst people of Pakistani and Indian origin with Type 2 diabetes. Health Educ. Res. 2006, 21, 43–54. [Google Scholar] [CrossRef]
- Silveira, E.A.; Mendonca, C.R.; Delpino, F.M.; Elias Souza, G.V.; Pereira de Souza Rosa, L.; de Oliveira, C.; Noll, M. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2022, 50, 63–73. [Google Scholar] [CrossRef]
- Balducci, S.; D’Errico, V.; Haxhi, J.; Sacchetti, M.; Orlando, G.; Cardelli, P.; Di Biase, N.; Bollanti, L.; Conti, F.; Zanuso, S.; et al. Level and correlates of physical activity and sedentary behavior in patients with type 2 diabetes: A cross-sectional analysis of the Italian Diabetes and Exercise Study_2. PLoS ONE 2017, 12, e0173337. [Google Scholar] [CrossRef]
- Lamb, M.J.E.; Westgate, K.; Brage, S.; Ekelund, U.; Long, G.H.; Griffin, S.J.; Simmons, R.K.; Cooper, A.J.M.; on behalf of the ADDITION-Plus Study Team. Prospective associations between sedentary time, physical activity, fitness and cardiometabolic risk factors in people with type 2 diabetes. Diabetologia 2016, 59, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Wijndaele, K.; Dunstan, D.W.; Shaw, J.E.; Salmon, J.; Zimmet, P.Z.; Owen, N. Objectively measured sedentary time, physical activity, and metabolic risk: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 2008, 31, 369–371. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.W.; Shivgulam, M.E.; Petterson, J.L.; Wu, Y.; Frayne, R.J.; Mekari, S.; Kimmerly, D.S. Habitual sedentary time and stationary time are inversely related to aerobic fitness. Sports Med. Health Sci. 2022, 4, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Emery, C.F.; Madden, D.J.; George, L.K.; Coleman, R.E.; Riddle, M.W.; McKee, D.C.; Reasoner, J.; Williams, R.S. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. J. Gerontol. 1989, 44, M147–M157. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.V.; Tobin, S.Y.; Mifflin, M.G.; Burns, R.D.; Bailey, R.R.; Purcell, S.A.; Melanson, E.L.; Cornier, M.-A.; Halliday, T.M. The effects of an acute bout of aerobic or resistance exercise on nonexercise physical activity. Exerc. Sport Mov. 2023, 1, e00004. [Google Scholar] [CrossRef] [PubMed]
- Domazet, S.L.; Tarp, J.; Thomsen, R.W.; Højlund, K.; Stidsen, J.V.; Brønd, J.C.; Grøntved, A.; Nielsen, J.S. Accelerometer-derived physical activity and sedentary behaviors in individuals with newly diagnosed type 2 diabetes: A cross-sectional study from the Danish nationwide DD2 cohort. Front. Sports Act. Living 2022, 4, 1089579. [Google Scholar] [CrossRef]
- Kaufman, A.; Augustson, E.M.; Patrick, H. Unraveling the relationship between smoking and weight: The role of sedentary behavior. J. Obes. 2012, 2012, 735465. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Gutor, S.; Dikalova, A.E. Pathological mechanisms of cigarette smoking, dietary, and sedentary lifestyle risks in vascular dysfunction: Mitochondria as a common target of risk factors. Pflugers Arch. 2023, 475, 857–866. [Google Scholar] [CrossRef]
- Ke, J.; Li, K.; Ke, T.; Zhong, X.; Zheng, Q.; Wang, Y.; Li, L.; Dai, Y.; Dong, Q.; Ji, B.; et al. Association of sedentary time and carotid atherosclerotic plaques in patients with type 2 diabetes. J. Diabetes 2022, 14, 64–72. [Google Scholar] [CrossRef]
- Deguchi, N.; Kojima, N.; Osuka, Y.; Sasai, H. Factors associated with passive sedentary behavior among community-dwelling older women with and without knee osteoarthritis: The Otassha Study. Int. J. Environ. Res. Public Health 2022, 19, 13765. [Google Scholar] [CrossRef]
- O’Leary, H.; Larkin, L.; Murphy, G.M.; Quinn, K. Relationship between pain and sedentary behavior in rheumatoid arthritis patients: A cross-sectional study. Arthritis Care Res. 2021, 73, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Steultjens, M.; Bell, K.; Hendry, G. The challenges of measuring physical activity and sedentary behaviour in people with rheumatoid arthritis. Rheumatol. Adv. Pract. 2023, 7, rkac101. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.A.M.; O’Brien, C.M.; Kitas, G.D.; Duda, J.L.; Veldhuijzen van Zanten, J.; Metsios, G.S. The behavioural epidemiology of sedentary behaviour in inflammatory arthritis: Where are we, and where do we need to go? Rheumatol. Adv. Pract. 2023, 7, rkac097. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Hermelink, R.; Leitzmann, M.F.; Markozannes, G.; Tsilidis, K.; Pukrop, T.; Berger, F.; Baurecht, H.; Jochem, C. Sedentary behavior and cancer-an umbrella review and meta-analysis. Eur. J. Epidemiol. 2022, 37, 447–460. [Google Scholar] [CrossRef]

| Variables | DM (n = 3900) | Non-DM (n = 13,932) | ||||
|---|---|---|---|---|---|---|
| Short ST | Long ST | p Value | Short ST | Long ST | p Value | |
| Unweighted number (n) | 1797 | 2103 | 7155 | 6777 | ||
| Weighted number (n) | 1,578,182 | 1,836,382 | 6,760,736 | 6,370,523 | ||
| Duration of diabetes (years) | 6.94 ± 0.23 | 8.19 ± 0.22 | <0.001 | |||
| Age groups | <0.001 | <0.001 | ||||
| 50–59 years | 35.06 (1.41) | 29.56 (1.22) | 52.46 (0.77) | 47.58 (0.85) | ||
| 60–69 years | 37.54 (1.32) | 30.28 (1.15) | 30.95 (0.65) | 26.22 (0.67) | ||
| 70–79 years | 23.51 (1.07) | 29.23 (1.07) | 13.62 (0.46) | 18.44 (0.54) | ||
| ≥80 years | 3.89 (0.46) | 10.93 (0.77) | 2.97 (0.22) | 7.76 (0.40) | ||
| Sex | 0.007 | 0.100 | ||||
| Men | 56.00 (1.32) | 50.90 (1.27) | 44.81 (0.61) | 46.41 (0.65) | ||
| Women | 44.00 (1.32) | 49.10 (1.27) | 55.19 (0.61) | 53.59 (0.65) | ||
| Education | 0.826 | <0.001 | ||||
| ≤9 years | 58.04 (1.48) | 58.47 (1.32) | 48.64 (0.87) | 43.51 (0.91) | ||
| >9 years | 41.96 (1.48) | 41.53 (1.32) | 51.36 (0.87) | 56.49 (0.91) | ||
| Occupation | <0.001 | <0.001 | ||||
| Employed | 59.25 (1.36) | 42.14 (1.27) | 63.06 (0.72) | 53.57 (0.78) | ||
| Unemployed | 40.75 (1.36) | 57.86 (1.27) | 36.94 (0.72) | 46.43 (0.78) | ||
| Marital status | 0.448 | 0.144 | ||||
| Married | 98.37 (0.36) | 97.94 (0.41) | 98.57 (0.17) | 98.21 (0.18) | ||
| Unmarried | 1.63 (0.36) | 2.06 (0.41) | 1.43 (0.17) | 1.79 (0.18) | ||
| Household composition | 0.025 | <0.001 | ||||
| Living with other | 85.41 (0.93) | 82.52 (0.96) | 89.99 (0.43) | 86.85 (0.53) | ||
| Living alone | 14.59 (0.93) | 17.48 (0.96) | 10.01 (0.43) | 13.15 (0.53) | ||
| Household Income | 0.004 | <0.001 | ||||
| Low | 29.59 (1.33) | 35.86 (1.37) | 20.47 (0.66) | 23.79 (0.74) | ||
| Lower-middle | 27.49 (1.24) | 25.62 (1.15) | 26.96 (0.72) | 22.93 (0.72) | ||
| Upper-middle | 22.64 (1.26) | 18.81 (1.03) | 26.40 (0.71) | 22.53 (0.72) | ||
| High | 20.29 (1.24) | 19.71 (1.14) | 26.16 (0.83) | 30.75 (0.92) | ||
| Residence | <0.001 | <0.001 | ||||
| Rural | 25.45 (1.96) | 17.82 (1.47) | 22.16 (1.42) | 16.30 (1.20) | ||
| Urban | 74.55 (1.96) | 82.18 (1.47) | 77.84 (1.42) | 83.70 (1.20) | ||
| Alcohol | 0.599 | 0.789 | ||||
| Non-excessive | 86.58 (0.98) | 85.80 (1.02) | 87.45 (0.49) | 87.63 (0.49) | ||
| Excessive | 13.42 (0.98) | 14.20 (1.02) | 12.55 (0.49) | 12.37 (0.49) | ||
| Smoking | 0.660 | 0.012 | ||||
| Never | 51.17 (1.36) | 51.99 (1.27) | 60.49 (0.65) | 57.43 (0.68) | ||
| Past | 30.72 (1.25) | 29.16 (1.21) | 24.36 (0.58) | 26.51 (0.63) | ||
| Current | 18.10 (1.11) | 18.85 (1.11) | 15.16 (0.55) | 16.06 (0.57) | ||
| Aerobic exercise | <0.001 | <0.001 | ||||
| Sufficient | 41.80 (1.38) | 30.94 (1.20) | 46.27 (0.77) | 37.15 (0.73) | ||
| Insufficient | 58.20 (1.38) | 69.06 (1.20) | 53.73 (0.77) | 62.85 (0.73) | ||
| Resistance exercise | 0.378 | 0.143 | ||||
| Sufficient | 18.04 (1.15) | 16.75 (0.98) | 21.61 (0.61) | 20.40 (0.59) | ||
| Insufficient | 81.96 (1.15) | 83.25 (0.98) | 78.39 (0.61) | 79.60 (0.59) | ||
| Obesity | 0.037 | 0.003 | ||||
| Underweight | 0.92 (0.24) | 1.08 (0.27) | 2.47 (0.21) | 2.96 (0.23) | ||
| Normal weight | 27.43 (1.25) | 24.95 (1.09) | 38.12 (0.71) | 35.62 (0.69) | ||
| Overweight | 26.70 (1.17) | 23.80 (1.13) | 26.85 (0.64) | 25.84 (0.64) | ||
| Obese | 44.94 (1.34) | 50.17 (1.28) | 32.56 (0.68) | 35.58 (0.72) | ||
| Presence of comorbidities | ||||||
| Hypertension | 59.82 (1.32) | 66.57 (1.27) | <0.001 | 39.73 (0.69) | 43.67 (0.75) | <0.001 |
| Cardiovascular disease | 10.11 (0.78) | 16.12 (0.90) | <0.001 | 5.32 (0.31) | 7.42 (0.36) | <0.001 |
| Chronic respiratory disease | 29.82 (1.31) | 29.08 (1.17) | 0.669 | 22.53 (0.59) | 23.14 (0.61) | 0.461 |
| Cancer | 7.37 (0.65) | 9.41 (0.78) | 0.044 | 6.67 (0.34) | 8.34 (0.39) | 0.001 |
| Arthritis | 19.64 (1.13) | 26.39 (1.15) | <0.001 | 17.78 (0.50) | 20.91 (0.55) | <0.001 |
| Depression | 4.91 (0.57) | 6.73 (0.62) | 0.033 | 4.99 (0.28) | 5.78 (0.34) | 0.061 |
| Variables | DM (n = 3900) | Non-DM (n = 13,932) | ||
|---|---|---|---|---|
| Unadjusted OR | Adjusted OR * | Unadjusted OR | Adjusted OR * | |
| Alcohol | ||||
| Non-excessive | Reference | Reference | Reference | Reference |
| Excessive | 1.07 (0.84–1.36) | 1.34 (1.02–1.74) | 0.98 (0.87–1.11) | 1.02 (0.89–1.16) |
| Smoking | ||||
| Never | Reference | Reference | Reference | Reference |
| Past | 0.93 (0.79–1.10) | 1.21 (0.91–1.59) | 1.15 (1.04–1.26) | 1.16 (1.01–1.35) |
| Current | 1.02 (0.83–−1.27) | 1.51 (1.11–2.05) | 1.12 (0.99–1.26) | 1.23 (1.05–1.45) |
| Aerobic exercise | ||||
| Sufficient | Reference | Reference | Reference | Reference |
| Insufficient | 1.60 (1.37–1.88) | 1.55 (1.30–1.84) | 1.46 (1.35–1.58) | 1.50 (1.37–1.63) |
| Resistance exercise | ||||
| Sufficient | Reference | Reference | Reference | Reference |
| Insufficient | 1.09 (0.90–1.34) | 0.91 (0.73–1.12) | 1.08 (0.98–1.19) | 1.05 (0.94–1.17) |
| Obesity | ||||
| Underweight | 1.29 (0.62–2.68) | 1.13 (0.51–2.50) | 1.28 (1.01–1.62) | 1.10 (0.86–1.42) |
| Normal weight | Reference | Reference | Reference | Reference |
| Overweight | 0.98 (0.80–1.21) | 1.01 (0.82–1.26) | 1.03 (0.93–1.14) | 1.08 (0.97–1.19) |
| Obese | 1.23 (1.03–1.46) | 1.28 (1.05–1.54) | 1.17 (1.06–1.29) | 1.24 (1.11–1.37) |
| Presence of comorbidities | ||||
| Hypertension | 1.34 (1.15–1.56) | 0.93 (0.78–−1.10) | 1.18 (1.09–1.27) | 0.96 (0.88–1.04) |
| Cardiovascular disease | 1.71 (1.38–2.11) | 1.47 (1.16–1.85) | 1.43 (1.22–1.67) | 1.13 (0.96–1.34) |
| Chronic respiratory disease | 0.96 (0.82–1.14) | 0.95 (0.80–1.14) | 1.04 (0.94–1.14) | 0.98 (0.89–1.09) |
| Cancer | 1.31 (1.01–1.69) | 1.27 (0.97–1.67) | 1.27 (1.10–1.47) | 1.24 (1.06–1.44) |
| Arthritis | 1.47 (1.23–1.75) | 1.28 (1.04–1.56) | 1.22 (1.12–1.34) | 1.15 (1.04–1.27) |
| Depression | 1.40 (1.03–1.91) | 1.25 (0.90–1.73) | 1.17 (0.99–1.37) | 1.13 (0.95–1.34) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, D.K.; Nam, H.S.; Park, M.; Kim, Y.H. Differences in Associated Factors of Sedentary Behavior by Diabetes Mellitus Status: A Nationwide Cross-Sectional Study. J. Clin. Med. 2023, 12, 5453. https://doi.org/10.3390/jcm12175453
Jang DK, Nam HS, Park M, Kim YH. Differences in Associated Factors of Sedentary Behavior by Diabetes Mellitus Status: A Nationwide Cross-Sectional Study. Journal of Clinical Medicine. 2023; 12(17):5453. https://doi.org/10.3390/jcm12175453
Chicago/Turabian StyleJang, Dong Kee, Hyung Seok Nam, Mina Park, and Yeo Hyung Kim. 2023. "Differences in Associated Factors of Sedentary Behavior by Diabetes Mellitus Status: A Nationwide Cross-Sectional Study" Journal of Clinical Medicine 12, no. 17: 5453. https://doi.org/10.3390/jcm12175453
APA StyleJang, D. K., Nam, H. S., Park, M., & Kim, Y. H. (2023). Differences in Associated Factors of Sedentary Behavior by Diabetes Mellitus Status: A Nationwide Cross-Sectional Study. Journal of Clinical Medicine, 12(17), 5453. https://doi.org/10.3390/jcm12175453






