Impact of Age on Endothelial Function of Saphenous Vein Grafts in Coronary Artery Bypass Grafting
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. Harvesting and Collection of the Graft
2.3. Preparation of the Graft
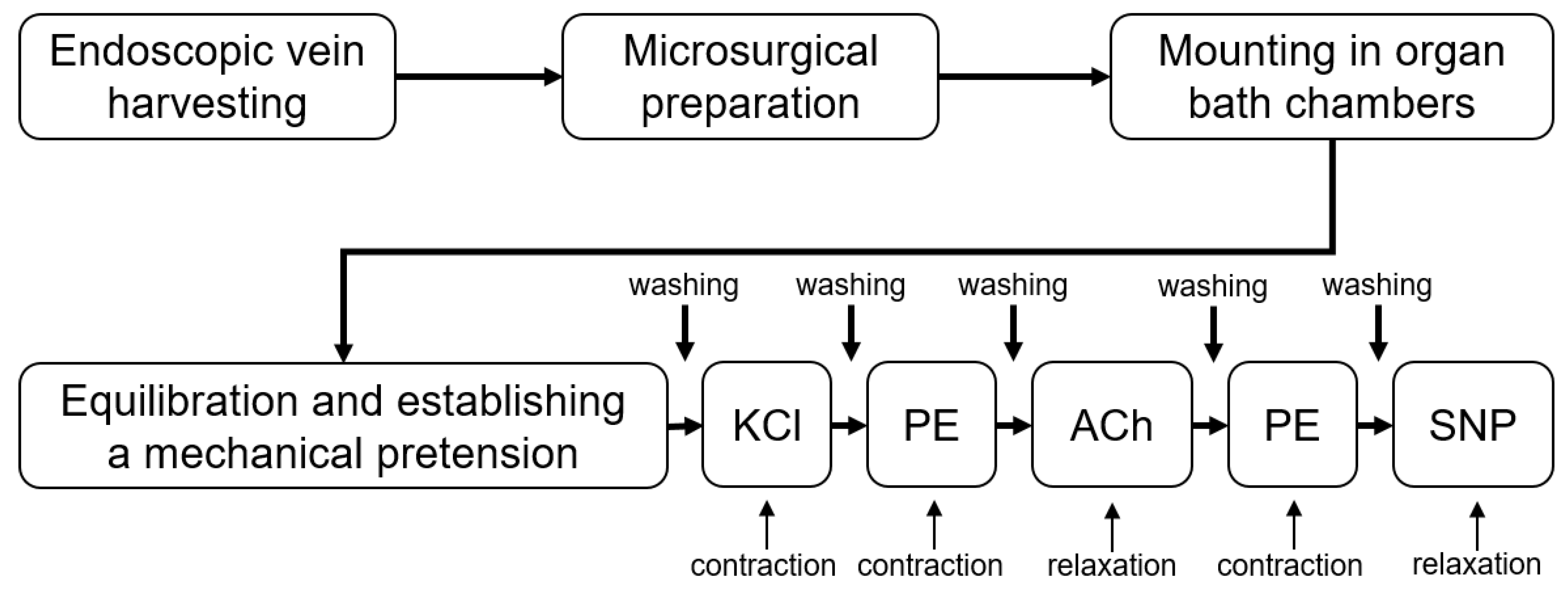
2.4. Functional Assessment
2.5. Statistics
3. Results
3.1. Patient Data Stratified by Age
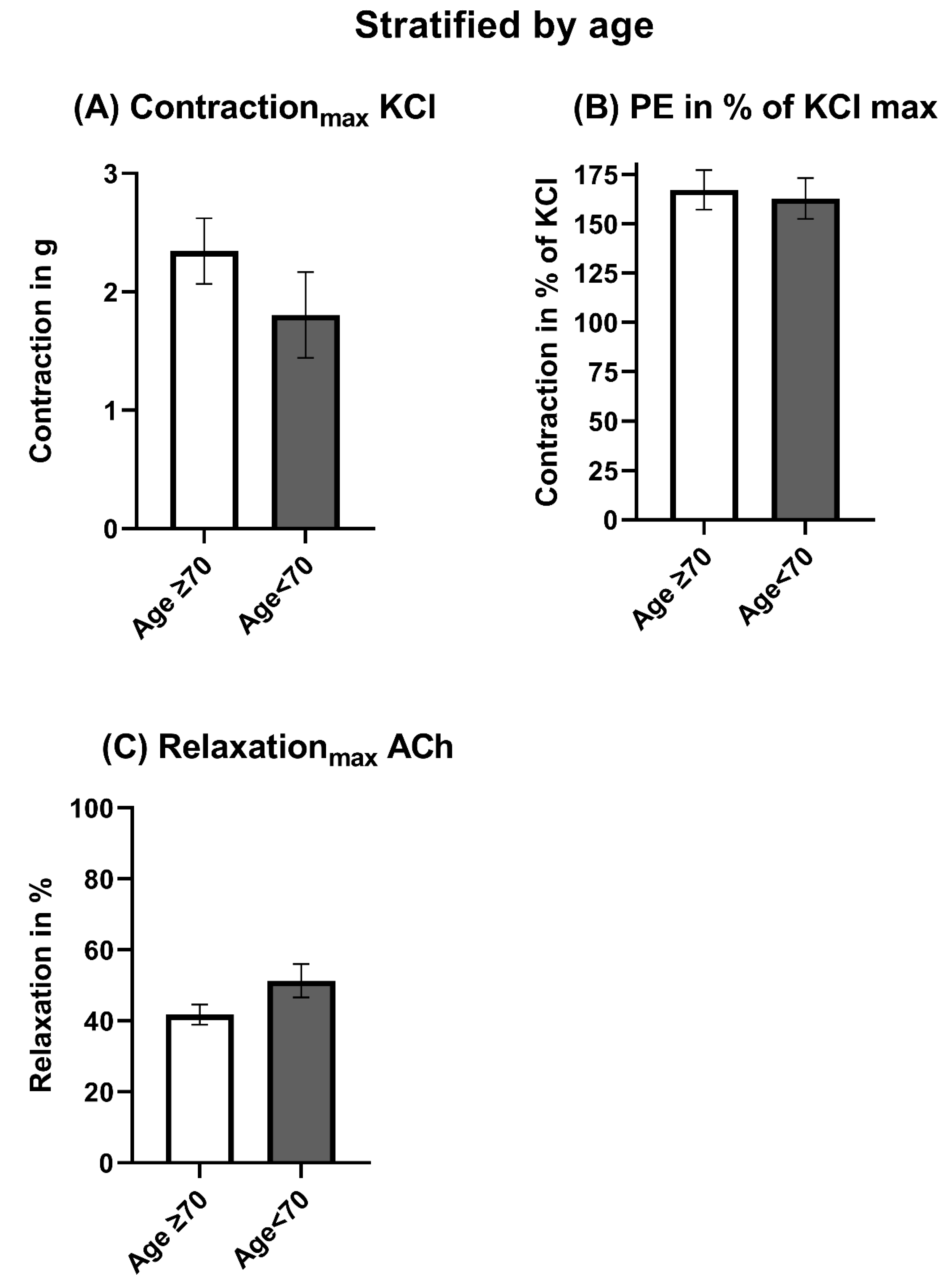
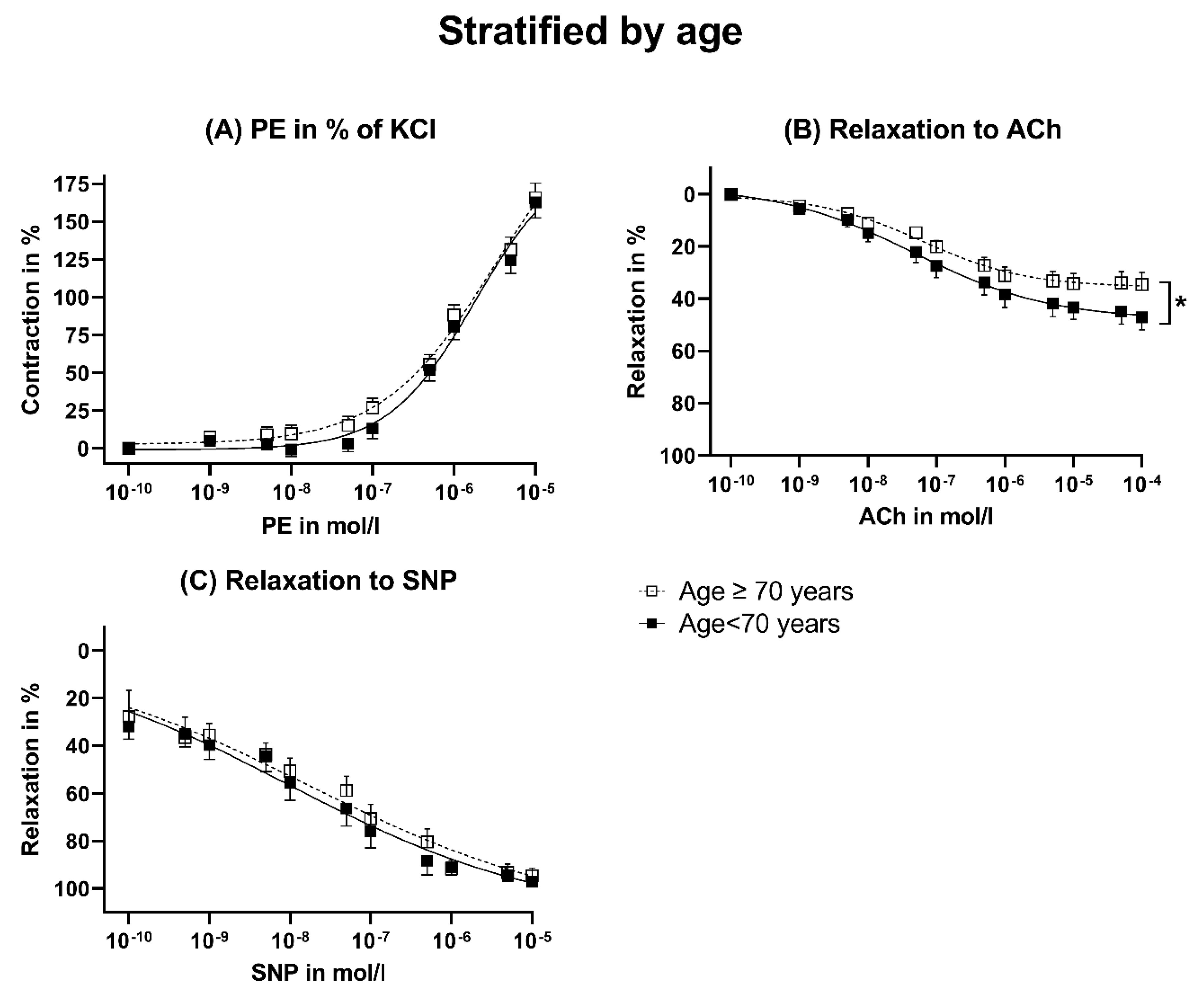
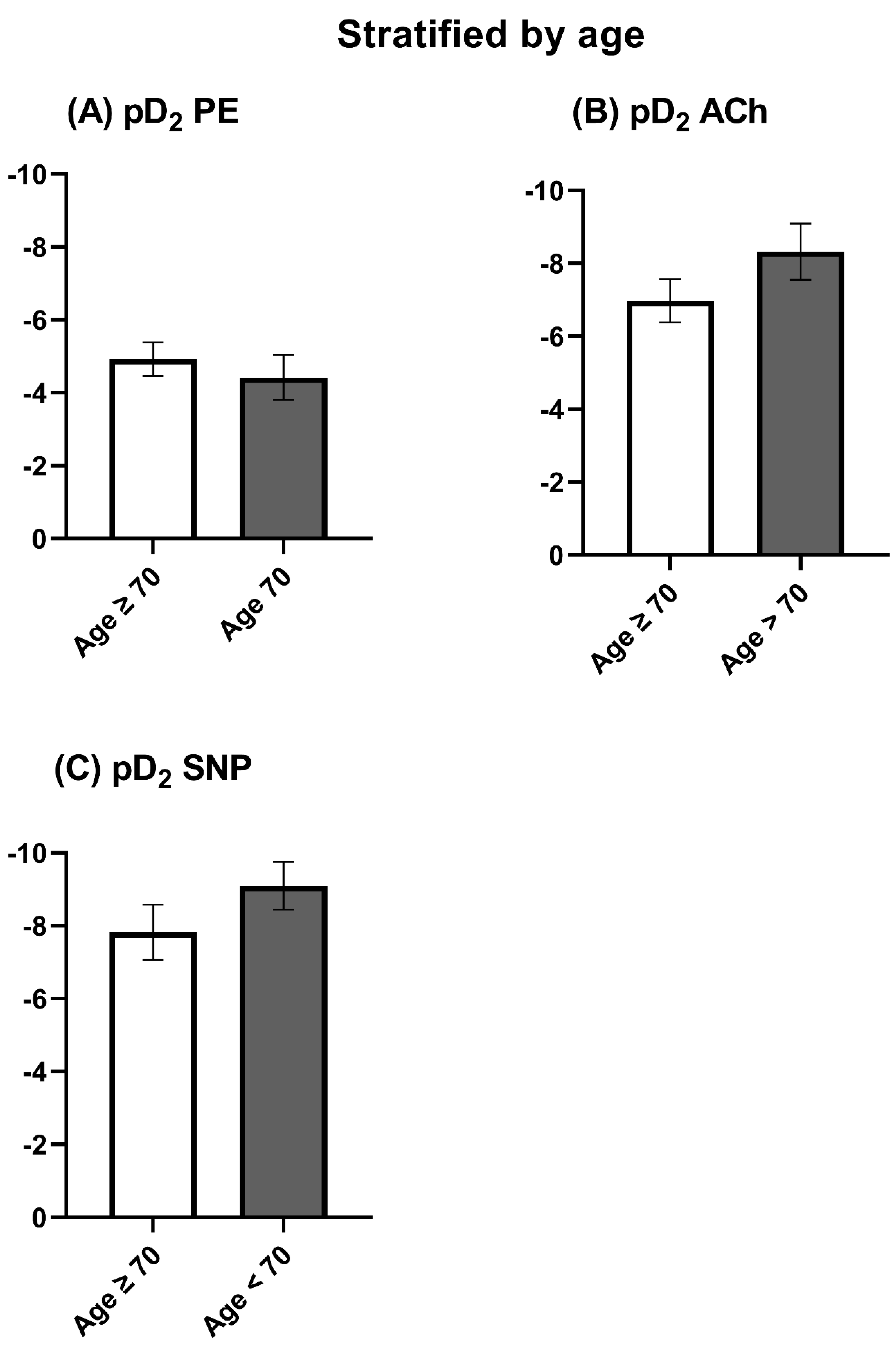
3.2. Vasomotor Function Stratified by Age
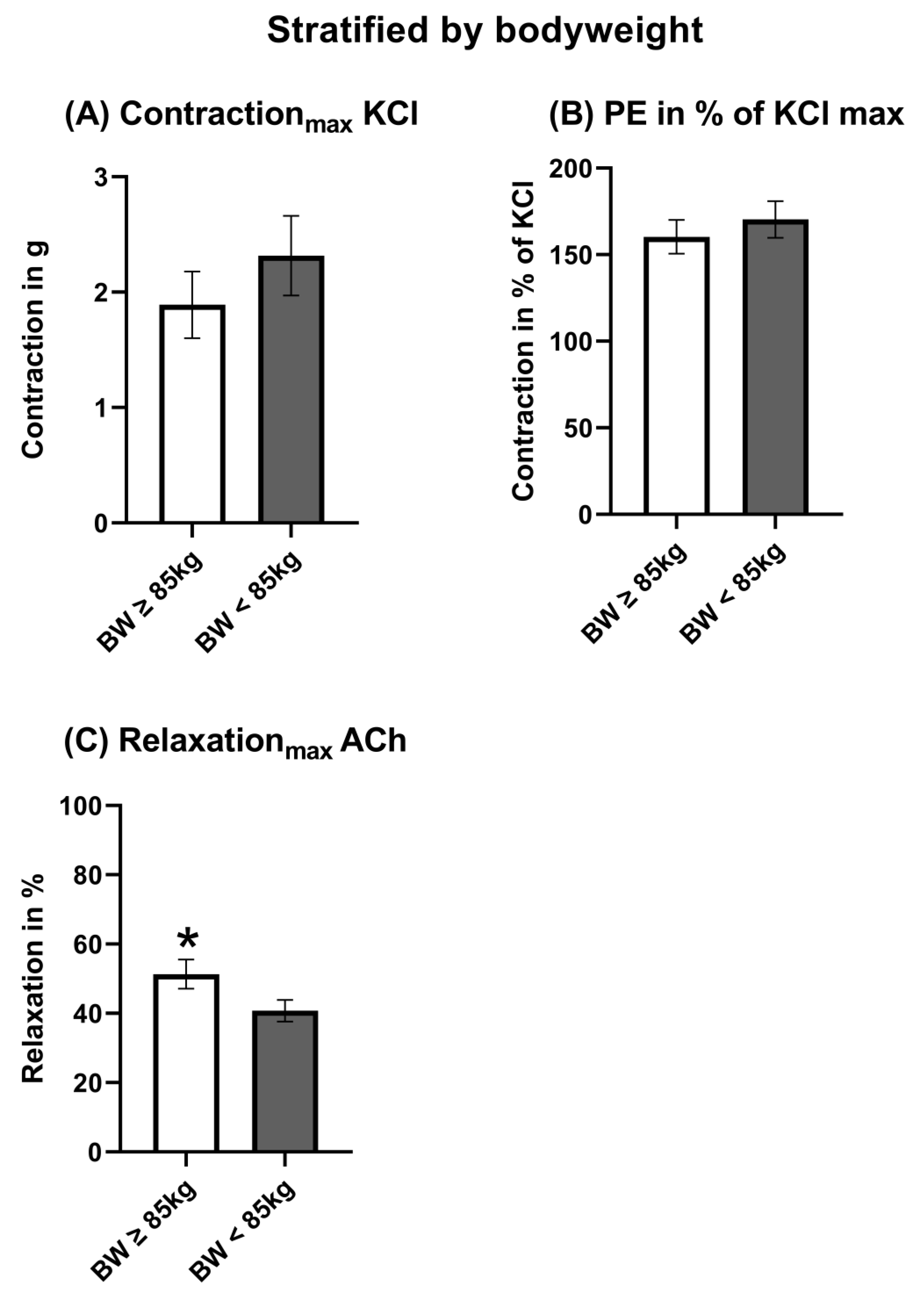
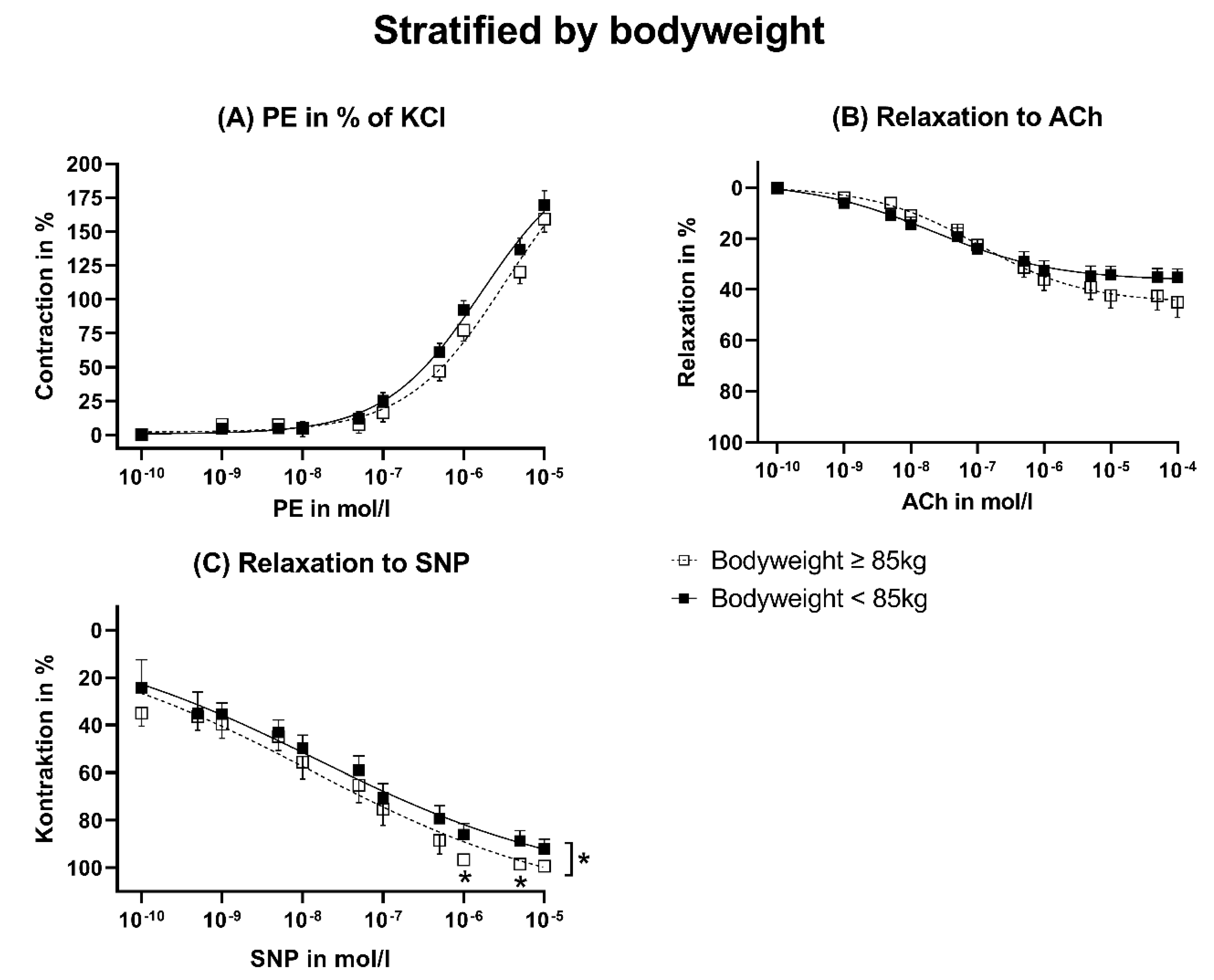
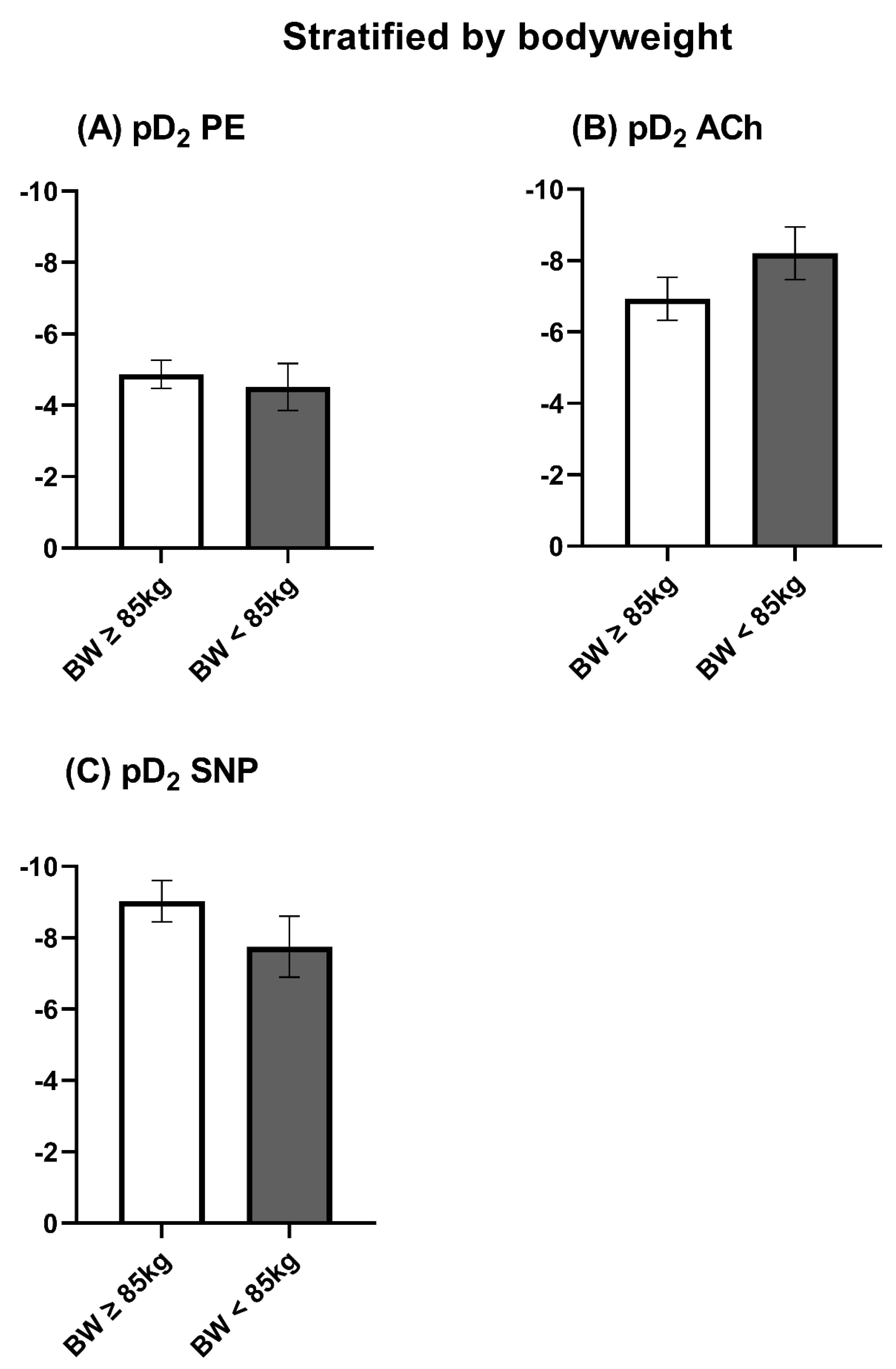
3.3. Stratification by Bodyweight
3.4. Patient Data
3.5. Vasomotor Function Stratified by Bodyweight
4. Discussion
4.1. Relevance of Initial Vascular Function of the Conduit
4.2. Age
4.3. Bodyweight
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AGE | Advanced glycation endproducts |
| CABG | Coronary artery bypass grafting |
| CAD | Coronary artery disease |
| KCl | Potassium chloride |
| KHS | Krebs–Henseleit solution |
| NO | Nitric oxide |
| PE | Phenylephrine |
| SNP | Sodium nitroprusside |
References
- Head, S.J.; Milojevic, M.; Daemen, J.; Ahn, J.-M.; Boersma, E.; Christiansen, E.H.; Domanski, M.J.; Farkouh, M.E.; Flather, M.; Fuster, V.; et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: A pooled analysis of individual patient data. Lancet 2018, 391, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Li, F.D.; Eagle, S.; Brophy, C.; Hocking, K.M.; Osgood, M.; Komalavilas, P.; Cheung-Flynn, J. Pressure control during preparation of saphenous veins. JAMA Surg. 2014, 149, 655–662. [Google Scholar] [CrossRef]
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Blaßfeld, D.; Böning, A. German Heart Surgery Report 2021: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2022, 70, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Steele, R.; Manning, W.J.; Khabbaz, K.R.; Rudski, L.G.; Langlois, Y.; Morin, J.-F.; Picard, M.H. Derivation and Validation of Prognosis-Based Age Cutoffs to Define Elderly in Cardiac Surgery. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, A.; Soto, C.; Salgueiro, L.; Ikegami, H.; Russo, M.J.; Lee, L.Y. The impact of age on outcomes of coronary artery bypass grafting. J. Cardiothorac. Surg. 2020, 15, 158. [Google Scholar] [CrossRef]
- Spertus, J.A.; Nerella, R.; Kettlekamp, R.; House, J.; Marso, S.; Borkon, A.M.; Rumsfeld, J.S. Risk of restenosis and health status outcomes for patients undergoing percutaneous coronary intervention versus coronary artery bypass graft surgery. Circulation 2005, 111, 768–773. [Google Scholar] [CrossRef]
- Caliskan, E.; de Souza, D.R.; Böning, A.; Liakopoulos, O.J.; Choi, Y.-H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.-B.; et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef]
- Reeves, B.C.; Ascione, R.; Chamberlain, M.H.; Angelini, G.D. Effect of body mass index on early outcomes in patients undergoing coronary artery bypass surgery. J. Am. Coll. Cardiol. 2003, 42, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.C.; Moreno, P.; Nabel, E.G.; Hachinski, V.; Fuster, V. Cellular senescence, vascular disease, and aging: Part 2 of a 2-part review: Clinical vascular disease in the elderly. Circulation 2011, 123, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Medici, D.; Shore, E.M.; Lounev, V.Y.; Kaplan, F.S.; Kalluri, R.; Olsen, B.R. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat. Med. 2010, 16, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, S.E. Ageing of the conduit arteries. J. Pathol. 2007, 211, 157–172. [Google Scholar] [CrossRef]
- Krasiński, Z.; Biskupski, P.; Dzieciuchowicz, Ł.; Kaczmarek, E.; Krasińska, B.; Staniszewski, R.; Pawlaczyk, K.; Stanisić, M.; Majewski, P.; Majewski, W. The influence of elastic components of the venous wall on the biomechanical properties of different veins used for arterial reconstruction. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 224–229. [Google Scholar] [CrossRef]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Bucala, R.; Tracey, K.J.; Cerami, A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J. Clin. Investig. 1991, 87, 432–438. [Google Scholar] [CrossRef]
- Kim, J.H.; Bugaj, L.J.; Oh, Y.J.; Bivalacqua, T.J.; Ryoo, S.; Soucy, K.G.; Santhanam, L.; Webb, A.; Camara, A.; Sikka, G.; et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J. Appl. Physiol. (1985) 2009, 107, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Eskurza, I.; Silver, A.E.; Levy, A.S.; Pierce, G.L.; Gates, P.E.; Seals, D.R. Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ. Res. 2007, 100, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, L.P.; Benhassen, L.; Modrau, I.S.; de Paoli, F.; Boedtkjer, E. Increased Contractile Function of Human Saphenous Vein Grafts Harvested by “No-Touch” Technique. Front. Physiol. 2017, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kurazumi, H.; Suzuki, R.; Matsuno, Y.; Mikamo, A.; Hamano, K. Preserving the endothelium in saphenous vein graft with both conventional and no-touch preparation. J. Cardiothorac. Surg. 2020, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- Layton, G.R.; Ladak, S.S.; Abbasciano, R.; McQueen, L.W.; George, S.J.; Murphy, G.J.; Zakkar, M. The Role of Preservation Solutions upon Saphenous Vein Endothelial Integrity and Function: Systematic Review and UK Practice Survey. Cells 2023, 12, 815. [Google Scholar] [CrossRef]
- Tsakok, M.; Montgomery-Taylor, S.; Tsakok, T. Storage of saphenous vein grafts prior to coronary artery bypass grafting: Is autologous whole blood more effective than saline in preserving graft function? Interact. Cardiovasc. Thorac. Surg. 2012, 15, 720–725. [Google Scholar] [CrossRef]
- Toto, F.; Torre, T.; Turchetto, L.; Lo Cicero, V.; Soncin, S.; Klersy, C.; Demertzis, S.; Ferrari, E. Efficacy of Intraoperative Vein Graft Storage Solutions in Preserving Endothelial Cell Integrity during Coronary Artery Bypass Surgery. J. Clin. Med. 2022, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Massad, M.G.; Sileri, P.; Zhang, W.; Jarzembowski, T.; Kpodonu, J.; Geha, A.S.; Benedetti, E. Effects of procurement and preservation media on cellular apoptosis in autologous saphenous vein grafts. J. Appl. Res. Clin. Exp. Ther. 2004, 4, 453–463. [Google Scholar]
- Murphy, G.J.; Johnson, T.W.; Chamberlain, M.H.; Rizvi, S.I.; Wyatt, M.; George, S.J.; Angelini, G.D.; Karsch, K.R.; Oberhoff, M.; Newby, A.C. Short- and long-term effects of cytochalasin D, paclitaxel and rapamycin on wall thickening in experimental porcine vein grafts. Cardiovasc. Res. 2007, 73, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Cheung-Flynn, J.; Song, J.; Voskresensky, I.; Wise, E.S.; Liu, Y.; Xiong, Y.; Eagle, S.S.; Brophy, C.M.; Flynn, C.R. Limiting Injury During Saphenous Vein Graft Preparation for Coronary Arterial Bypass Prevents Metabolic Decompensation. Sci. Rep. 2017, 7, 14179. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.O.; Caputo, M.; Angelini, G.D.; George, S.J.; Zakkar, M. Activation and inflammation of the venous endothelium in vein graft disease. Atherosclerosis 2017, 265, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kulik, A.; Voisine, P.; Mathieu, P.; Masters, R.G.; Mesana, T.G.; Le May, M.R.; Ruel, M. Statin therapy and saphenous vein graft disease after coronary bypass surgery: Analysis from the CASCADE randomized trial. Ann. Thorac. Surg. 2011, 92, 1284–1290, discussion 1290–1291. [Google Scholar] [CrossRef] [PubMed]
- Saemann, L.; Naujoks, P.; Hartrumpf, L.; Pohl, S.; Simm, A.; Szabó, G. Sex-Specific Protection of Endothelial Function after Vascular Ischemia/Reperfusion Injury by the Senomorphic Agent Ruxolitinib. Int. J. Mol. Sci. 2023, 24, 11727. [Google Scholar] [CrossRef]
| Parameter | Category | Age ≥ 70 Years n = 40 (%) | Age < 70 Years n = 33 (%) | p-Value |
|---|---|---|---|---|
| Sex | female (n = 16) | 9 (12.3) | 7 (9.6) | 0.9 |
| male (n = 57) | 31 (42.5) | 26 (35.6) | ||
| Bodyweight in kg | 79.9 ± 2.3 | 91.3 ± 3.0 | 0.003 | |
| ≥85 kg (n = 37) | 17 (23.3) | 20 (27.4) | 0.12 | |
| <85 kg (n = 36) | 23 (31.5) | 13 (17.8) | ||
| BMI | 27.5 ± 0.7 | 30.5 ± 0.7 | 0.89 | |
| Graft characteristics | ||||
| Storage solution | Tiprotec® (n = 21) | 13 (18.1) | 8 (11.1) | 0.34 |
| NaCl-heparin (n = 16) | 8 (11.1) | 8 (11.1) | ||
| Ringer-heparin (n = 13) | 6 (8.3) | 7 (9.7) | ||
| Blood-heparin (n = 6) | 1 (1.4) | 5 (6.9) | ||
| NaCl (n = 11) | 8 (11.1) | 3 (4.2) | ||
| Ringer (n = 5) | 3 (4.2) | 2 (2.8) | ||
| Graft diameter in mm | 3.4 ± 0.2 | 3.1 ± 0.2 | 0.3 | |
| ≥3 mm (n = 28) | 18 (26.5) | 20 (29.4) | 0.97 | |
| <3 mm (n = 38) | 19 (27.9) | 11 (16.1) | ||
| Comorbidities | ||||
| Arterial hypertension | Existent (n= 69) | 38 (52.1) | 31 (42.5) | 0.84 |
| Non-existent (n = 4) | 2 (2.7) | 2 (2.7) | ||
| NYHA-classification | NYHA I (n = 3) | 1 (1.4) | 2 (2.7) | 0.52 |
| NYHA II (n = 13) | 6 (8.2) | 7 (9.5) | ||
| NYHA III (n = 18) | 8 (11.0) | 10 (13.7) | ||
| NYHA IV (n = 2) | 1 (1.4) | 1 (1.4) | ||
| N/A (n = 37) | 24 (32.9) | 13 (17.8) | ||
| LVEF in % | <30% (n = 4) | 3 (4.1) | 1 (1.4) | 0.71 |
| 30–50% (n = 28) | 15 (20.5) | 13 (17.8) | ||
| >50% (n = 41) | 22 (30.1) | 19 (26) | ||
| PAD | Existent (n = 6) | 2 (2.7) | 4 (5.5) | 0.27 |
| Non-existent (n = 67) | 38 (52.0) | 29 (39.7) | ||
| Obesity | Class 1 (n = 21) | 8 (11.0) | 13 (17.8) | 0.36 |
| Class 2 (n= 6) | 3 (4.1) | 3 (4.1) | ||
| Class 3 (n = 1) | 0 (0) | 1 (1.3) | ||
| Non-existent (n = 34) | 20 (27.4) | 14 (19.2) | ||
| Hyperlipoproteinemia | Existent (n = 32) | 19 (26.0) | 13 (17.8) | 0.49 |
| Non-existent (n = 40) | 21 (28.8) | 20 (27.4) | ||
| Chronic kidney insufficiency | Stage 1 (n = 5) | 5 (6.8) | 1 (1.4) | 0.48 |
| Stage 2 (n = 5) | 3 (4.1) | 2 (2.7) | ||
| Stage 3 (n = 15) | 7 (9.6) | 8 (11.0) | ||
| Non-existent (n = 47) | 25 (34.2) | 22 (30.1) | ||
| Diabetes mellitus | Type 1 (n = 1) | 1 (1.4) | 0 (0) | 0.48 |
| Type 2 (n = 32) | 19 (26.0) | 13 (17.8) | ||
| Non-existent (n = 40) | 20 (27.4) | 20 (27.4) | ||
| Medications | ||||
| Oral antidiabetics | Administered (n = 24) | 14 (19.2) | 10 (13.7) | 0.26 |
| Not administered (n = 6) | 5 (6.8) | 1 (1.4) | ||
| No diabetes (n = 43) | 21 (28.8) | 22 (30.1) | ||
| Antiplatelet drugs | Administered (n = 59) | 32 (43.8) | 27 (37.0) | 0.84 |
| Not administered (n = 14) | 8 (11.0) | 6 (8.2) | ||
| NOACs | Administered (n = 10) | 5 (6.8) | 5 (6.8) | 0.74 |
| Not administered (n = 63) | 35 (47.9) | 28 (38.4) | ||
| Diuretics | Administered (n = 47) | 27 (37.0) | 20 (27.4) | 0.54 |
| Not administered (n = 26) | 13 (17.8) | 13 (17.8) | ||
| Statins | Administered (n = 55) | 27 (37.0) | 28 (38.4) | 0.09 |
| Not administered (n = 18) | 13 (17.8) | 5 (6.8) | ||
| Parameter | Category | BW ≥ 85 kg n = 37 (%) | BW < 85 kg n = 36 (%) | p-Wert |
|---|---|---|---|---|
| Sex | Female (n = 16) | 2 (2.7) | 14 (19.2) | 0.001 |
| Male (n = 57) | 35 (47.9) | 22 (30.1) | ||
| Bodyweight in kg | 69.3 ± 1.6 | 70.9 ± 1.4 | 0.43 | |
| ≥70 years (n = 40) | 17 (23.3) | 23 (31.5) | 0.12 | |
| <70 years (n = 33) | 20 (27.4) | 13 (17.8) | ||
| BMI | 31.3 ± 0.6 | 26.1 ± 0.7 | 0.16 | |
| Graft characteristics | ||||
| Storage solution | Tiprotec® (n = 21) | 11 (15.3) | 10 (13.9) | 0.57 |
| NaCl-heparin (n = 16) | 10 (13.9) | 6 (8.3) | ||
| Ringer-heparin (n = 13) | 4 (5.6) | 9 (12.5) | ||
| Blood-heparin (n = 6) | 4 (5.6) | 2 (2.8) | ||
| NaCl (n = 11) | 6 (8.3) | 5 (6.9) | ||
| Ringer (n = 5) | 2 (2.8) | 3 (4.2) | ||
| Graft diameter in mm | 3.2 ± 0.2 | 3.3 ± 0.2 | 0.93 | |
| ≥3 mm (n = 28) | 18 (27.3) | 20 (30.3) | 0.62 | |
| <3 mm (n = 38) | 15 (22.7) | 13 (19.7) | ||
| Comorbidities | ||||
| Arterial hypertension | Existent (n = 69) | 34 (46.6) | 35 (47.9) | 0.32 |
| Non-existent (n = 4) | 3 (4.1) | 1 (1.3) | ||
| NYHA-classification | NYHA I (n = 3) | 1 (1.3) | 2 (2.7) | 0.55 |
| NYHA II (n = 13) | 7 (9.5) | 6 (8.2) | ||
| NYHA III (n = 18) | 12 (16.4) | 6 (8.2) | ||
| NYHA IV (n = 2) | 1 (1.3) | 1 (1.3) | ||
| N/A (n = 37) | 16 (21.9) | 21 (28.8) | ||
| LVEF in % | <30% (n = 4) | 1 (1.4) | 3 (4.1) | 0.004 |
| 30–50% (n = 28) | 21 (28.8) | 7 (9.6) | ||
| >50% (n = 41) | 15 (20.5) | 26 (35.6) | ||
| PAD | Existent (n = 6) | 2 (2.7) | 4 (5.4) | 0.38 |
| Non-existent (n = 67) | 35 (47.9) | 32 (43.8) | ||
| Obesity | Class 1 (n = 21) | 17 (23.3) | 4 (5.5) | 0.001 |
| Class 2 (n = 6) | 5 (6.8) | 1 (1.3) | ||
| Class 3 (n = 1) | 1 (1.3) | 0 (0) | ||
| Non-existent (n = 45) | 14 (19.2) | 31 (42.5) | ||
| Hyperlipoproteinemia | Existent (n = 32) | 15 (20.5) | 17 (23.3) | 0.57 |
| Non-existent (n = 41) | 22 (30.1) | 19 (26.0) | ||
| Chronic kidney insufficiency | Stage 1 (n = 6) | 1 (1.4) | 5 (6.8) | 0.30 |
| Stage 2 (n = 5) | 2 (2.7) | 3 (4.1) | ||
| Stage 3 (n = 15) | 9 (12.3) | 6 (8.2) | ||
| Non-existent (n = 47) | 25 (34.2) | 22 (30.1) | ||
| Diabetes mellitus | Type 1 (n = 1) | 0 (0) | 1 (1.4) | 0.47 |
| Type 2 (n = 32) | 15 (20.5) | 17 (23.3) | ||
| Non-existent (n = 40) | 22 (30.1) | 18 (24.7) | ||
| Medications | ||||
| Oral antidiabetics | Administered (n = 24) | 12 (16.4) | 12 (16.4) | 0.99 |
| Not administered (n = 6) | 3 (4.1) | 3 (4.1) | ||
| No diabetes (n = 43) | 22 (30.1) | 21 (28.8) | ||
| Antiplatelet drugs | Administered (n = 59) | 29 (39.7) | 30 (41.1) | 0.59 |
| Not administered (n = 14) | 8 (11.0) | 6 (8.2) | ||
| NOACs | Administered (n = 10) | 5 (6.8) | 5 (6.8) | 0.96 |
| Not administered (n = 63) | 32 (43.8) | 31 (42.5) | ||
| Diuretics | Administered (n = 47) | 24 (32.9) | 23 (31.5) | 0.93 |
| Not administered (n = 26) | 13 (17.8) | 13 (17.8) | ||
| Statins | Administered (n = 55) | 30 (41.1) | 25 (34.2) | 0.25 |
| Not administered (n = 18) | 7 (9.6) | 11 (15.1) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saemann, L.; Wernstedt, L.; Pohl, S.; Stiller, M.; Willsch, J.; Hofmann, B.; Veres, G.; Simm, A.; Szabó, G. Impact of Age on Endothelial Function of Saphenous Vein Grafts in Coronary Artery Bypass Grafting. J. Clin. Med. 2023, 12, 5454. https://doi.org/10.3390/jcm12175454
Saemann L, Wernstedt L, Pohl S, Stiller M, Willsch J, Hofmann B, Veres G, Simm A, Szabó G. Impact of Age on Endothelial Function of Saphenous Vein Grafts in Coronary Artery Bypass Grafting. Journal of Clinical Medicine. 2023; 12(17):5454. https://doi.org/10.3390/jcm12175454
Chicago/Turabian StyleSaemann, Lars, Lena Wernstedt, Sabine Pohl, Markus Stiller, Jan Willsch, Britt Hofmann, Gábor Veres, Andreas Simm, and Gábor Szabó. 2023. "Impact of Age on Endothelial Function of Saphenous Vein Grafts in Coronary Artery Bypass Grafting" Journal of Clinical Medicine 12, no. 17: 5454. https://doi.org/10.3390/jcm12175454
APA StyleSaemann, L., Wernstedt, L., Pohl, S., Stiller, M., Willsch, J., Hofmann, B., Veres, G., Simm, A., & Szabó, G. (2023). Impact of Age on Endothelial Function of Saphenous Vein Grafts in Coronary Artery Bypass Grafting. Journal of Clinical Medicine, 12(17), 5454. https://doi.org/10.3390/jcm12175454





