Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion (PETLIF): Current Techniques, Clinical Outcomes, and Narrative Review
Abstract
:1. Introduction
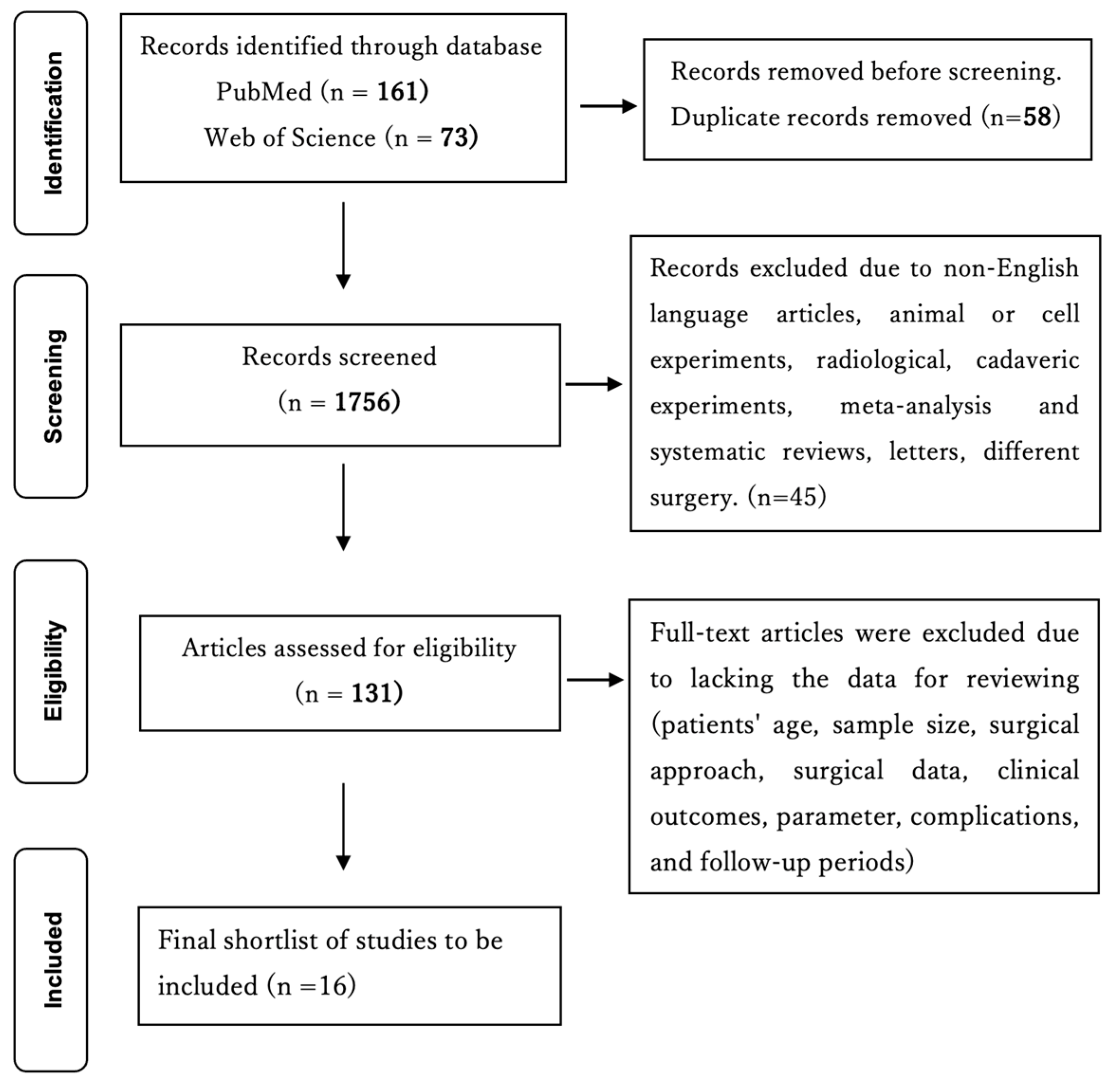
2. Materials and Methods
2.1. Literature Search
2.2. Data Extraction from the Manuscripts
2.3. Patient Population
2.4. Quality Assessment
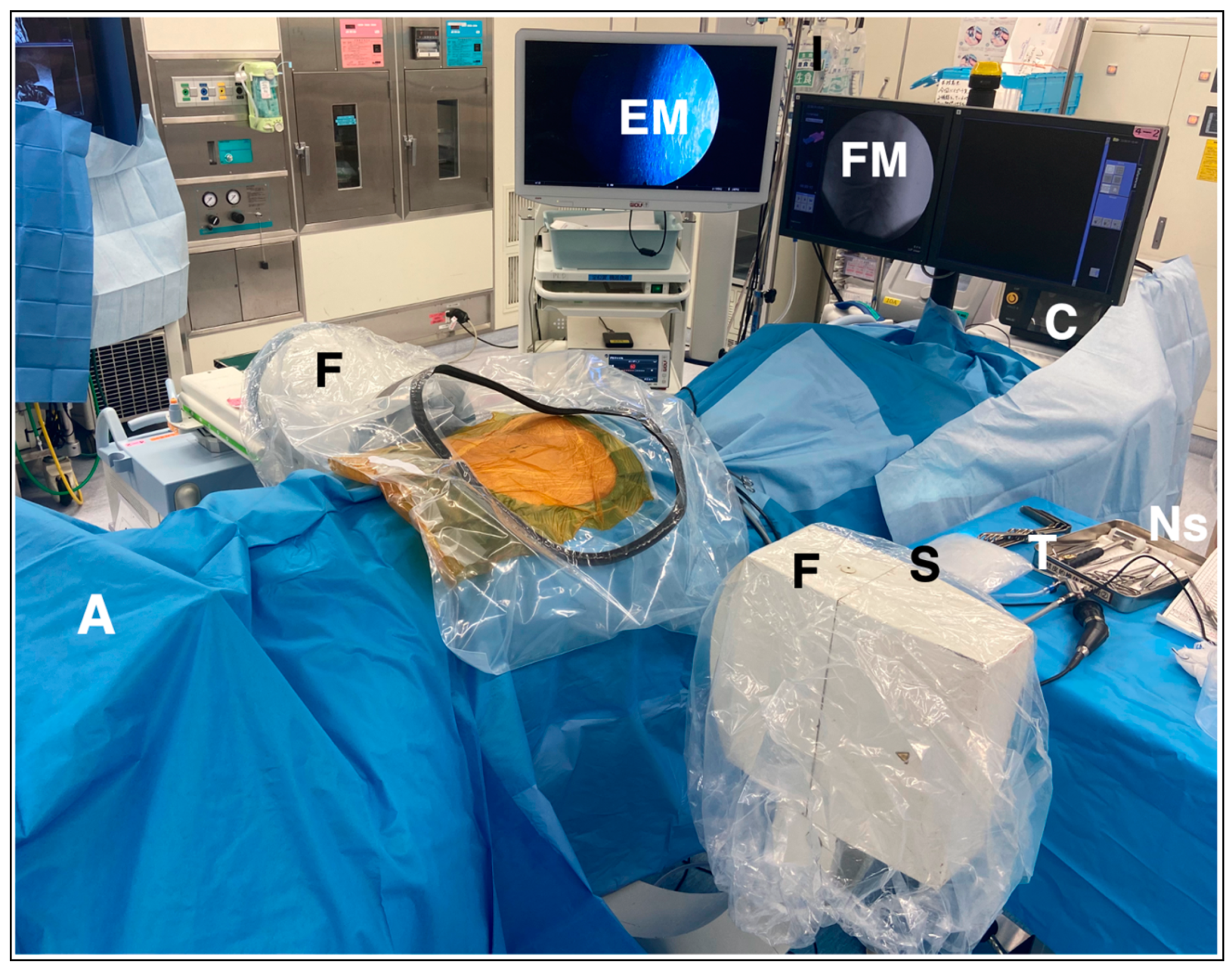
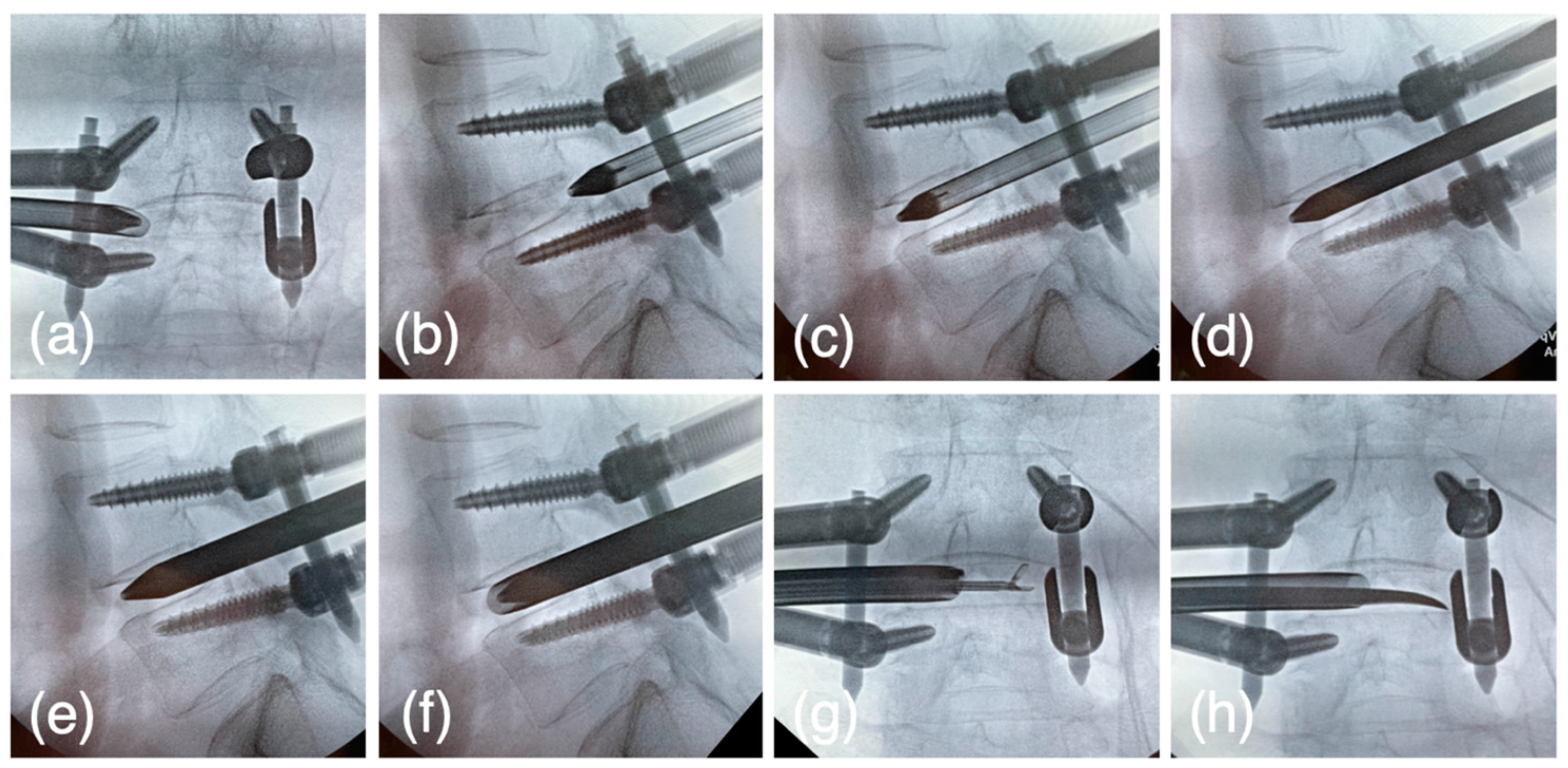
2.5. Surgical Technique of PETLIF
2.5.1. Preparation and Planning
2.5.2. Bone Harvesting
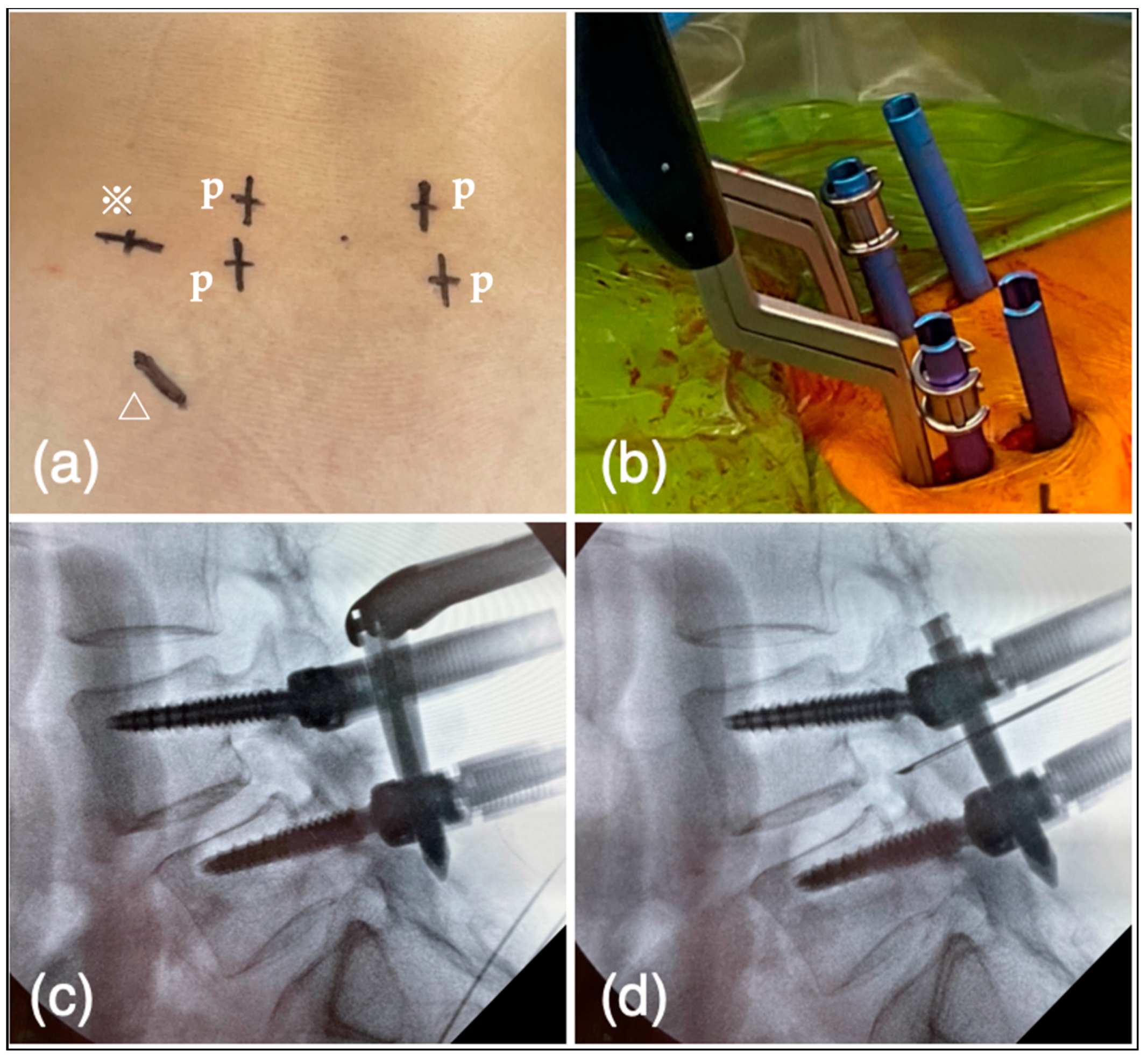
2.5.3. Reduction of Spondylolisthesis Using PPS
2.5.4. Expansion of Intervertebral Foramen
2.5.5. The First Step for Acquiring the Disc Height
2.5.6. The Second Step for Acquiring the Disc Height
2.5.7. The Third Step for Acquiring the Disc Height
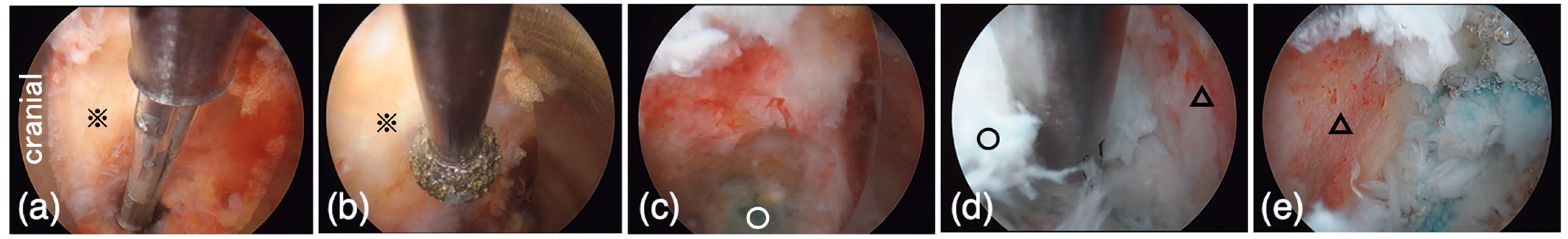
2.5.8. Intervertebral Disk Curettage
2.5.9. Cage Insertion
2.5.10. End of the Surgery
2.6. Statistical Analysis
3. Clinical Data of the Previous Literature (Table 1)
4. Results
4.1. Patients’ Characteristics and Clinical Outcomes
4.2. Complications
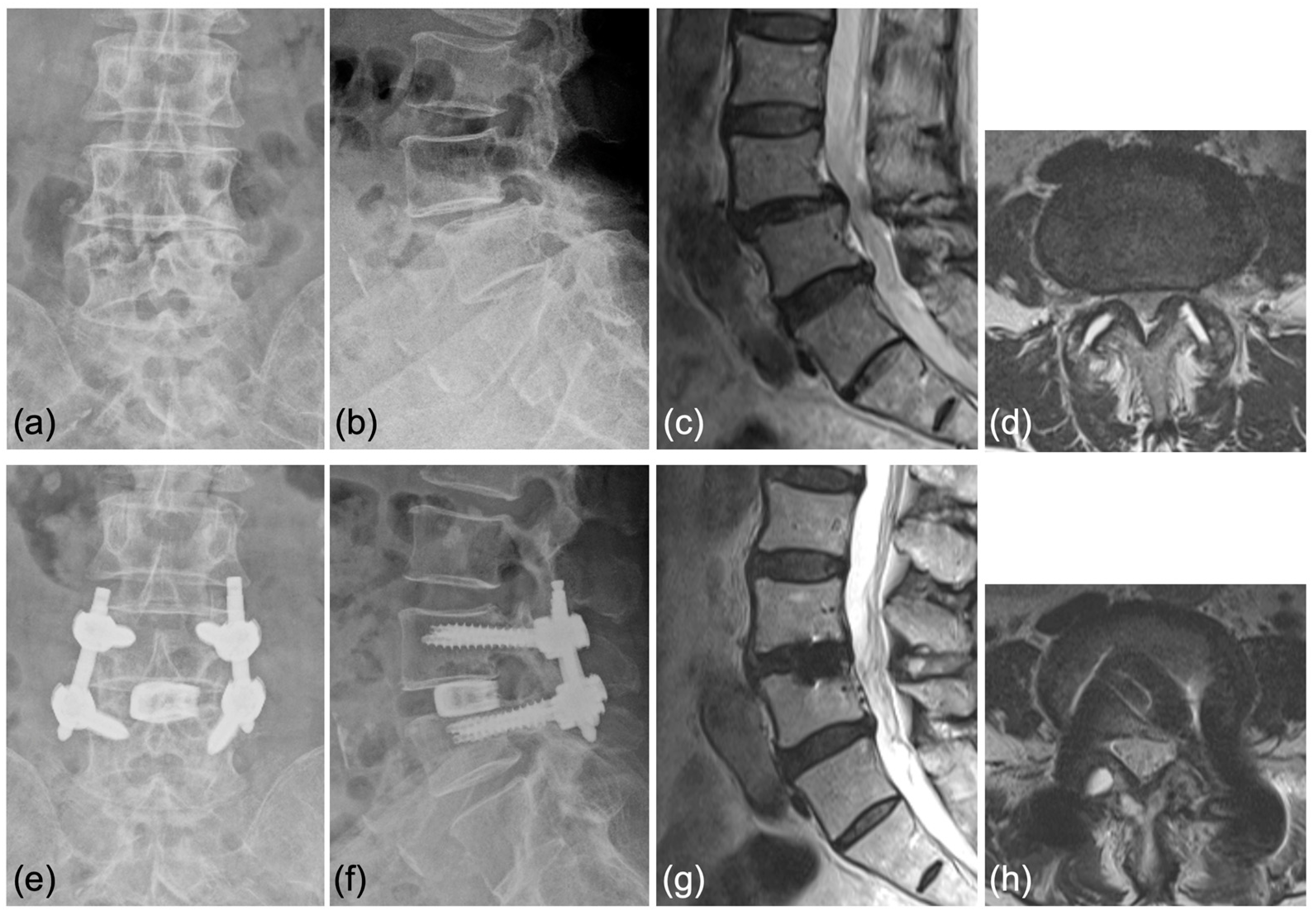
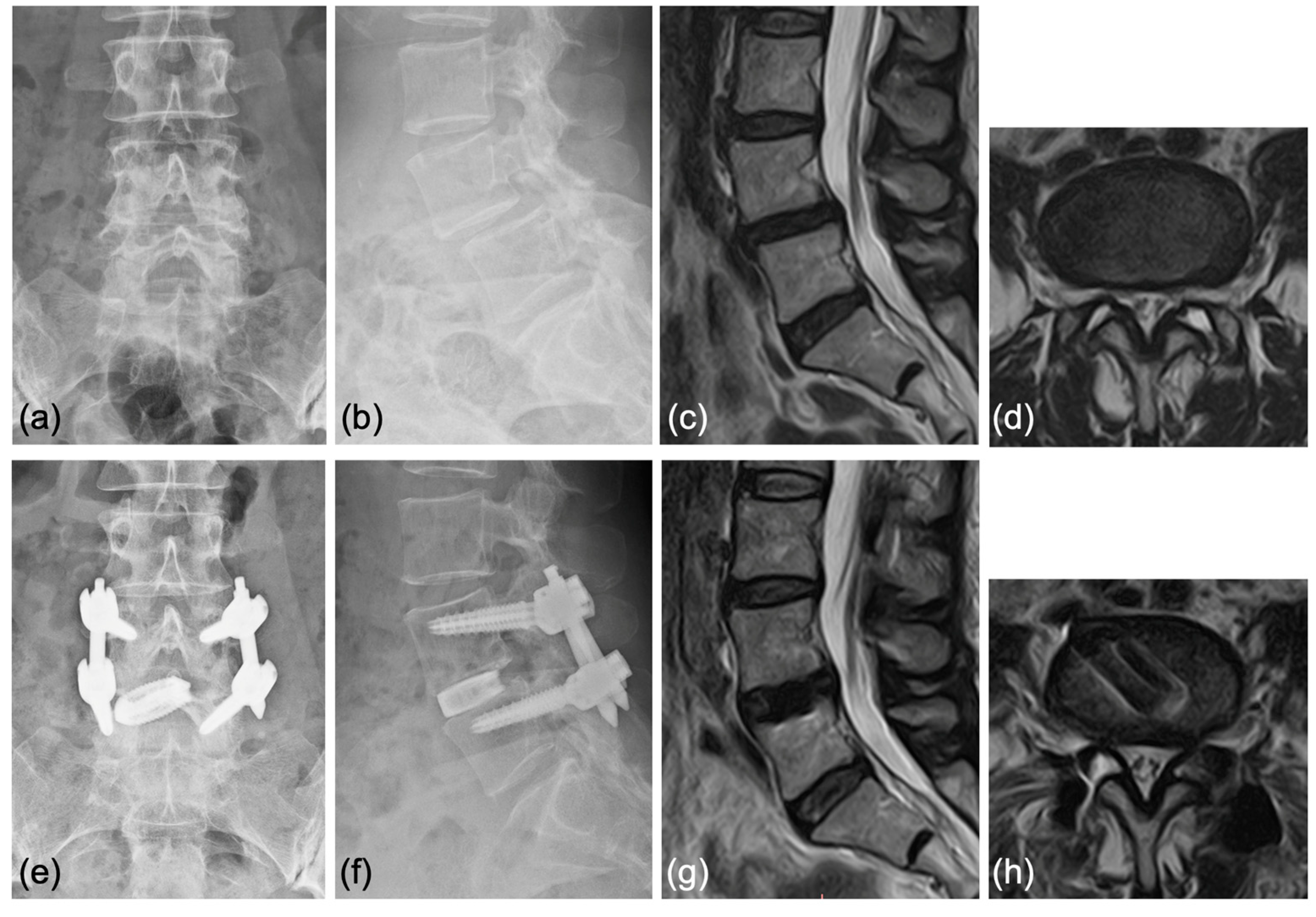
4.3. Representative Cases
5. Discussion
- Obtaining indirect decompression with reduced lumbar spondylolisthesis and disc height using PPS first and then an oval-shaped sleeve and dilator placed by an endoscopic transforaminal approach [14].
- Curettage of the affected intervertebral disc is performed under fluoroscopic and endoscopic guidance using special instruments dedicated to PETLIF [14].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaina, F.; Tomkins-Lane, C.; Carragee, E.; Negrini, S. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst. Rev. 2016, 2016, CD010264. [Google Scholar] [CrossRef]
- Deyo, R.A.; Mirza, S.K.; Martin, B.I.; Kreuter, W.; Goodman, D.C.; Jarvik, J.G. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010, 303, 1259–1265. [Google Scholar] [CrossRef]
- de Kunder, S.L.; van Kuijk, S.M.J.; Rijkers, K.; Caelers, I.; van Hemert, W.L.W.; de Bie, R.A.; van Santbrink, H. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: A systematic review and meta-analysis. Spine J. 2017, 17, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, T.; Herno, A.; Paljarvi, L.; Airaksinen, O.; Partanen, J.; Tapaninaho, A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine 1993, 18, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.T.; Holly, L.T.; Schwender, J.D. Minimally invasive lumbar fusion. Spine 2003, 28, S26–S35. [Google Scholar] [CrossRef]
- Ozgur, B.M.; Aryan, H.E.; Pimenta, L.; Taylor, W.R. Extreme Lateral Interbody Fusion (XLIF): A novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006, 6, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Katoh, H.; Sakai, D.; Tanaka, M.; Sato, M.; Watanabe, M. Short-term comparison of preoperative and postoperative pain after indirect decompression surgery and direct decompression surgery in patients with degenerative spondylolisthesis. Sci. Rep. 2020, 10, 18887. [Google Scholar] [CrossRef]
- Epstein, N.E. Review of Risks and Complications of Extreme Lateral Interbody Fusion (XLIF). Surg. Neurol. Int. 2019, 10, 237. [Google Scholar] [CrossRef]
- Kou, Y.; Chang, J.; Guan, X.; Chang, Q.; Feng, H. Endoscopic Lumbar Interbody Fusion and Minimally Invasive Transforaminal Lumbar Interbody Fusion for the Treatment of Lumbar Degenerative Diseases: A Systematic Review and Meta-Analysis. World Neurosurg. 2021, 152, e352–e368. [Google Scholar] [CrossRef]
- Brusko, G.D.; Wang, M.Y. Endoscopic Lumbar Interbody Fusion. Neurosurg. Clin. N. Am. 2020, 31, 17–24. [Google Scholar] [CrossRef]
- Ahn, Y.; Youn, M.S.; Heo, D.H. Endoscopic transforaminal lumbar interbody fusion: A comprehensive review. Expert. Rev. Med. Devices. 2019, 16, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Huang, Z.; Liu, H.; Zhang, C.; Zheng, W.; Li, C.; Tang, Y.; Zhou, Y. A Modified Endoscopic Transforaminal Lumbar Interbody Fusion Technique: Preliminary Clinical Results of 96 Cases. Front. Surg. 2021, 8, 676847. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, D.; Ono, K.; Kenji, T.; Majima, T. A Narrative Review of Full-Endoscopic Lumbar Discectomy Using Interlaminar Approach. World Neurosurg. 2022, 168, 324–332. [Google Scholar] [CrossRef]
- Nagahama, K.; Ito, M.; Abe, Y.; Murota, E.; Hiratsuka, S.; Takahata, M. Early Clinical Results of Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion: A New Modified Technique for Treating Degenerative Lumbar Spondylolisthesis. Spine Surg. Relat. Res. 2019, 3, 327–334. [Google Scholar] [CrossRef]
- Chen, W.J.; Lai, P.L.; Niu, C.C.; Chen, L.H.; Fu, T.S.; Wong, C.B. Surgical treatment of adjacent instability after lumbar spine fusion. Spine 2001, 26, E519–E524. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Miyauchi, A.; Oda, T.; Haku, T.; Yamamoto, T.; Iwasaki, M. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J. Neurosurg. Spine 2006, 4, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Kolcun, J.P.G.; Brusko, G.D.; Wang, M.Y. Endoscopic transforaminal lumbar interbody fusion without general anesthesia: Technical innovations and outcomes. Ann. Transl. Med. 2019, 7, S167. [Google Scholar] [CrossRef]
- Heo, D.H.; Son, S.K.; Eum, J.H.; Park, C.K. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: Technical note and preliminary clinical results. Neurosurg. Focus. 2017, 43, E8. [Google Scholar] [CrossRef]
- Ishihama, Y.; Morimoto, M.; Tezuka, F.; Yamashita, K.; Manabe, H.; Sugiura, K.; Takeuchi, M.; Takata, Y.; Sakai, T.; Maeda, T.; et al. Full-Endoscopic Trans-Kambin Triangle Lumbar Interbody Fusion: Surgical Technique and Nomenclature. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2022, 83, 308–313. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Hai, Y.; Yin, P.; Zhou, L.; Zhang, Y.; Pan, A.; Zhang, Y.; Zhang, L.; Ding, Y.; et al. Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion for the Treatment of Lumbar Spinal Stenosis: Preliminary Report of Seven Cases with 12-Month Follow-Up. Biomed. Res. Int. 2019, 2019, 3091459. [Google Scholar] [CrossRef]
- Yin, P.; Ding, Y.; Zhou, L.; Xu, C.; Gao, H.; Pang, D.; Hai, Y.; Yang, J. Innovative Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion of Lumbar Spinal Stenosis with Degenerative Instability: A Non-Randomized Clinical Trial. J. Pain. Res. 2021, 14, 3685–3693. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Alcantara, T.; Nogueira, M.P. PERCUTANEOUS ENDOSCOPIC LUMBAR INTERBODY FUSION: RESULTS OVER 47 MONTHS OF FOLLOW-UP. Acta Ortop. Bras. 2022, 30, e249489. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yan, H.; Chen, B.; Tang, J. Percutaneous Pedicle Screw Fixation with Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion in the Treatment of Degenerative Lumbar Spondylolisthesis with Instability. World Neurosurg. 2023, S1878–S8750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, C.; Wang, C.; Zhu, K.; Tu, Q.; Kong, M.; Zhao, C.; Ma, X. Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion: Technique Note and Comparison of Early Outcomes with Minimally Invasive Transforaminal Lumbar Interbody Fusion for Lumbar Spondylolisthesis. Int. J. Gen. Med. 2021, 14, 549–558. [Google Scholar] [CrossRef] [PubMed]
- He, L.M.; Chen, K.T.; Chen, C.M.; Chang, Q.; Sun, L.; Zhang, Y.N.; Chang, J.J.; Feng, H.Y. Comparison of percutaneous endoscopic and open posterior lumbar interbody fusion for the treatment of single-segmental lumbar degenerative diseases. BMC Musculoskelet. Disord. 2022, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yin, S.; Wei, J.; Zhao, W.; Wang, X.; Zhang, Y.; Hao, D.; Du, H. Full-Endoscopic Posterior Lumbar Interbody Fusion with Epidural Anesthesia: Technical Note and Initial Clinical Experience with One-Year Follow-Up. J. Pain. Res. 2021, 14, 3815–3826. [Google Scholar] [CrossRef]
- Jin, M.; Xu, G.; Shen, T.; Zhang, J.; Shao, H.; Liu, J.; Zhao, T.; Huang, Y. Minimally invasive surgery for low-grade spondylolisthesis: Percutaneous endoscopic or oblique lumbar interbody fusion. J. Comp. Eff. Res. 2020, 9, 639–650. [Google Scholar] [CrossRef]
- Wang, J.C.; Li, Z.Z.; Cao, Z.; Zhao, H.L.; Zhang, M. Technical Notes of Full Endoscopic Lumbar Interbody Fusion with Anterior Expandable Cylindrical Fusion Cage: Clinical and Radiographic Outcomes at 1-Year Follow-Up. World Neurosurg. 2022, 158, e618–e626. [Google Scholar] [CrossRef]
- Ao, S.; Zheng, W.; Wu, J.; Tang, Y.; Zhang, C.; Zhou, Y.; Li, C. Comparison of Preliminary clinical outcomes between percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion for lumbar degenerative diseases in a tertiary hospital: Is percutaneous endoscopic procedure superior to MIS-TLIF? A prospective cohort study. Int. J. Surg. 2020, 76, 136–143. [Google Scholar] [CrossRef]
- Lin, L.; Liu, X.Q.; Shi, L.; Cheng, S.; Wang, Z.Q.; Ge, Q.J.; Gao, D.Z.; Ismail, A.C.; Ke, Z.Y.; Chu, L. Comparison of Postoperative Outcomes Between Percutaneous Endoscopic Lumbar Interbody Fusion and Minimally Invasive Transforaminal Lumbar Interbody Fusion for Lumbar Spinal Stenosis. Front. Surg. 2022, 9, 916087. [Google Scholar] [CrossRef]
- Kim, H.S.; Wu, P.H.; An, J.W.; Lee, Y.J.; Lee, J.H.; Kim, M.H.; Lee, I.; Park, J.S.; Lee, J.H.; Park, J.H.; et al. Evaluation of Two Methods (Inside-Out/Outside-In) Inferior Articular Process Resection for Uniportal Full Endoscopic Posterolateral Transforaminal Lumbar Interbody Fusion: Technical Note. Brain Sci. 2021, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Ohtori, S.; Orita, S.; Yamauchi, K.; Eguchi, Y.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Aoki, Y.; Nakamura, J.; Ishikawa, T.; et al. Mini-Open Anterior Retroperitoneal Lumbar Interbody Fusion: Oblique Lateral Interbody Fusion for Lumbar Spinal Degeneration Disease. Yonsei Med. J. 2015, 56, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Winder, M.J.; Gambhir, S. Comparison of ALIF vs. XLIF for L4/5 interbody fusion: Pros, cons, and literature review. J. Spine Surg. 2016, 2, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Wang, L.; Yuan, S.; Tian, Y.; Zhao, Y.; Liu, X. Percutaneous Endoscopic Robot-Assisted Transforaminal Lumbar Interbody Fusion (PE RA-TLIF) for Lumbar Spondylolisthesis: A Technical Note and Two Years Clinical Results. Pain. Physician 2022, 25, e73–e86. [Google Scholar] [PubMed]
- Orita, S.; Shiga, Y.; Inage, K.; Eguchi, Y.; Maki, S.; Furuya, T.; Aoki, Y.; Inoue, M.; Hynes, R.A.; Koda, M.; et al. Technical and Conceptual Review on the L5-S1 Oblique Lateral Interbody Fusion Surgery (OLIF51). Spine Surg. Relat. Res. 2021, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.X.; Chen, C.M.; Rui, G.; Kim, J.S. A pilot study of endoscope-assisted MITLIF with fluoroscopy-guided technique: Intraoperative objective and subjective evaluation of disc space preparation. BMC Surg. 2022, 22, 109. [Google Scholar] [CrossRef]
- Sharma, M.; Chhawra, S.; Jain, R.; Sharma, S. Full Endoscopic Lumbar Transforaminal Interbody Fusion in DDD Lumbar Degenerative Disc Disease: A Latest Technique. Int. J. Spine Surg. 2021, 14, S71–S77. [Google Scholar] [CrossRef]
- Li, Y.; Dai, Y.; Wang, B.; Li, L.; Li, P.; Xu, J.; Jiang, B.; Lü, G. Full-Endoscopic Posterior Lumbar Interbody Fusion Via an Interlaminar Approach Versus Minimally Invasive Transforaminal Lumbar Interbody Fusion: A Preliminary Retrospective Study. World Neurosurg. 2020, 144, e475–e482. [Google Scholar] [CrossRef]
- He, L.M.; Li, J.R.; Wu, H.R.; Chang, Q.; Guan, X.M.; Ma, Z.; Feng, H.Y. Percutaneous Endoscopic Posterior Lumbar Interbody Fusion with Unilateral Laminotomy for Bilateral Decompression Vs. Open Posterior Lumbar Interbody Fusion for the Treatment of Lumbar Spondylolisthesis. Front. Surg. 2022, 9, 915522. [Google Scholar] [CrossRef]
- Choi, J.M.; Choi, M.K.; Kim, S.B. Perioperative Results and Complications after Posterior Lumbar Interbody Fusion for Spinal Stenosis in Geriatric Patients over than 70 Years Old. J. Korean Neurosurg. Soc. 2017, 60, 684–690. [Google Scholar] [CrossRef]
- Schwab, F.; Patel, A.; Ungar, B.; Farcy, J.P.; Lafage, V. Adult spinal deformity-postoperative standing imbalance: How much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine 2010, 35, 2224–2231. [Google Scholar] [CrossRef] [PubMed]


| First Author | Year | Study Design | Disease Type | Surgical Technique | Approach | Sample Size | Age | Sex (M/F) | Op Time | Anesthesia | EBL | Post-Op Hospital Stay | Follow–Up Periods | Decompression (Direct/Indirect) | Order of PPS and Cage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nagahama, K | 2019 [14] | RS | DLS | PETLIF | TF | 25 | 68.4 | 5/20 | 125.4 | general | 64.8 | - | 22.7 | indirect | PPS → cage |
| Lin, L | 2022 [15] | RS | LSCS | PE-LIF | IL | 41 | 61.9 | 15/26 | 193.4 | general | 122.2 | 8.9 | 14.1 | direct | cage → PPS |
| MIS-TLIF | - | 48 | 63.0 | 18/30 | 167.3 | general | 157.9 | 9.4 | 13.7 | direct | |||||
| He, LM | 2022 [16] | RS | DLS | PE-PLIF | IL | 28 | 59.8 | 14/14 | 221.2 | general | 169.2 | 3.5 | 18.4 | direct | cage → PPS |
| Open PLIF | - | 28 | 54.2 | 13/15 | 138.4 | general | 649.6 | 7.3 | 18.9 | direct | |||||
| He, LM | 2022 [17] | PS | LDH, DLS, LDD | PE-PLIF | IL | 30 | 51.6 | 18/2 | 179.8 | general | 63.3 | 3.3 | 24.7 | direct | cage → PPS |
| Open PLIF | - | 30 | 55.9 | 18/12 | 125.8 | general | 313.3 | 7.0 | 25.3 | direct | |||||
| Wang, JC | 2022 [18] | RS | TF | TF | 14 | 35.0 | 3/11 | 121.8 | local | - | - | >12 | indirect | cage → PPS | |
| IL | IL | 18 | 43.1 | 8/10 | 129.7 | general | - | - | >12 | direct | cage → PPS | ||||
| Silva, AC | 2022 [19] | RS | PELIF | TF | 19 | 36.1 | 17/2 | 355 | general | 215.8 | 3.0 | 47 | indirect | cage → PPS | |
| Xue, Y | 2021 [20] | RS | PETLIF | TF | 41 | 46.3 | 11/9 | 140.3 | general | 65.6 | 2.4 | 16.1 | direct | cage → PPS | |
| MISTLIF | - | 48 | 47.1 | 12/8 | 170.6 | general | 140.5 | 4.5 | 15.8 | direct | |||||
| Kim, HS | 2021 [21] | RS | ETLIF(I) | TF | 48 | 65.0 | - | 102.6 | general | - | - | 14.7 | direct | cage → PPS | |
| ETLIF(O) | TF | 38 | 68.4 | - | 87.5 | general | - | - | 11.6 | direct | |||||
| He, L | 2021 [22] | RS | PE-PLIF | IL | 35 | 52.3 | 21/14 | 179.6 | general | 68.6 | 3.1 | >12 | direct | cage → PPS | |
| Jiang, C | 2021 [23] | RS | LSCS, DLS | Endo-PLIF | IL | 24 | 59.5 | 10/14 | 209.2 | epi | 43.3 | 8.7 | 15.2 | direct | cage → PPS |
| Yin, P | 2021 [24] | PS | LSCS | PETLIF | TF | 56 | 60.5 | 10/46 | 204.2 | general/epi | 105.6 | - | 15.3 | direct | cage → PPS |
| PLIF | - | 58 | 60.6 | 10/48 | 99.8 | general/epi | 241.6 | - | 15.8 | direct | |||||
| Zhang, H | 2021 [25] | RS | DLS | Endo-TLIF | TF | 32 | 53.1 | 12/20 | 202.6 | general | 73.0 | 1.6 | >12 | direct | cage → PPS |
| MIS-TLIF | - | 30 | 55.7 | 14/16 | 192.1 | general | 129.0 | 2.3 | >12 | direct | |||||
| Jin, M | 2020 [26] | RS | PELIF | TF | 16 | 61.2 | 9/7 | 155.2 | local | 35.0 | 4.9 | 22.6 | direct | cage → PPS | |
| OLIF | - | 32 | 63.9 | 18/14 | 160.3 | general | 80.0 | 4.3 | 24.2 | indirect | |||||
| Ao, S | 2020 [27] | PS | DLS, LDH, LSCS | PETLIF | TF | 35 | 52.8 | 16/19 | 143.0 | general | 492.7 | 3.1 | >14 | ? | cage → PPS |
| MIS-TLIF | - | 40 | 53.6 | 22/18 | 103.6 | general | 698.1 | 5.2 | >14 | direct | |||||
| Yang, J | 2019 [28] | RS | LSCS | PE-TLIF | TF | 7 | 55.3 | 1/6 | 285.7 | general/epi/local | 117.1 | 4.0 | >12 | direct | cage → PPS |
| Cheng, X | 2023 [29] | RS | DLS | PE-TLIF | TF | 27 | 70.7 | 22/5 | 157.59 | local | 47.41 | 7.85 | >12 | direct | PPS → cage |
| Characteristic | Value |
|---|---|
| Case number | 24 |
| Age (years) | |
| Mean | 70.5 ± 2.19 |
| Range | 51–86 |
| Sex | |
| Male | 13 |
| Female | 11 |
| Diagnosis | |
| Degenerative spondylolisthesis | 7 |
| Lumbar spinal canal stenosis | 17 |
| Surgical levels | |
| L2–3 | 1 |
| L3–4 | 9 |
| L4–5 | 14 |
| L5–S1 | 0 |
| Operation time (mins) | 130.8 ± 9.2 |
| Intraoperative blood loss (mL) | 24.0 ± 4.9 |
| Postoperative hospitalization time (days) | 9.4 ± 0.6 |
| Follow up periods (month) | 21.5 ± 0.5 |
| Case No. | Diagnosis | Surgical Procedure | Endplate Injury | Cage Subsidence | Exiting Nerve Injury | Bone Fusion |
|---|---|---|---|---|---|---|
| 1 | Degenerative spondylolisthesis | PETLIF | − | + | − | + |
| 2 | Lumbar spinal canal stenosis | PETLIF | − | + | − | + |
| 3 | Lumbar spinal canal stenosis | PETLIF | − | − | − | + |
| 4 | Lumbar spinal canal stenosis | PETLIF | − | + | − | + |
| 5 | Lumbar spinal canal stenosis | PETLIF | − | + | − | − |
| 6 | Lumbar spinal canal stenosis | PETLIF | − | − | − | + |
| 7 | Lumbar spinal canal stenosis | PETLIF | − | − | − | − |
| 8 | Lumbar spinal canal stenosis | PETLIF | − | + | − | + |
| 9 | Degenerative spondylolisthesis | PETLIF | − | + | − | + |
| 10 | Degenerative spondylolisthesis | PETLIF | − | − | + | + |
| 11 | Degenerative spondylolisthesis | PETLIF | − | − | − | − |
| 12 | Degenerative spondylolisthesis | PETLIF | + | − | − | + |
| 13 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| 14 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| 15 | Degenerative spondylolisthesis | PETLIF | − | + | − | + |
| 16 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| 17 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| 18 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| 19 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| 20 | Degenerative spondylolisthesis | PETLIF | − | − | + | + |
| 21 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| 22 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| 23 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| 24 | Degenerative spondylolisthesis | PETLIF | − | − | − | + |
| Scores | Preop | 1M | 3M | 6M | Final |
|---|---|---|---|---|---|
| JOA score | 17.4 ± 0.8 | 25.7 ± 0.4 * | 27.0 ± 0.4 * | 26.9 ± 0.3 * | 26.9 ± 0.3 * |
| VAS of low back pain | 51.0 ± 5.1 | 15.6 ± 4.2 * | 7.3 ± 2.6 * | 6.9 ± 2.1 * | 6.5 ± 2.0 * |
| VAS of leg pain | 74.4 ± 3.7 | 10.8 ± 2.9 * | 11.7 ± 3.6 * | 9.5 ± 2.6 * | 10.8 ± 3.5 * |
| Parameters | Preop | 1M | 3M | 6M | Final |
|---|---|---|---|---|---|
| Lumbar lordosis (LL) | 33.3 | 32.6 | 33.4 | 33.0 | 33.3 |
| Pelvic incidence (PI) | 48.9 | 48.9 | 48.9 | 48.9 | 48.9 |
| PI-LL | 15.6 | 16.3 | 15.5 | 15.9 | 15.6 |
| Local lordosis | 9.3 | 10.0 | 9.9 | 9.2 | 9.0 |
| % Slip | 17.3 | n.a. | n.a. | n.a. | 4.5 * |
| Disc height | 5.1 | n.a. | n.a. | n.a. | 7.3 * |
| Spinal canal area | 43.1 | n.a. | n.a. | n.a. | 108.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, K.; Fukuhara, D.; Nagahama, K.; Abe, Y.; Takahashi, K.; Majima, T. Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion (PETLIF): Current Techniques, Clinical Outcomes, and Narrative Review. J. Clin. Med. 2023, 12, 5391. https://doi.org/10.3390/jcm12165391
Ono K, Fukuhara D, Nagahama K, Abe Y, Takahashi K, Majima T. Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion (PETLIF): Current Techniques, Clinical Outcomes, and Narrative Review. Journal of Clinical Medicine. 2023; 12(16):5391. https://doi.org/10.3390/jcm12165391
Chicago/Turabian StyleOno, Koichiro, Daisuke Fukuhara, Ken Nagahama, Yuichiro Abe, Kenji Takahashi, and Tokifumi Majima. 2023. "Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion (PETLIF): Current Techniques, Clinical Outcomes, and Narrative Review" Journal of Clinical Medicine 12, no. 16: 5391. https://doi.org/10.3390/jcm12165391
APA StyleOno, K., Fukuhara, D., Nagahama, K., Abe, Y., Takahashi, K., & Majima, T. (2023). Percutaneous Endoscopic Transforaminal Lumbar Interbody Fusion (PETLIF): Current Techniques, Clinical Outcomes, and Narrative Review. Journal of Clinical Medicine, 12(16), 5391. https://doi.org/10.3390/jcm12165391





