Is the Pattern Changing? Atrial Fibrillation and Screening with Holter Electrocardiograms among Ischemic Stroke Patients in Greenland from 2016 to 2021
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Data Extraction
2.3. Analysis
3. Results
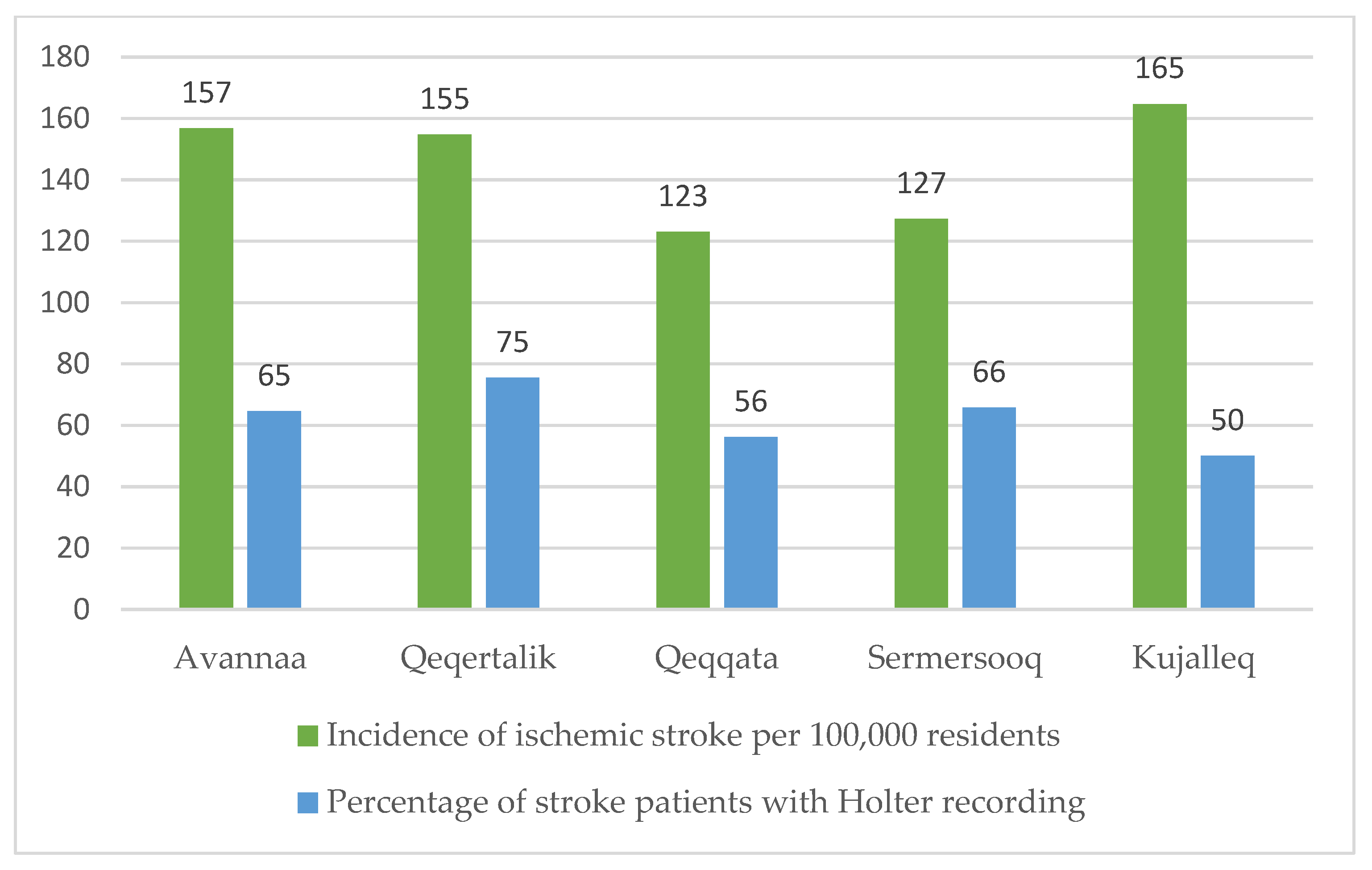
3.1. IS Incidence
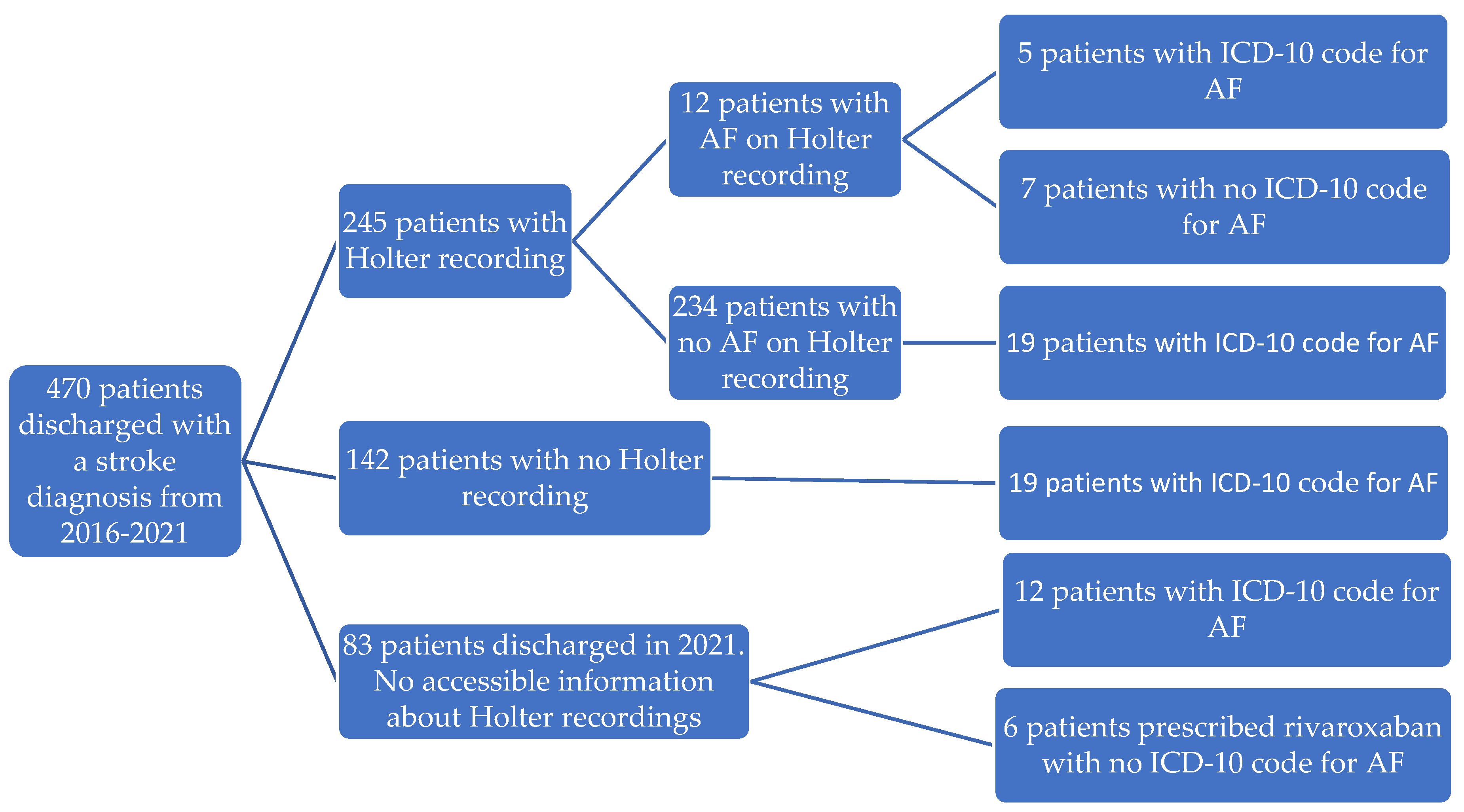
3.2. Holter Recordings
3.3. AF among IS Patients
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2021, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, X.; Yu, X.; Liu, Z.; Jiang, Y.; Fang, Y.; Zong, M.; Suo, C.; Man, Q.; Xiong, L. Global Burden, Risk Factors Analysis, and Prediction Study of Ischemic Stroke, 1990–2030. Neurology 2023, 101, e137–e150. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, H.; Pu, L.; Zhao, T.; Zhang, S.; Han, K.; Han, L. Global Burden of Ischemic Stroke in Young Adults in 204 Countries and Territories. Neurology 2023, 100, E422–E434. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; Singh, K.; Kenny, T.A.; Chan, H.M. Prevalence of Heart Attack and Stroke and Associated Risk Factors among Inuit in Canada: A Comparison with the General Canadian Population. Int. J. Hyg. Environ. Health 2019, 222, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Atzema, C.L.; Khan, S.; Lu, H.; Allard, Y.E.; Russell, S.J.; Gravelle, M.R.; Klein-Geltink, J.; Austin, P.C. Cardiovascular Disease Rates, Outcomes, and Quality of Care in Ontario Métis: A Population-Based Cohort Study. PLoS ONE 2015, 10, e0121779. [Google Scholar] [CrossRef]
- Schumacher, C.; Davidson, M.; Ehrsam, G. International Journal of Circumpolar Health Cardiovascular Disease among Alaska Natives: A Review of the Literature. Int. J. Circumpolar Health 2003, 62, 343–362. [Google Scholar] [CrossRef]
- Bjorn-Mortensen, K.; Lynggaard, F.; Pedersen, M.L. Incidence of Greenlandic Stroke-Survivors in Greenland: A 2-Year Cross-Sectional Study. Int. J. Circumpolar Health 2013, 72, 22626. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Jolly, S.E.; Dewland, T.A.; Tseng, Z.H.; Nah, G.; Vittinghoff, E.; Marcus, G.M. Incident Strokes Among American Indian Individuals With Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e019581. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial Fibrillation as an Independent Risk Factor for Stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial Fibrillation: A Major Contributor to Stroke in the Elderly: The Framingham Study. Arch. Intern. Med. 1987, 147, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, S.K.; Frost, L.; Eagle, K.A.; Johnsen, S.P. Atrial Fibrillation in Patients with Ischemic Stroke: A Population-Based Study. Clin. Epidemiol. 2009, 1, 55–65. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M.; Andresen, D.; Camm, A.J.; Davies, W.; Capucci, A.; Olsson, B.; et al. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach: The Euro Heart Survey on Atrial Fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Allan, V.; Honarbakhsh, S.; Casas, J.P.; Wallace, J.; Hunter, R.; Schilling, R.; Perel, P.; Morley, K.; Banerjee, A.; Hemingway, H. Are Cardiovascular Risk Factors Also Associated with the Incidence of Atrial Fibrillation?: A Systematic Review and Field Synopsis of 23 Factors in 32 Population-Based Cohorts of 20 Million Participants. Thromb. Haemost. 2017, 117, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Stefanick, M.L.; Greenland, P.; Soliman, E.Z.; Manson, J.E.; Parikh, N.; Martin, L.W.; Larson, J.C.; Hlatky, M.; Nassir, R.; et al. Racial and Ethnic Differences in Atrial Fibrillation Risk Factors and Predictors in Women: Findings from the Women’s Health Initiative. Am. Heart J. 2016, 176, 70–77. [Google Scholar] [CrossRef]
- Alonso, A.; Roetker, N.S.; Soliman, E.Z.; Chen, L.Y.; Greenland, P.; Heckbert, S.R. Prediction of Atrial Fibrillation in a Racially Diverse Cohort: The Multi-Ethnic Study of Atherosclerosis (Mesa). J. Am. Heart Assoc. 2016, 5, e003077. [Google Scholar] [CrossRef]
- Martinez, C.; Katholing, A.; Wallenhorst, C.; Granziera, S.; Cohen, A.T.; Freedman, S.B. Increasing Incidence of Non-Valvular Atrial Fibrillation in the UK from 2001 to 2013. Heart 2015, 101, 1748–1754. [Google Scholar] [CrossRef]
- Wong, C.X.; Brooks, A.G.; Cheng, Y.H.; Lau, D.H.; Rangnekar, G.; Roberts-Thomson, K.C.; Kalman, J.M.; Brown, A.; Sanders, P. Atrial Fibrillation in Indigenous and Non-Indigenous Australians: A Cross-Sectional Study. BMJ Open 2014, 4, e006242. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.M.; Woods, J.A.; Teng, T.H.K.; Thompson, S.C. Atrial Fibrillation in the Indigenous Populations of Australia, Canada, New Zealand, and the United States: A Systematic Scoping Review. BMC Cardiovasc. Disord. 2015, 15, 87. [Google Scholar] [CrossRef]
- Albertsen, N.; Riahi, S.; Pedersen, M.L.; Skovgaard, N.; Andersen, S. The Prevalence of Atrial Fibrillation in Greenland: A Register-Based Cross-Sectional Study Based on Disease Classifications and Prescriptions of Oral Anticoagulants. Int. J. Circumpolar Health 2022, 81, 2030522. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Bjorn-Mortensen, K.; Lynggaard, F.; Pedersen, M.L. High Prevalence of Atrial Fibrillation among Greenlanders with Ischemic Stroke—Atrial Fibrillation Found in More than 30% of Cases. Int. J. Circumpolar Health 2013, 72, 22628. [Google Scholar] [CrossRef] [PubMed]
- Trifork Medical Diagnosekoder.Dk. Available online: http://diagnosekoder.dk/#/search/DI649 (accessed on 31 July 2023).
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.; Rosenqvist, M.; Lindgren, A.; Terént, A.; Norrving, B.; Asplund, K. High Prevalence of Atrial Fibrillation among Patients with Ischemic Stroke. Stroke 2014, 45, 2599–2605. [Google Scholar] [CrossRef] [PubMed]
- Danish Medicines Agency Nyt Om Pradaxa®(Dabigatran Etexilat) Og Xarelto® (Rivaroxaban). Available online: https://laegemiddelstyrelsen.dk/da/nyheder/2013/~/media/7AEFE599582A4F268E4F757B86D4799E.ashx (accessed on 14 March 2023).
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Lip, G.Y.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation CHEST Guideline and Expert Panel Report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef]
- Larsen, T.B.; Skjøth, F.; Nielsen, P.B.; Kjældgaard, J.N.; Lip, G.Y.H. Comparative Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants and Warfarin in Patients with Atrial Fibrillation: Propensity Weighted Nationwide Cohort Study. BMJ 2016, 353, i3189. [Google Scholar] [CrossRef]
- Haas, S.; ten Cate, H.; Accetta, G.; Angchaisuksiri, P.; Bassand, J.-P.; Camm, A.J.; Corbalan, R.; Darius, H.; Fitzmaurice, D.A.; Goldhaber, S.Z.; et al. Quality of Vitamin K Antagonist Control and 1-Year Outcomes in Patients with Atrial Fibrillation: A Global Perspective from the GARFIELD-AF Registry. PLoS ONE 2016, 11, e0164076. [Google Scholar] [CrossRef]
- Sposato, L.A.; Cipriano, L.E.; Saposnik, G.; Vargas, E.R.; Riccio, P.M.; Hachinski, V. Diagnosis of Atrial Fibrillation after Stroke and Transient Ischaemic Attack: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 377–387. [Google Scholar] [CrossRef]
- RKKP. Styregruppe og formandskab for Dansk Apopleksiregister Dansk Apopleksiregister Årsrapport 2021. 2022, pp. 1–267. Available online: https://www.sundhed.dk/content/cms/69/4669_dap_aarsrapport-2021_270622.pdf (accessed on 15 April 2023).
- Nielsen, M.H.; Backe, M.B.; Pedersen, M.L. Prevalence of Patients Using Antihypertensive Medication in Greenland, and an Assessment of the Importance of Diagnosis for the Associated Quality of Care—A Cross-Sectional Study. Int. J. Circumpolar Health 2022, 81, 2110675. [Google Scholar] [CrossRef] [PubMed]
- Viskum, C.; Larsen, L.; Hansen, C.B.; Ingemann, C.; Eika, M.; Ingelise, J.; Ivalu, O.; Sørensen, K.; Koch, A.; Backer, V.; et al. Befolkningsundersøgelsen i Grønland 2018-Levevilkår, Livsstil Og Helbred Oversigt over Indikatorer for Folkesundheden Statens Institut for Folkesundhed; Syddansk Universitet: Odense, Denmark, 2019; ISBN 9788778994554. [Google Scholar]
- Senftleber, N.K.; Overvad, M.; Dahl-Petersen, I.K.; Bjerregaard, P.; Jørgensen, M.E. Diet and Physical Activity in Greenland: Genetic Interactions and Associations with Obesity and Diabetes. Appl. Physiol. Nutr. Metab. 2021, 46, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Jørsboe, E.; Andersen, M.K.; Skotte, L.; Stæger, F.F.; Færgeman, N.J.; Hanghøj, K.; Santander, C.G.; Senftleber, N.K.; Diaz, L.J.; Overvad, M.; et al. An LDLR Missense Variant Poses High Risk of Familial Hypercholesterolemia in 30% of Greenlanders and Offers Potential of Early Cardiovascular Disease Intervention. Hum. Genet. Genom. Adv. 2022, 3, 100118. [Google Scholar] [CrossRef]
- Bundgaard, J.S.; Jørgensen, M.E.; Andersen, K.; Bundgaard, H.; Geisler, U.W.; Pedersen, M.L. Dyslipidemia and the Preventive Potential in the Greenlandic Population. Atheroscler. Plus 2023, 51, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.E.; Borch-Johnsen, K.; Witte, D.R.; Bjerregaard, P. Diabetes in Greenland and Its Relationship with Urbanization. Diabet. Med. 2012, 29, 755–760. [Google Scholar] [CrossRef]
- Thuesen, A.C.B.; Stæger, F.F.; Kaci, A.; Solheim, M.H.; Aukrust, I.; Jørsboe, E.; Santander, C.G.; Andersen, M.K.; Li, Z.; Gilly, A.; et al. A Novel Splice-Affecting HNF1A Variant with Large Population Impact on Diabetes in Greenland. Lancet Reg. Health-Eur. 2023, 24, 100529. [Google Scholar] [CrossRef]
- Moltke, I.; Grarup, N.; Jørgensen, M.E.; Bjerregaard, P.; Treebak, J.T.; Fumagalli, M.; Korneliussen, T.S.; Andersen, M.A.; Nielsen, T.S.; Krarup, N.T.; et al. A Common Greenlandic TBC1D4 Variant Confers Muscle Insulin Resistance and Type 2 Diabetes. Nature 2014, 512, 190–193. [Google Scholar] [CrossRef]
- Tvermosegaard, M.; Rønn, P.F.; Pedersen, M.L.; Bjerregaard, P.; Dahl Pedersen, I.; Larsen, C.V.L.; Jørgensen, M.E. Validation of Cardiovascular Diagnoses in the Greenlandic Hospital Discharge Register for Epidemiological Use. Int. J. Circumpolar Health 2018, 77, 1422668. [Google Scholar] [CrossRef]
- Pezawas, T. ECG Smart Monitoring versus Implantable Loop Recorders for Atrial Fibrillation Detection after Cryptogenic Stroke—An Overview for Decision Making. J. Cardiovasc. Dev. Dis. 2023, 10, 306. [Google Scholar] [CrossRef]
| Age | <55 Years | 55–64 Years | 65–74 Years | 75+ Years | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | |
| Stroke, n (% of all patients) a | 58 (27) | 65 (25) | 72 (34) | 97 (38) | 43 (20) | 72 (28) | 40 (19) | 23 (8.9) | 213 (45) | 257 (55) |
| AF, n (% of patients in age group) a | 2 (3.4) | 7 (10.8) | 8 (11.1) | 15 (15.5) | 4 (9.3) | 16 (22.2) | 13 (33) | 3 (13) | 27 (12.7) | 41 (16.0) |
| Holter, n (% of patients in age group) b | 34 (63) | 37 (64) | 34 (63) | 44 (59) | 22 (69) | 43 (70) | 21 (62) | 10 (53) | 111 (64) | 134 (63) |
| Rivaroxaban, n (% of patients with AF in age group) a | 2 (100) | 7 (100) | 7 (87.5) | 12 (80) | 4 (100) | 9 (56.3) | 9 (69.2) | 3 (100) | 22 (81.5) | 31 (75.6) |
| Warfarin, n (% of patients with AF in age group) a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6.3) | 0 (0) | 0 (0) | 0 (0) | 1 (2.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albertsen, N.; Hansen, A.S.; Skovgaard, N.; Pedersen, M.L.; Andersen, S.; Riahi, S. Is the Pattern Changing? Atrial Fibrillation and Screening with Holter Electrocardiograms among Ischemic Stroke Patients in Greenland from 2016 to 2021. J. Clin. Med. 2023, 12, 5378. https://doi.org/10.3390/jcm12165378
Albertsen N, Hansen AS, Skovgaard N, Pedersen ML, Andersen S, Riahi S. Is the Pattern Changing? Atrial Fibrillation and Screening with Holter Electrocardiograms among Ischemic Stroke Patients in Greenland from 2016 to 2021. Journal of Clinical Medicine. 2023; 12(16):5378. https://doi.org/10.3390/jcm12165378
Chicago/Turabian StyleAlbertsen, Nadja, Anne Sofie Hansen, Nils Skovgaard, Michael Lynge Pedersen, Stig Andersen, and Sam Riahi. 2023. "Is the Pattern Changing? Atrial Fibrillation and Screening with Holter Electrocardiograms among Ischemic Stroke Patients in Greenland from 2016 to 2021" Journal of Clinical Medicine 12, no. 16: 5378. https://doi.org/10.3390/jcm12165378
APA StyleAlbertsen, N., Hansen, A. S., Skovgaard, N., Pedersen, M. L., Andersen, S., & Riahi, S. (2023). Is the Pattern Changing? Atrial Fibrillation and Screening with Holter Electrocardiograms among Ischemic Stroke Patients in Greenland from 2016 to 2021. Journal of Clinical Medicine, 12(16), 5378. https://doi.org/10.3390/jcm12165378






