Treatment of Vulvovaginal Candidiasis—An Overview of Guidelines and the Latest Treatment Methods
Abstract
:1. Introduction
2. Materials and Methods
3. Symptoms and Diagnosis of VVC
4. Treatment of Uncomplicated VVC
4.1. Conventional Treatment Methods for Uncomplicated VVC
4.2. Unconventional Treatment Methods for Uncomplicated VVC
5. Treatment of Complicated VVC
5.1. Treatment of VVC Caused by Non-Albicans Species
5.2. Treatment of Vulvovaginal Candidiasis in Patients with Diabetes Mellitus
5.3. Treatment of VVC in HIV-Positive Patients
5.4. Treatment of VVC with Azole Intolerance or Resistance
5.5. Treatment of Recurrent VVC
5.6. Treatment of VVC in Pregnant Women
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nyirjesy, P.; Brookhart, C.; Lazenby, G.; Schwebke, J.; Sobel, J.D. Vulvovaginal Candidiasis: A Review of the Evidence for the 2021 Centers for Disease Control and Prevention of Sexually Transmitted Infections Treatment Guidelines. Clin. Infect. Dis. 2022, 74, S162–S168. [Google Scholar] [CrossRef]
- Nyirjesy, P. Vulvovaginal Candidiasis and Bacterial Vaginosis. Infect. Dis. Clin. N. Am. 2008, 22, 637–652. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent Vulvovaginal Candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef]
- Cole, A.M. Innate Host Defense of Human Vaginal and Cervical Mucosae. Curr. Top. Microbiol. Immunol. 2006, 306, 199–230. [Google Scholar] [CrossRef] [PubMed]
- Felix, T.C.; de Brito Röder, D.V.D.; Dos Santos Pedroso, R. Alternative and Complementary Therapies for Vulvovaginal Candidiasis. Folia Microbiol. 2019, 64, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.; Watson, C.; Deckx, L.; Pirotta, M.; Smith, J.; Van Driel, M.L. Treatment for Recurrent Vulvovaginal Candidiasis (Thrush). Cochrane Database Syst. Rev. 2022, 2022. [Google Scholar] [CrossRef]
- Nyirjesy, P.; Sobel, J.D. Vulvovaginal Candidiasis. Obstet. Gynecol. Clin. N. Am. 2003, 30, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.; Lee, A.; Fischer, G. Quality of Life in Patients with Chronic Vulvovaginal Candidiasis: A before and after Study on the Impact of Oral Fluconazole Therapy. Australas. J. Dermatol. 2017, 58, e176–e181. [Google Scholar] [CrossRef] [PubMed]
- Crouss, T.; Sobel, J.D.; Smith, K.; Nyirjesy, P. Long-Term Outcomes of Women With Recurrent Vulvovaginal Candidiasis After a Course of Maintenance Antifungal Therapy. J. Low. Genit. Tract Dis. 2018, 22, 382–386. [Google Scholar] [CrossRef]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global Burden of Recurrent Vulvovaginal Candidiasis: A Systematic Review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Leusink, P.; Van De Pasch, S.; Teunissen, D.; Laan, E.T.; Lagro-Janssen, A.L. The Relationship Between Vulvovaginal Candidiasis and Provoked Vulvodynia: A Systematic Review. J. Sex. Med. 2018, 15, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, A.T.; Wiederhold, N.P.; Flowers, S.A.; Zhang, Q.; Kelly, S.L.; Morschhäuser, J.; Yates, C.M.; Hoekstra, W.J.; Schotzinger, R.J.; Garvey, E.P.; et al. In Vitro Activities of the Novel Investigational Tetrazoles VT-1161 and VT-1598 Compared to the Triazole Antifungals against Azole-Resistant Strains and Clinical Isolates of Candida Albicans. Antimicrob. Agents Chemother. 2019, 63, e00341-19. [Google Scholar] [CrossRef]
- Larkin, E.L.; Long, L.; Isham, N.; Borroto-Esoda, K.; Barat, S.; Angulo, D.; Wring, S.; Ghannoum, M. A Novel 1,3-Beta-d-Glucan Inhibitor, Ibrexafungerp (Formerly SCY-078), Shows Potent Activity in the Lower PH Environment of Vulvovaginitis. Antimicrob. Agents Chemother. 2019, 63, e02611-18. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Sobel, J.D.; Nyirjesy, P.; Sobel, R.; Williams, V.L.; Yu, Q.; Noverr, M.C.; Fidel, P.L. Current Patient Perspectives of Vulvovaginal Candidiasis: Incidence, Symptoms, Management and Post-Treatment Outcomes. BMC Womens Health 2019, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Eckert, L. Vulvovaginal Candidiasis: Clinical Manifestations, Risk Factors, Management Algorithm. Obstet. Gynecol. 1998, 92, 757–765. [Google Scholar] [CrossRef]
- Mtibaa, L.; Fakhfakh, N.; Kallel, A.; Belhadj, S.; Belhaj Salah, N.; Bada, N.; Kallel, K. Vulvovaginal Candidiasis: Etiology, Symptomatology and Risk Factors. J. Mycol. Médicale 2017, 27, 153–158. [Google Scholar] [CrossRef]
- Anderson, M.R. Evaluation of Vaginal Complaints. JAMA 2004, 291, 1368. [Google Scholar] [CrossRef]
- Abbott, J. Clinical and Microscopic Diagnosis of Vaginal Yeast Infection: A Prospective Analysis. Ann. Emerg. Med. 1995, 25, 587–591. [Google Scholar] [CrossRef]
- Schaaf, V.M.; Perez-Stable, E.J.; Borchardt, K. The Limited Value of Symptoms and Signs in the Diagnosis of Vaginal Infections. Arch. Intern. Med. 1990, 150, 1929–1933. [Google Scholar] [CrossRef]
- Geiger, A.M.; Foxman, B. Risk Factors for Vulvovaginal Candidiasis: A Case- Control Study among University Students. Epidemiology 1996, 7, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.; Spinillo, A.; Osnengo, G.; Penna, C.; Guaschino, S.; Beltrame, A.; Blasi, N.; Festa, A. An Epidemiological Survey of Vulvovaginal Candidiasis in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, 66–72. [Google Scholar] [CrossRef]
- Linhares, I.M.; Witkin, S.S.; Miranda, S.D.; Fonseca, A.M.; Pinotti, J.A.; Ledger, W.J. Differentiation Between Women With Vulvovaginal Symptoms Who Are Positive or Negative for Candida Species by Culture. Infect. Dis. Obstet. Gynecol. 2001, 9, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Aniebue, U.U.; Nwankwo, T.O.; Nwafor, M.I. Vulvovaginal Candidiasis in Reproductive Age Women in Enugu Nigeria, Clinical versus Laboratory-Assisted Diagnosis. Niger. J. Clin. Pract. 2018, 21, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Gaydos, C.A.; Nyirjesy, P.; Paradis, S.; Kodsi, S.; Cooper, C.K. Diagnostic Performance of a Molecular Test versus Clinician Assessment of Vaginitis. J. Clin. Microbiol. 2018, 56, e00252-18. [Google Scholar] [CrossRef] [PubMed]
- Farr, A.; Effendy, I.; Frey Tirri, B.; Hof, H.; Mayser, P.; Petricevic, L.; Ruhnke, M.; Schaller, M.; Schaefer, A.P.A.; Sustr, V.; et al. Guideline: Vulvovaginal Candidosis (AWMF 015/072, Level S2k). Mycoses 2021, 64, 583–602. [Google Scholar] [CrossRef]
- Naglik, J.R.; Gaffen, S.L.; Hube, B. Candidalysin: Discovery and Function in Candida Albicans Infections. Curr. Opin. Microbiol. 2019, 52, 100–109. [Google Scholar] [CrossRef]
- Jafarzadeh, L.; Ranjbar, M.; Nazari, T.; Naeimi Eshkaleti, M.; Aghaei Gharehbolagh, S.; Sobel, J.D.; Mahmoudi, S. Vulvovaginal Candidiasis: An Overview of Mycological, Clinical, and Immunological Aspects. J. Obstet. Gynaecol. Res. 2022, 48, 1546–1560. [Google Scholar] [CrossRef]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef]
- Morton, R.S.; Rashid, S. Candidal Vaginitis: Natural History, Predisposing Factors and Prevention. Proc. R. Soc. Med. 1977, 70 (Suppl. S4), 3–6. [Google Scholar] [CrossRef]
- Wiesenfeld, H.C.; Macio, I. The Infrequent Use of Office-Based Diagnostic Tests for Vaginitis. Am. J. Obstet. Gynecol. 1999, 181, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, C.A.; Beqaj, S.; Schwebke, J.R.; Lebed, J.; Smith, B.; Davis, T.E.; Fife, K.H.; Nyirjesy, P.; Spurrell, T.; Furgerson, D.; et al. Clinical Validation of a Test for the Diagnosis of Vaginitis. Obstet. Gynecol. 2017, 130, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bolan, G.A. Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2015, 64, S759–S762. [Google Scholar]
- Sobel, J.D.; Wiesenfeld, H.C.; Martens, M.; Danna, P.; Hooton, T.M.; Rompalo, A.; Sperling, M.; Livengood, C.; Horowitz, B.; Von Thron, J.; et al. Maintenance Fluconazole Therapy for Recurrent Vulvovaginal Candidiasis. N. Engl. J. Med. 2004, 351, 876–883. [Google Scholar] [CrossRef]
- Denison, H.J.; Worswick, J.; Bond, C.M.; Grimshaw, J.M.; Mayhew, A.; Gnani Ramadoss, S.; Robertson, C.; Schaafsma, M.E.; Watson, M.C. Oral versus Intra-Vaginal Imidazole and Triazole Anti-Fungal Treatment of Uncomplicated Vulvovaginal Candidiasis (Thrush). Cochrane Database Syst. Rev. 2020, 8, CD002845. [Google Scholar] [CrossRef]
- Sexually Transmitted Infections Treatment Guidelines. Vulvovaginal Candidiasis (VVC). 2021. Available online: https://www.cdc.gov/std/treatment-guidelines/candidiasis.htm (accessed on 22 July 2023).
- Mendling, W. Guideline: Vulvovaginal Candidosis (AWMF 015/072), S2k (Excluding Chronic Mucocutaneous Candidosis). Mycoses 2015, 58, 1–15. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K.; Calandra, T.F.; Edwards, J.E.; Filler, S.G.; Fisher, J.F.; Kullberg, B.-J.; Zeichner, L.O.; et al. Clinical Practice Guidelines for the Management Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Wang, Y.; Bandara, H.M.H.N.; Mayer, M.P.A.; Samaranayake, L.P. Probiotic Lactobacilli Inhibit Early Stages of Candida Albicans Biofilm Development by Reducing Their Growth, Cell Adhesion, and Filamentation. Appl. Microbiol. Biotechnol. 2016, 100, 6415–6426. [Google Scholar] [CrossRef]
- Stabile, G.; Gentile, R.M.; Carlucci, S.; Restaino, S.; De Seta, F. A New Therapy for Uncomplicated Vulvovaginal Candidiasis and Its Impact on Vaginal Flora. Healthcare 2021, 9, 1555. [Google Scholar] [CrossRef]
- Marrazzo, J.M.; Dombrowski, J.C.; Wierzbicki, M.R.; Perlowski, C.; Pontius, A.; Dithmer, D.; Schwebke, J. Safety and Efficacy of a Novel Vaginal Anti-Infective, TOL-463, in the Treatment of Bacterial Vaginosis and Vulvovaginal Candidiasis: A Randomized, Single-Blind, Phase 2, Controlled Trial. Clin. Infect. Dis. 2019, 68, 803–809. [Google Scholar] [CrossRef]
- Mirzaeei, S.; Zangeneh, M.; Veisi, F.; Parsa, S.; Hematti, M. Chlorhexidine, clotrimazole, metronidazole and combination therapy in the treatment of vaginal infections. J. Med. Life 2021, 14, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J. Treatment of Non-Albicans Candida Vaginitis with Amphotericin B Vaginal Suppositories. Am. J. Obstet. Gynecol. 2005, 192, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.P.; Sanglard, D.; Donnelly, S.M.; Shanley, D.B.; Sullivan, D.J.; Coleman, D.C. Identification and Expression of Multidrug Transporters Responsible for Fluconazole Resistance in Candida Dubliniensis. Antimicrob. Agents Chemother. 1998, 42, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
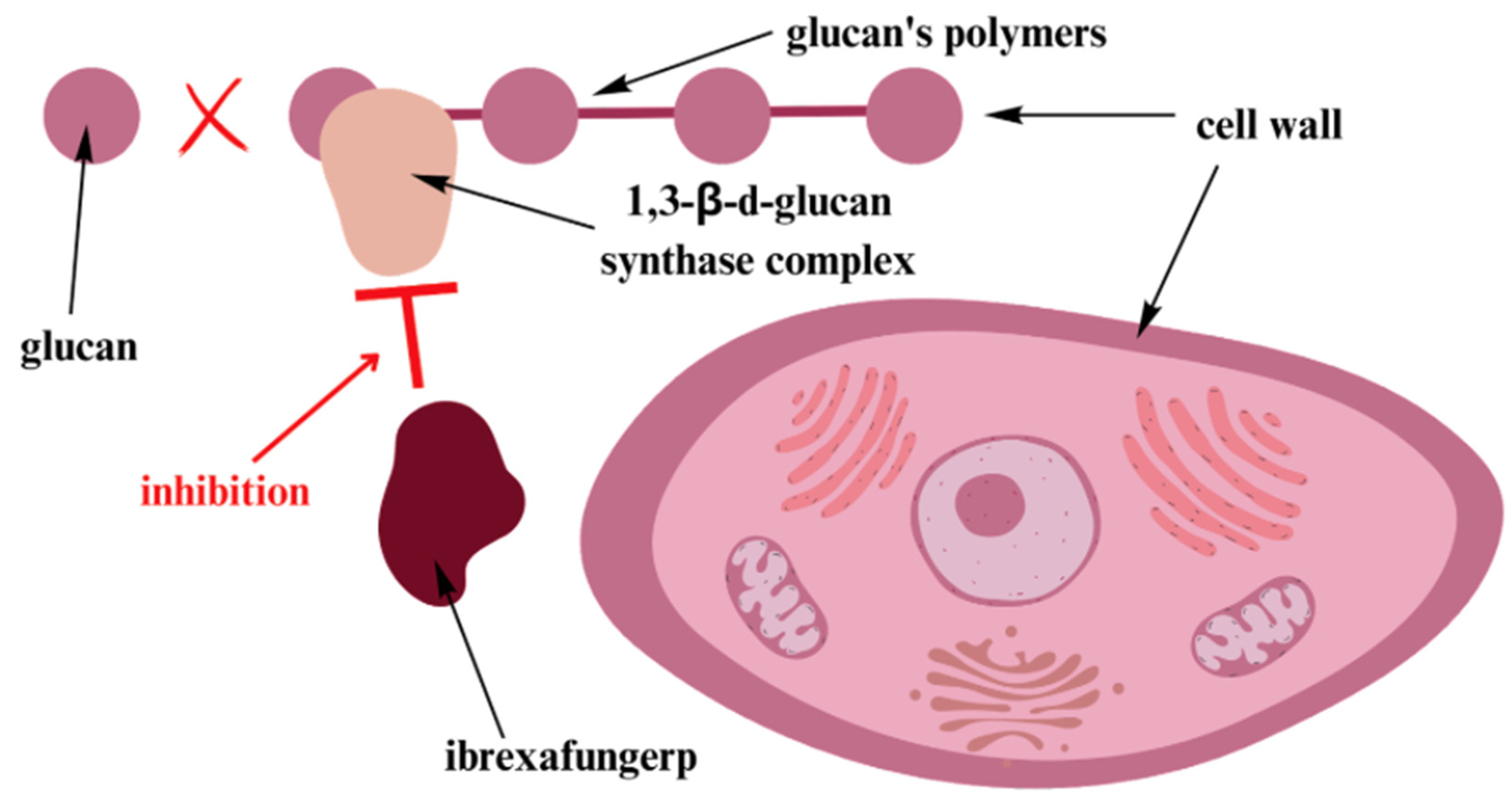
- Azie, N.; Angulo, D.; Dehn, B.; Sobel, J.D. Oral Ibrexafungerp: An Investigational Agent for the Treatment of Vulvovaginal Candidiasis. Expert Opin. Investig. Drugs 2020, 29, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. New Antifungals for Vulvovaginal Candidiasis: What Is Their Role? Clin. Infect. Dis. 2023, 76, 783–785. [Google Scholar] [CrossRef]
- Donders, G.; Sziller, I.O.; Paavonen, J.; Hay, P.; De Seta, F.; Bohbot, J.M.; Kotarski, J.; Vives, J.A.; Szabo, B.; Cepuliené, R.; et al. Management of Recurrent Vulvovaginal Candidosis: Narrative Review of the Literature and European Expert Panel Opinion. Front. Cell. Infect. Microbiol. 2022, 12, 934353. [Google Scholar] [CrossRef]
- Guideline Development Group; Edwards, A.; Rautemaa-Richardson, R.; Owen, C.; Nathan, B.; Palmer, B.; Wood, C.; Ahmed, H. Sameena Ahmad Patient Representatives; FitzGerald, M. British Association for Sexual Health and HIV National Guideline for the Management of Vulvovaginal Candidiasis (2019). Int. J. STD AIDS 2020, 31, 1124–1144. [Google Scholar] [CrossRef]
- Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-oi/guidelines-adult-adolescent-oi.pdf (accessed on 22 July 2023).
- Nasrollahi Omran, A.; Vakili, L.; Jafarpur, M. The Determination of Vaginal Candidiasis InWomen Referred to Shahid Rajaei Hospital in Tonekabon (2009–2010). mljgoums 2011, 5, 1–7. [Google Scholar]
- Paiva, L.C.F.; Vidigal, P.G.; Donatti, L.; Svidzinski, T.I.E.; Consolaro, M.E.L. Assessment of in Vitro Biofilm Formation by Candida Species Isolates from Vulvovaginal Candidiasis and Ultrastructural Characteristics. Micron 2012, 43, 497–502. [Google Scholar] [CrossRef]
- Desai, J.V.; Mitchell, A.P. Candida albicans Biofilm Development and Its Genetic Control. Microbiol. Spectr. 2015, 3, 99–114. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Pharmacodynamics, Mechanisms of Action and Resistance, and Spectrum of Activity of New Antifungal Agents. J. Fungi 2022, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.G.S.; Hull, C.M.; Parker, J.E.; Garvey, E.P.; Hoekstra, W.J.; Moore, W.R.; Schotzinger, R.J.; Kelly, D.E.; Kelly, S.L. The Clinical Candidate VT-1161 Is a Highly Potent Inhibitor of Candida Albicans CYP51 but Fails To Bind the Human Enzyme. Antimicrob. Agents Chemother. 2014, 58, 7121–7127. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.R.; Sobel, J.D.; Nyirjesy, P.; Ghannoum, M.A.; Schotzinger, R.J.; Degenhardt, T.P. A Randomized Phase 2 Study of VT-1161 for the Treatment of Acute Vulvovaginal Candidiasis. Clin. Infect. Dis. 2021, 73, e1518–e1524. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.G.; Maximos, B.; Degenhardt, T.; Person, K.; Curelop, S.; Ghannoum, M.; Flynt, A.; Brand, S.R. Phase 3 Study Evaluating the Safety and Efficacy of Oteseconazole in the Treatment of Recurrent Vulvovaginal Candidiasis and Acute Vulvovaginal Candidiasis Infections. Am. J. Obstet. Gynecol. 2022, 227, 880.e1–880.e11. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Sobel, R.; Gersten, J.K.; Sussman, S.A.; Lederman, S.N.; Jacobs, M.A.; Chappell, B.T.; Weinstein, D.L.; Moffett, A.H.; Azie, N.E.; et al. Ibrexafungerp Versus Placebo for Vulvovaginal Candidiasis Treatment: A Phase 3, Randomized, Controlled Superiority Trial (VANISH 303). Clin. Infect. Dis. 2022, 74, 1979–1985. [Google Scholar] [CrossRef]
- Morris, G.C.; Dean, G.; Soni, S.; Sundaram, S.; Fearnley, N.; Wilson, J.D. Outcomes and Experiences of Using Oral Voriconazole with or without Concomitant Topical Agents to Treat Refractory Vulvovaginal Yeast Infections. Int. J. STD AIDS 2022, 33, 1134–1141. [Google Scholar] [CrossRef]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Woodburn, K.W.; Clemens, L.E.; Jaynes, J.; Joubert, L.-M.; Botha, A.; Nazik, H.; Stevens, D.A. Designed Antimicrobial Peptides for Recurrent Vulvovaginal Candidiasis Treatment. Antimicrob. Agents Chemother. 2019, 63, e02690-18. [Google Scholar] [CrossRef]
- Lírio, J.; Giraldo, P.C.; Amaral, R.L.; Sarmento, A.C.A.; Costa, A.P.F.; Gonçalves, A.K. Antifungal (Oral and Vaginal) Therapy for Recurrent Vulvovaginal Candidiasis: A Systematic Review Protocol. BMJ Open 2019, 9, e027489. [Google Scholar] [CrossRef]
- Philips, N.; Burchill, D.; O’Donoghue, D.; Keller, T.; Gonzalez, S. Identification of Benzene Metabolites in Dermal Fibroblasts as Nonphenolic: Regulation of Cell Viability, Apoptosis, Lipid Peroxidation and Expression of Matrix Metalloproteinase 1 and Elastin by Benzene Metabolites. Skin Pharmacol. Physiol. 2004, 17, 147–152. [Google Scholar] [CrossRef]
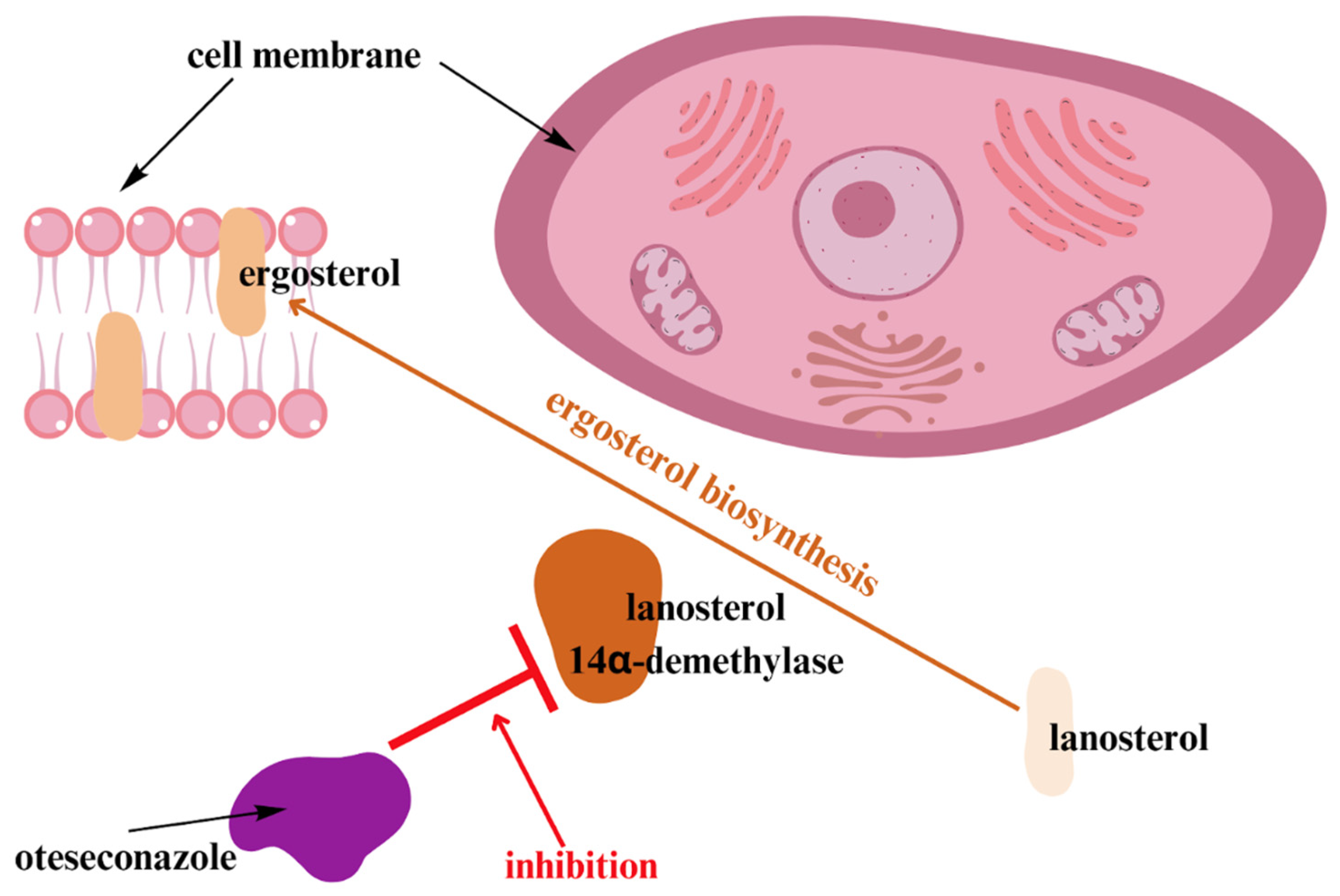
- Sobel, J.D.; Nyirjesy, P. Oteseconazole: An Advance in Treatment of Recurrent Vulvovaginal Candidiasis. Future Microbiol. 2021, 16, 1453–1461. [Google Scholar] [CrossRef]
- Grant, L.M.; Orenstein, R. Treatment of Recurrent Vulvovaginal Candidiasis With Ibrexafungerp. J. Investig. Med. High Impact Case Rep. 2022, 10, 232470962211231. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Schwartz, M.M.; Schmidt, C.S.; Sobel, J.D.; Nyirjesy, P.; Schodel, F.; Marchus, E.; Lizakowski, M.; DeMontigny, E.A.; Hoeg, J.; et al. A Fungal Immunotherapeutic Vaccine (NDV-3A) for Treatment of Recurrent Vulvovaginal Candidiasis—A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2018, 66, 1928–1936. [Google Scholar] [CrossRef]
- Russo, R.; Superti, F.; Karadja, E.; De Seta, F. Randomised Clinical Trial in Women with Recurrent Vulvovaginal Candidiasis: Efficacy of Probiotics and Lactoferrin as Maintenance Treatment. Mycoses 2019, 62, 328–335. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lin, Z.; Robb, S.; Ezeamama, A. Serum Vitamin D Levels and Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 4555–4577. [Google Scholar] [CrossRef] [PubMed]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef]
- Chew, S.Y.; Than, L.T.L. Vulvovaginal Candidosis: Contemporary Challenges and the Future of Prophylactic and Therapeutic Approaches. Mycoses 2016, 59, 262–273. [Google Scholar] [CrossRef]
- Alsaad, A.M.S.; Kaplan, Y.C.; Koren, G. Exposure to Fluconazole and Risk of Congenital Malformations in the Offspring: A Systematic Review and Meta-Analysis. Reprod. Toxicol. 2015, 52, 78–82. [Google Scholar] [CrossRef]
- Mittelstaedt, R.; Kretz, A.; Levine, M.; Handa, V.L.; Ghanem, K.G.; Sobel, J.D.; Powell, A.; Tuddenham, S. Data on Safety of Intravaginal Boric Acid Use in Pregnant and Nonpregnant Women: A Narrative Review. Sex. Transm. Dis. 2021, 48, e241–e247. [Google Scholar] [CrossRef]
- Mendling, W.; Weissenbacher, E.R.; Gerber, S.; Prasauskas, V.; Grob, P. Use of Locally Delivered Dequalinium Chloride in the Treatment of Vaginal Infections: A Review. Arch. Gynecol. Obstet. 2016, 293, 469–484. [Google Scholar] [CrossRef]
- Abdelmonem, A.M.; Rasheed, S.M.; Mohamed, A.S. Bee-Honey and Yogurt: A Novel Mixture for Treating Patients with Vulvovaginal Candidiasis during Pregnancy. Arch. Gynecol. Obstet. 2012, 286, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lijuan, D.; Lu, Y.; Liping, W.; Yaya, S.; Xiaoyun, L.; Xiangrong, L.I. Effectiveness of Redcore Lotion in Patients with Vulvovaginal Candidiasis: A Systematic Review and Meta-Analysis. J. Tradit. Chin. Med. 2022, 42, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Gamal, A.; Chu, S.; McCormick, T.S.; Borroto-Esoda, K.; Angulo, D.; Ghannoum, M.A. Ibrexafungerp, a Novel Oral Triterpenoid Antifungal in Development: Overview of Antifungal Activity Against Candida Glabrata. Front. Cell. Infect. Microbiol. 2021, 11, 642358. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Rodriguez-Garcia, M.; Wira, C.R. The Immune System in Menopause: Pros and Cons of Hormone Therapy. J. Steroid Biochem. Mol. Biol. 2014, 142, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Y.; Feng, D.; Wei, D.M.; Mei, L.; Chen, H.; Wang, X.; Fang, F. Probiotics for Vulvovaginal Candidiasis in Non-Pregnant Women. Cochrane Database Syst. Rev. 2017, 11, CD010496. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.; Gottlieb, A. Probiotics for Oral and Vulvovaginal Candidiasis: A Review. Dermatol. Ther. 2019, 32, e12970. [Google Scholar] [CrossRef]
| Author of the Study | Year of Publication | Medical Treatment | Dose | Number of Patients | Time until Symptoms Subside | Side Effects |
|---|---|---|---|---|---|---|
| Stabile G et al. [40] | 2021 | Unilen® Microbio+ | 1 tablet containing Saccharomyces cerevisiae in the morning and 1 tablet containing melatonin and GLA-14 in the evening. | 40 | 15 and 30 days | Microscopic wet-mount analysis at 1 and 3 months showed an increase in Lactobacillus count and a reduction in the polymorphonucleate cells in the Unilen® Microbio+ group. |
| Marrazzo JM et al. [41] | 2019 | TOL-463 | Medication was administered vaginally once nightly for 7 days as either a 5 g dose of gel or a 2 g unit-dose insert. | 36 patients with VVC | 9–12 days | Headache, vulvovaginal burning sensation, vulvovaginal pruritus. |
| Mirzaee S et al. [42] | 2021 | Chlorhexidine | Clotrimazole vaginal cream or 0.5% chlorhexidine vaginal gel was administered. | 34 patients with VVC | 5 days | Vaginal burning, nausea, vomiting, cutaneous lesions. |
| Author of the Study | Year of Publication | Number of Patients | Dose | Time until Symptoms Subside | Side Effects |
|---|---|---|---|---|---|
| Brand SR et al. [55] | 2021 | 55 | 300 mg once daily of VT-1161 for 3 days, 600 mg q.d. for 3 days, or 600 mg twice daily for 3 days or receiving a single dose of fluconazole 150 mg. | 28 days | Infections: nasopharyngitis, urinary tract infection, vaginitis bacterial, nausea. |
| Martens MG et al. [56] | 2022 | 219 | 600 mg of oral oteseconazole on day 1 (4 × 150 mg) and 450 mg on day 2 (3 × 150 mg), with matching placebo capsules, or 3 sequential oral doses of fluconazole. | 2 weeks | Urinary tract infection, bacterial vaginosis, headache, nausea, diarrhea, upper respiratory tract infection, pyrexia. |
| Author of the Study | Year of Publication | Number of Patients | Dose | Time until Symptoms Subside | Side Effects |
|---|---|---|---|---|---|
| Schwebke et al. [57] | 2022 | 366 | Patients were randomly assigned 2:1 to receive ibrexafungerp (300 mg twice per day) or placebo. | 25 days | Treatment-related diarrhea, nausea, vomiting, dizziness, pneumonia, bronchial hyperactivity. |
| Grant LM et al. [64] | 2022 | 1 | Ibrexafungerp 375 mg twice daily for 3 days, followed by 375 mg twice daily on day 14. | 7 days, but patient’s symptoms recurred prior to day 14 of this regimen | Fatigue, nausea. |
| Author of the Study | Year of Publication | Number of Patients | Dose | Time until Symptoms Subside | Side Effects |
|---|---|---|---|---|---|
| Mendling et al. [72] | 2015 | 60 | 10 mg of dequalinium chloride. | No data | No side effects. |
| Abdelmonem et al. [73] | 2012 | 129 | Mixture of bee honey and yogurt—30 g twice daily for 7 days. | 25 days | Soiling of underclothes; local irritation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satora, M.; Grunwald, A.; Zaremba, B.; Frankowska, K.; Żak, K.; Tarkowski, R.; Kułak, K. Treatment of Vulvovaginal Candidiasis—An Overview of Guidelines and the Latest Treatment Methods. J. Clin. Med. 2023, 12, 5376. https://doi.org/10.3390/jcm12165376
Satora M, Grunwald A, Zaremba B, Frankowska K, Żak K, Tarkowski R, Kułak K. Treatment of Vulvovaginal Candidiasis—An Overview of Guidelines and the Latest Treatment Methods. Journal of Clinical Medicine. 2023; 12(16):5376. https://doi.org/10.3390/jcm12165376
Chicago/Turabian StyleSatora, Małgorzata, Arkadiusz Grunwald, Bartłomiej Zaremba, Karolina Frankowska, Klaudia Żak, Rafał Tarkowski, and Krzysztof Kułak. 2023. "Treatment of Vulvovaginal Candidiasis—An Overview of Guidelines and the Latest Treatment Methods" Journal of Clinical Medicine 12, no. 16: 5376. https://doi.org/10.3390/jcm12165376
APA StyleSatora, M., Grunwald, A., Zaremba, B., Frankowska, K., Żak, K., Tarkowski, R., & Kułak, K. (2023). Treatment of Vulvovaginal Candidiasis—An Overview of Guidelines and the Latest Treatment Methods. Journal of Clinical Medicine, 12(16), 5376. https://doi.org/10.3390/jcm12165376







