Burden of Disease; the Current Status of the Diagnosis and Management of Atopic Dermatitis in China
Abstract
:1. Introduction
2. Material and Methods
3. Result and Discussion
3.1. Prevalence of AD in China
3.2. Disease Burden of AD in China
3.3. Clinical Features of Chinese AD Patients
3.4. Diagnosis of AD in China
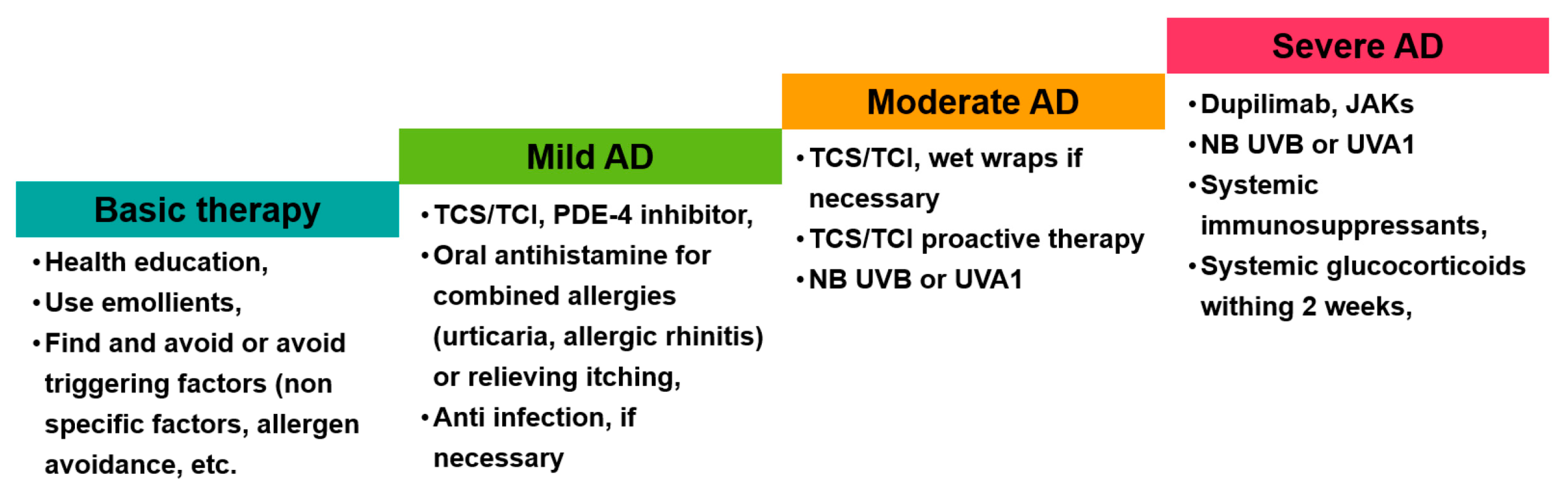
3.5. Management of AD in China
4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silverberg, J.I.; Greenland, P. Eczema and cardiovascular risk factors in 2 US adult population studies. J. Allergy Clin. Immunol. 2015, 135, 721–728.e6. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Y.M.; Egeberg, A.; Gislason, G.H.; Hansen, P.R.; Skov, L.; Thyssen, J.P. Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 310–312.e3. [Google Scholar] [CrossRef] [PubMed]
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A multicenter study on the prevalence of clinical patterns and clinical phenotypes in adult atopic dermatitis. J. Investig. Allergol. Clin. Immunol 2020, 30, 448–450. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Vakharia, P.P.; Chopra, R.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y. Phenotypical differences of childhood-and adult-onset atopic dermatitis. J. Allergy Clin. Immunol. Pract. 2018, 6, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, C. The impact of atopic dermatitis on quality of life. Ann. Nutr. Metab. 2015, 66 (Suppl. 1), 34–40. [Google Scholar] [CrossRef]
- Blome, C.; Radtke, M.A.; Eissing, L.; Augustin, M. Quality of life in patients with atopic dermatitis: Disease burden, measurement, and treatment benefit. Am. J. Clin. Dermatol. 2016, 17, 163–169. [Google Scholar] [CrossRef]
- Zuberbier, T.; Orlow, S.J.; Paller, A.S.; Taïeb, A.; Allen, R.; Hernanz-Hermosa, J.M.; Ocampo-Candiani, J.; Cox, M.; Langeraar, J.; Simon, J.C. Patient perspectives on the management of atopic dermatitis. J. Allergy Clin. Immunol. 2006, 118, 226–232. [Google Scholar] [CrossRef]
- Patruno, C.; Potestio, L.; Napolitano, M. Clinical phenotypes of adult atopic dermatitis and related therapies. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 242–249. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Hanifin, J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: A US population–based study. J. Allergy Clin. Immunol. 2013, 132, 1132–1138. [Google Scholar] [CrossRef]
- Drucker, A.; Li, W.Q.; Lin, L.; Cho, E.; Li, T.; Camargo, C., Jr.; Qureshi, A. Atopic dermatitis (eczema) in US female nurses: Lifestyle risk factors and atopic comorbidities. Br. J. Dermatol. 2016, 174, 1395–1397. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Torres, T. Atopic dermatitis: The new therapeutic revolution in dermatology. Acta Medica Port. 2017, 30, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Blomberg, M.; Rifas-Shiman, S.L.; Camargo, C.A., Jr.; Gold, D.R.; Thyssen, J.P.; Litonjua, A.A.; Oken, E.; Asgari, M.M. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J. Investig. Dermatol. 2019, 139, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Oganisian, A.; Spieker, A.J.; Hoffstad, O.J.; Mitra, N.; Margolis, D.J.; Takeshita, J. Racial/ethnic variation in use of ambulatory and emergency care for atopic dermatitis among US children. J. Investig. Dermatol. 2019, 139, 1906–1913.e1. [Google Scholar] [CrossRef]
- Milam, E.C.; Jacob, S.E.; Cohen, D.E. Contact dermatitis in the patient with atopic dermatitis. J. Allergy Clin. Immunol. Pract. 2019, 7, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66 (Suppl. 1), 8–16. [Google Scholar] [CrossRef]
- Kapur, S.; Watson, W.; Carr, S. Atopic dermatitis. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2018, 14 (Suppl. 2), 52. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, Y.; Mu, Z.-L.; Lu, Q.-J.; Zhang, L.; Yao, X.; Zheng, M.; Tang, Y.-W.; Lu, X.-X.; Xia, X.-J. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis. Chin. Med. J. 2016, 129, 757–762. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Liu, Q.; Wei, F.; Tang, J.; Li, P.; Han, X.; Zou, X.; Xu, G.; Xu, Z.; et al. Phenotypic analysis of atopic dermatitis in children aged 1–12 months: Elaboration of novel diagnostic criteria for infants in China and estimation of prevalence. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1569–1576. [Google Scholar] [CrossRef]
- Li, M.; Cheng, R.; Shi, M.; Liu, J.; Zhang, G.; Liu, Q.; Yu, H.; Yao, Z. Analyses of FLG mutation frequency and filaggrin expression in isolated ichthyosis vulgaris (IV) and atopic dermatitis-associated IV. Br. J. Dermatol. 2013, 168, 1335–1338. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, B.; Chen, J.; Wu, H.; Xu, Y.; Yang, B.; Lu, Q. Thymic stromal lymphopoietin epigenetically upregulates Fc receptor γ subunit-related receptors on antigen-presenting cells and induces T(H)2/T(H)17 polarization through dectin-2. J. Allergy Clin. Immunol. 2019, 144, 1025–1035.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, R.; Gu, C.; Zou, Y.; Yin, H.; Xu, J.; Li, W. Distinct clinical features and serum cytokine pattern of elderly atopic dermatitis in China. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, Y.; Li, W.; Liu, J.; Chen, M.; Gu, H.; Wang, B.; Yao, X. DC-SIGN promotes allergen uptake and activation of dendritic cells in patients with atopic dermatitis. J. Dermatol. Sci. 2016, 84, 128–136. [Google Scholar] [CrossRef]
- Luo, L.; Luo, Y.; Xu, J.; Zhu, R.; Wu, J.; Liu, X.; Li, W.; Yao, X. Heterogeneous origin of IgE in atopic dermatitis and psoriasis revealed by B cell receptor repertoire analysis. Allergy 2022, 77, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Luo, Y.; Sun, J.; Liu, X.; Ling, S.; Xu, B.; Zhang, Y.; Liu, J.; Li, W.; Wang, B.; et al. Transglutaminase 3 Promotes Skin Inflammation in Atopic Dermatitis by Activating Monocyte-Derived Dendritic Cells via DC-SIGN. J. Investig. Dermatol. 2020, 140, 370–379.e8. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yan, S.; Li, F.; Cai, M.; Chai, W.; Wu, M.; Fu, C.; Zhao, Z.; Kan, H.; Kang, K. Prevalence of childhood atopic dermatitis: An urban and rural community-based study in Shanghai, China. PLoS ONE 2012, 7, e36174. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhu, Z.; Liu, X.; Chen, B.; Yin, H.; Gu, C.; Fang, X.; Zhu, R.; Yu, T.; Mi, W.; et al. A dysregulated sebum-microbial metabolite-IL-33 axis initiates skin inflammation in atopic dermatitis. J. Exp. Med. 2022, 219, e20212397. [Google Scholar] [CrossRef]
- Li, W.; Xu, X.; Wen, H.; Wang, Z.; Ding, C.; Liu, X.; Gao, Y.; Su, H.; Zhang, J.; Han, Y.; et al. Inverse Association Between the Skin and Oral Microbiota in Atopic Dermatitis. J. Investig. Dermatol. 2019, 139, 1779–1787.e12. [Google Scholar] [CrossRef]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119.e12. [Google Scholar] [CrossRef]
- Yao, X.; Song, Z.-Q.; Li, W.; Liang, Y.-S.; Zhao, Y.; Cao, H.; Chen, T.; Chen, X.; Feng, A.-P.; Geng, S.-M.; et al. Guidelines for Diagnosis and Treatment of Atopic Dermatitis in China (2020)#. Int. J. Dermatol. Venereol. 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Guo, Y.; Li, P.; Tang, J.; Han, X.; Zou, X.; Xu, G.; Xu, Z.; Wei, F.; Liu, Q.; Wang, M.; et al. Prevalence of atopic dermatitis in Chinese children aged 1–7 ys. Sci. Rep. 2016, 6, 29751. [Google Scholar] [CrossRef] [PubMed]
- Katayama, I.; Aihara, M.; Ohya, Y.; Saeki, H.; Shimojo, N.; Shoji, S.; Taniguchi, M.; Yamada, H.; Allergology, J.S.O. Japanese guidelines for atopic dermatitis 2017. Allergol. Int. 2017, 66, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K. The prevalence of atopic dermatitis in Korean children. Allergy Asthma Immunol. Res. 2016, 8, 1–2. [Google Scholar] [CrossRef]
- Takeuchi, S.; Esaki, H.; Furue, M. Epidemiology of atopic dermatitis in Japan. J. Dermatol. 2014, 41, 200–204. [Google Scholar] [CrossRef]
- Choi, W.J.; Ko, J.Y.; Kim, J.W.; Lee, K.H.; Park, C.W.; Kim, K.H.; Kim, M.N.; Lee, A.Y.; Cho, S.H.; Park, Y.L.; et al. Prevalence and risk factors for atopic dermatitis: A cross-sectional study of 6,453 Korean preschool children. Acta Derm.-Venereol. 2012, 92, 467–471. [Google Scholar] [CrossRef]
- Gu, H.; Yan, Y.; Chen, K. Survey on atopic dermatitis in China. Chin. J. Dermatol. 1994, 33, 379–382. [Google Scholar]
- Dong, W.-L.; An, J.; Yu, M.; Yin, P.; Xu, T.-L.; Liu, B.; Zuberbier, T.; Zhao, Z.-T.; Zhou, M.-G. The prevalence and year lived with disability of atopic dermatitis in China: Findings from the global burden of disease study 2019. World Allergy Organ. J. 2021, 14, 100604. [Google Scholar] [CrossRef]
- Liu, W.; Cai, J.; Sun, C.; Zou, Z.; Zhang, J.; Huang, C. Time-trends for eczema prevalences among children and adults from 1985 to 2015 in China: A systematic review. BMC Public Health 2022, 22, 1294. [Google Scholar] [CrossRef]
- Bylund, S.; Kobyletzki, L.B.; Svalstedt, M.; Svensson, A. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Tarmizi, A.I.; Khalid, K.A.; Gajdacs, M.; Aslam, A.; Jamshed, S. The Epidemiology and Global Burden of Atopic Dermatitis: A Narrative Review. Life 2021, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Wang, A.R.; Li, W.-Q.; Sevetson, E.; Block, J.K.; Qureshi, A.A. The burden of atopic dermatitis: Summary of a report for the National Eczema Association. J. Investig. Dermatol. 2017, 137, 26–30. [Google Scholar] [CrossRef]
- Whiteley, J.; Emir, B.; Seitzman, R.; Makinson, G. The burden of atopic dermatitis in US adults: Results from the 2013 National Health and Wellness Survey. Curr. Med. Res. Opin. 2016, 32, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Laughter, M.; Maymone, M.; Mashayekhi, S.; Arents, B.; Karimkhani, C.; Langan, S.; Dellavalle, R.; Flohr, C. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990–2017. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Corn, G.; Wohlfahrt, J.; Melbye, M.; Bager, P. Retrospective markers of paediatric atopic dermatitis persistence after hospital diagnosis: A nationwide cohort study. Clin. Exp. Allergy 2019, 49, 1455–1463. [Google Scholar] [CrossRef]
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global skin disease morbidity and mortality: An update from the global burden of disease study 2013. JAMA Dermatol. 2017, 153, 406–412. [Google Scholar] [CrossRef]
- Dong, W.; Li, Y.; Liu, S.; Jiang, Y.; Mao, F.; Qi, L.; Zeng, X.; Zhou, M. The disease burden for low back pain in China, 1990 and 2013. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.] 2017, 51, 132–136. [Google Scholar]
- Murray, C.J.L.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; Abu-Raddad, L.J.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef]
- Andersen, L.; Nyeland, M.E.; Nyberg, F. Increasing severity of atopic dermatitis is associated with a negative impact on work productivity among adults with atopic dermatitis in France, Germany, the UK and the USA. Br. J. Dermatol. 2020, 182, 1007–1016. [Google Scholar] [CrossRef]
- Bieber, T.; D’Erme, A.M.; Akdis, C.A.; Traidl-Hoffmann, C.; Lauener, R.; Schäppi, G.; Schmid-Grendelmeier, P. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin. Immunol. 2017, 139, S58–S64. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Pansare, M. New insights and treatments in atopic dermatitis. Immunol. Allergy Clin. 2021, 41, 653–665. [Google Scholar] [CrossRef]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gu, C.; Wang, S.; Yin, H.; Qiu, Z.; Luo, Y.; Li, Z.; Wang, C.; Yao, X.; Li, W. Serum biomarker-based endotypes of atopic dermatitis in China and prediction for efficacy of dupilumab. Br. J. Dermatol. 2023, 188, 649–660. [Google Scholar] [CrossRef]
- Wang, I.J.; Lin, T.J.; Kuo, C.F.; Lin, S.L.; Lee, Y.L.; Chen, P.C. Filaggrin polymorphism P478S, IgE level, and atopic phenotypes. Br. J. Dermatol. 2011, 164, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Pugliarello, S.; Cozzi, A.; Gisondi, P.; Girolomoni, G. Phenotypes of atopic dermatitis. JDDG J. Dtsch. Dermatol. Ges. 2011, 9, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Deleuran, M.; Vestergaard, C. Clinical heterogeneity and differential diagnosis of atopic dermatitis. Br. J. Dermatol. 2014, 170, 2–6. [Google Scholar] [CrossRef]
- Silverberg, N.B. Typical and atypical clinical appearance of atopic dermatitis. Clin. Dermatol. 2017, 35, 354–359. [Google Scholar] [CrossRef]
- Girolomoni, G.; de Bruin-Weller, M.; Aoki, V.; Kabashima, K.; Deleuran, M.; Puig, L.; Bansal, A.; Rossi, A.B. Nomenclature and clinical phenotypes of atopic dermatitis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211002979. [Google Scholar] [CrossRef]
- Williamson, S.; Merritt, J.; De Benedetto, A. Atopic dermatitis in the elderly: A review of clinical and pathophysiological hallmarks. Br. J. Dermatol. 2020, 182, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, M.; De, A.; Godse, K.; Shankar, D.K.; Zawar, V.; Sharma, N.; Mukherjee, S.; Sarda, A.; Dhar, S. Guidelines on management of atopic dermatitis in India: An evidence-based review and an expert consensus. Indian J. Dermatol. 2019, 64, 166. [Google Scholar] [PubMed]
- Sathishkumar, D.; Gupta, A.; Saini, K. Atopic dermatitis in children: An update for pediatricians. Curr. Med. Issues 2020, 18, 317. [Google Scholar] [CrossRef]
- Reynolds, M.; Gorelick, J.; Bruno, M. Atopic Dermatitis: A Review of Current Diagnostic Criteria and a Proposed Update to Management. J. Drugs Dermatol. 2020, 19, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Saeki, H.; Oiso, N.; Honma, M.; Iizuka, H.; Kawada, A.; Tamaki, K. Prevalence of atopic dermatitis in Japanese adults and community validation of the U.K. diagnostic criteria. J. Dermatol. Sci. 2009, 55, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Vakharia, P.P.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y.; Silverberg, J.I. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br. J. Dermatol. 2017, 177, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Firooz, A.; Davoudi, S.M.; Farahmand, A.N.; Majdzadeh, R.; Kashani, M.N.; Dowlati, Y. Validation of the diagnostic criteria for atopic dermatitis. Arch. Dermatol. 1999, 135, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, D.; Todd, G.; Saxe, N.; Milne, J.; Tolosana, S.; Ngcelwane, P.; Hlaba, B.; Mngomeni, L.; Nonxuba, T.; Williams, H. Validation of the UK Working Party diagnostic criteria for atopic eczema in a Xhosa-speaking African population. Br. J. Dermatol. 2007, 156, 111–116. [Google Scholar] [CrossRef]
- Brenninkmeijer, E.; Schram, M.; Leeflang, M.; Bos, J.; Spuls, P.I. Diagnostic criteria for atopic dermatitis: A systematic review. Br. J. Dermatol. 2008, 158, 754–765. [Google Scholar] [CrossRef]
- Lan, C.-C.E.; Lee, C.-H.; Lu, Y.-W.; Lin, C.-L.; Chiu, H.-H.; Chou, T.-C.; Hu, S.C.-S.; Wu, C.-Y.; Kim, Y.-Y.; Yang, H.-J.; et al. Prevalence of adult atopic dermatitis among nursing staff in a Taiwanese medical center: A pilot study on validation of diagnostic questionnaires. J. Am. Acad. Dermatol. 2009, 61, 806–812. [Google Scholar] [CrossRef]
- Jøhnke, H.; Vach, W.; Norberg, L.; Bindslev-Jensen, C.; Høst, A.; Andersen, K.E. A comparison between criteria for diagnosing atopic eczema in infants. Br. J. Dermatol. 2005, 153, 352–358. [Google Scholar] [CrossRef]
- Cheng, R.; Zhang, H.; Zong, W.; Tang, J.; Han, X.; Zhang, L.; Zhang, X.; Gu, H.; Shu, Y.; Peng, G.; et al. Development and validation of new diagnostic criteria for atopic dermatitis in children of China. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Cheng, D.; Sun, Z.; Shen, Y.; Wang, S.; Liu, X.; Pei, X.; Deng, S.; Pan, H.; Liao, Z.; et al. Validation of diagnostic criteria for atopic dermatitis and proposal of novel diagnostic criteria for adult and elderly Chinese populations: A multicentre, prospective, clinical setting-based study. Br. J. Dermatol. 2022, 188, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Guo, Y.; Huang, L.; Hao, F.; Gao, X.; Bieber, T.; Yao, Z. Current status in diagnosis of atopic dermatitis in China. Allergy 2017, 72, 1277–1278. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, H.; Chen, X.; Guo, Y.; Tao, J.; Qi, H.; Gan, J.; Jiang, A.; Yu, H.; Liang, J.; et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J. Eur. Acad. Dermatol. Venereol. JEADV 2011, 25, 1206–1212. [Google Scholar] [CrossRef]
- Ashenager, M.S.; Grgela, T.; Aragane, Y.; Kawada, A. Inhibition of cytokine-induced expression of T-cell cytokines by antihistamines. J. Investig. Allergol. Clin. Immunol. 2007, 17, 20–26. [Google Scholar]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Avila Valle, G.; Barbarot, S.; Bieber, T.; Brough, H.A.; Calzavara Pinton, P.; Christen-Zäch, S.; et al. European guideline (EuroGuiDerm) on atopic eczema—Part II: Non-systemic treatments and treatment recommendations for special AE patient populations. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, 1904–1926. [Google Scholar] [CrossRef]
- Tan, H.Y.; Zhang, A.L.; Chen, D.; Xue, C.C.; Lenon, G.B. Chinese herbal medicine for atopic dermatitis: A systematic review. J. Am. Acad. Dermatol. 2013, 69, 295–304. [Google Scholar] [CrossRef]
- Li, L.; Mou, X.; Xie, H.; Zhang, A.; Li, J.; Wang, R.; Seid, A.; Tang, L.Y.; Wang, L.; Leung, P.C.; et al. In vitro tests to evaluate embryotoxicity and irritation of Chinese herbal medicine (Pentaherbs formulation) for atopic dermatitis. J. Ethnopharmacol. 2023, 305, 116149. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, L.; Lu, Q.; Gao, X.; Zhu, X.; Yao, X.; Li, L.; Li, W.; Ding, Y.; Song, Z.; et al. The efficacy and safety of dupilumab in Chinese patients with moderate-to-severe atopic dermatitis: A randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2022, 186, 633–641. [Google Scholar] [CrossRef]
- Gu, C.; Wu, Y.; Luo, Y.; Wang, S.; Yin, H.; Gao, Y.; Wang, C.; Yao, X.; Li, W. Real-world efficacy and safety of dupilumab in Chinese patients with atopic dermatitis: A single-centre, prospective, open-label study. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, 1064–1073. [Google Scholar] [CrossRef]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Thyssen, J.P.; Fahrbach, K.; Mickle, K.; Cappelleri, J.C.; Romero, W.; Cameron, M.C.; Myers, D.E.; Clibborn, C.; DiBonaventura, M. Comparative efficacy and safety of systemic therapies used in moderate-to-severe atopic dermatitis: A systematic literature review and network meta-analysis. J. Eur. Acad. Dermatol. Venereol. JEADV 2021, 35, 1797–1810. [Google Scholar] [CrossRef]
- De Bruin-Weller, M.; Biedermann, T.; Bissonnette, R.; Deleuran, M.; Foley, P.; Girolomoni, G.; Hercogová, J.; Hong, C.H.; Katoh, N.; Pink, A.E.; et al. Treat-to-Target in Atopic Dermatitis: An International Consensus on a Set of Core Decision Points for Systemic Therapies. Acta Derm.-Venereol. 2021, 101, adv00402. [Google Scholar] [CrossRef]
- Napolitano, M.; Maffei, M.; Patruno, C.; Leone, C.A.; Di Guida, A.; Potestio, L.; Scalvenzi, M.; Fabbrocini, G. Dupilumab effectiveness for the treatment of patients with concomitant atopic dermatitis and chronic rhinosinusitis with nasal polyposis. Dermatol. Ther. 2021, 34, e15120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, C.; Yao, X.; Li, W. Burden of Disease; the Current Status of the Diagnosis and Management of Atopic Dermatitis in China. J. Clin. Med. 2023, 12, 5370. https://doi.org/10.3390/jcm12165370
Gu C, Yao X, Li W. Burden of Disease; the Current Status of the Diagnosis and Management of Atopic Dermatitis in China. Journal of Clinical Medicine. 2023; 12(16):5370. https://doi.org/10.3390/jcm12165370
Chicago/Turabian StyleGu, Chaoying, Xu Yao, and Wei Li. 2023. "Burden of Disease; the Current Status of the Diagnosis and Management of Atopic Dermatitis in China" Journal of Clinical Medicine 12, no. 16: 5370. https://doi.org/10.3390/jcm12165370
APA StyleGu, C., Yao, X., & Li, W. (2023). Burden of Disease; the Current Status of the Diagnosis and Management of Atopic Dermatitis in China. Journal of Clinical Medicine, 12(16), 5370. https://doi.org/10.3390/jcm12165370






