Is the Combination of Plain X-ray and Probe-to-Bone Test Useful for Diagnosing Diabetic Foot Osteomyelitis? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Requirements
2.3. Literature Screening
2.4. Data Extraction
2.5. Quality Evaluation of Included Studies (STROBE Guidelines)
2.6. Statistical Analyses
3. Results
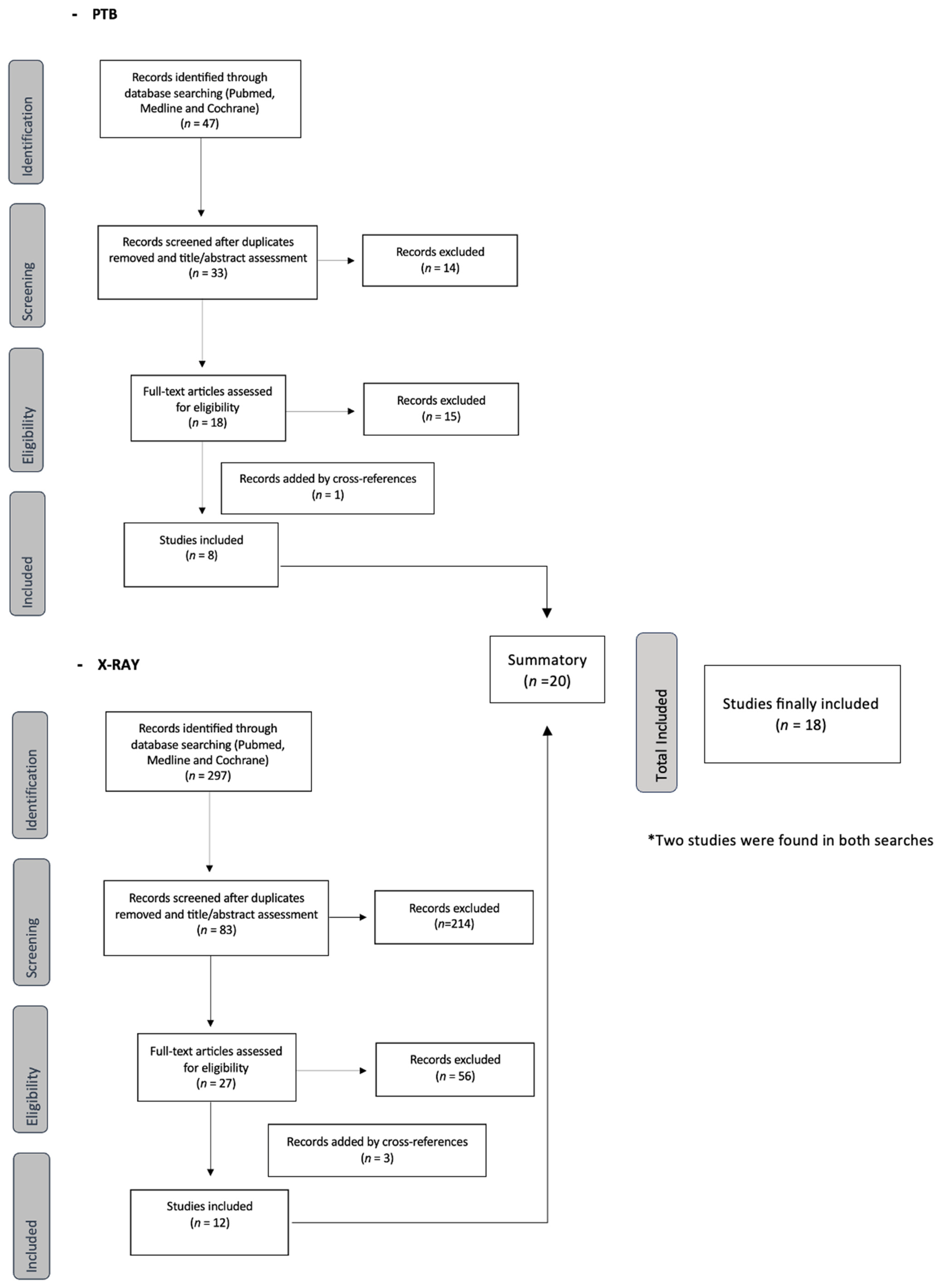
3.1. Literature Retrieval
3.2. Quality of the Reporting
3.3. Outcome Measures
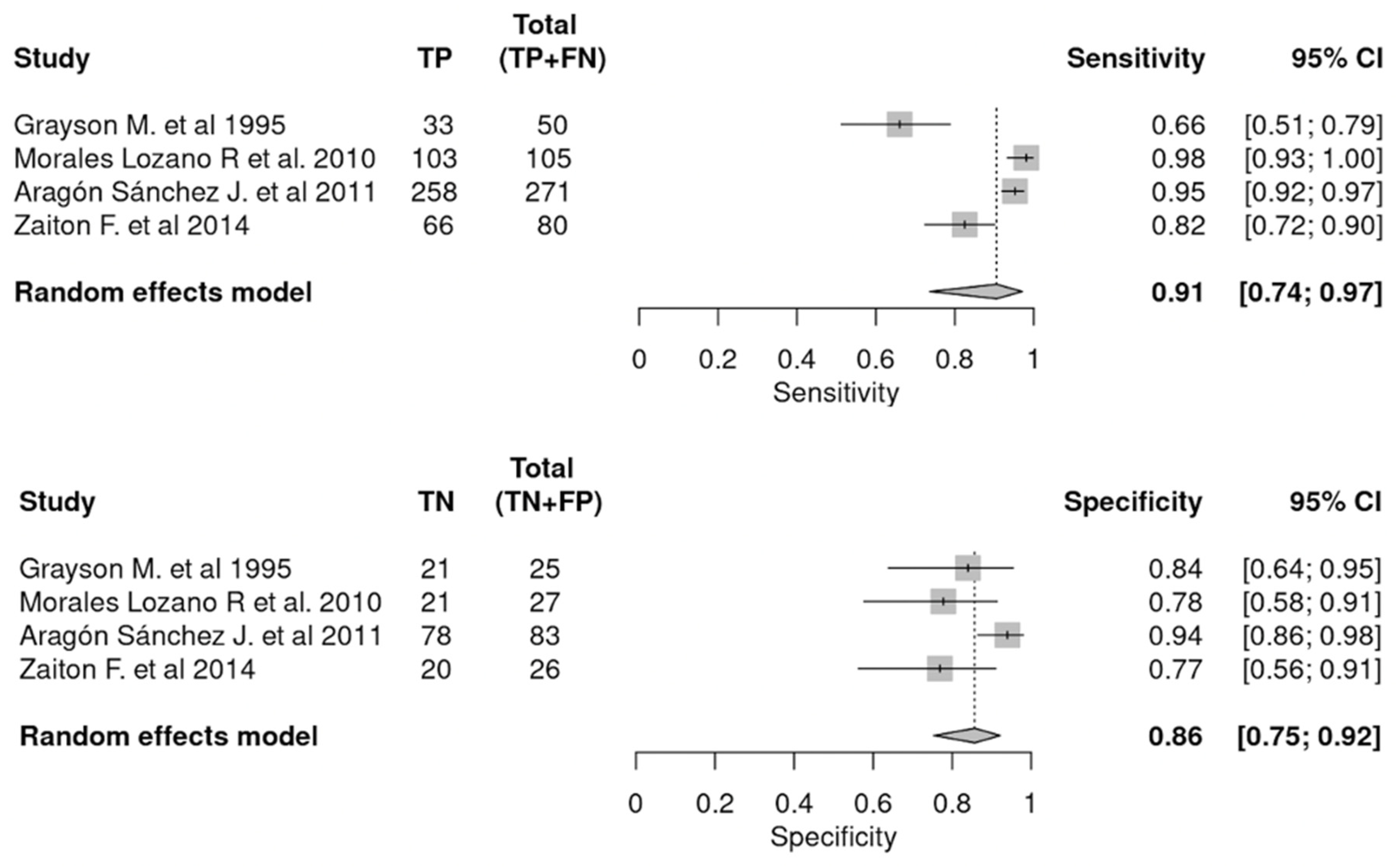
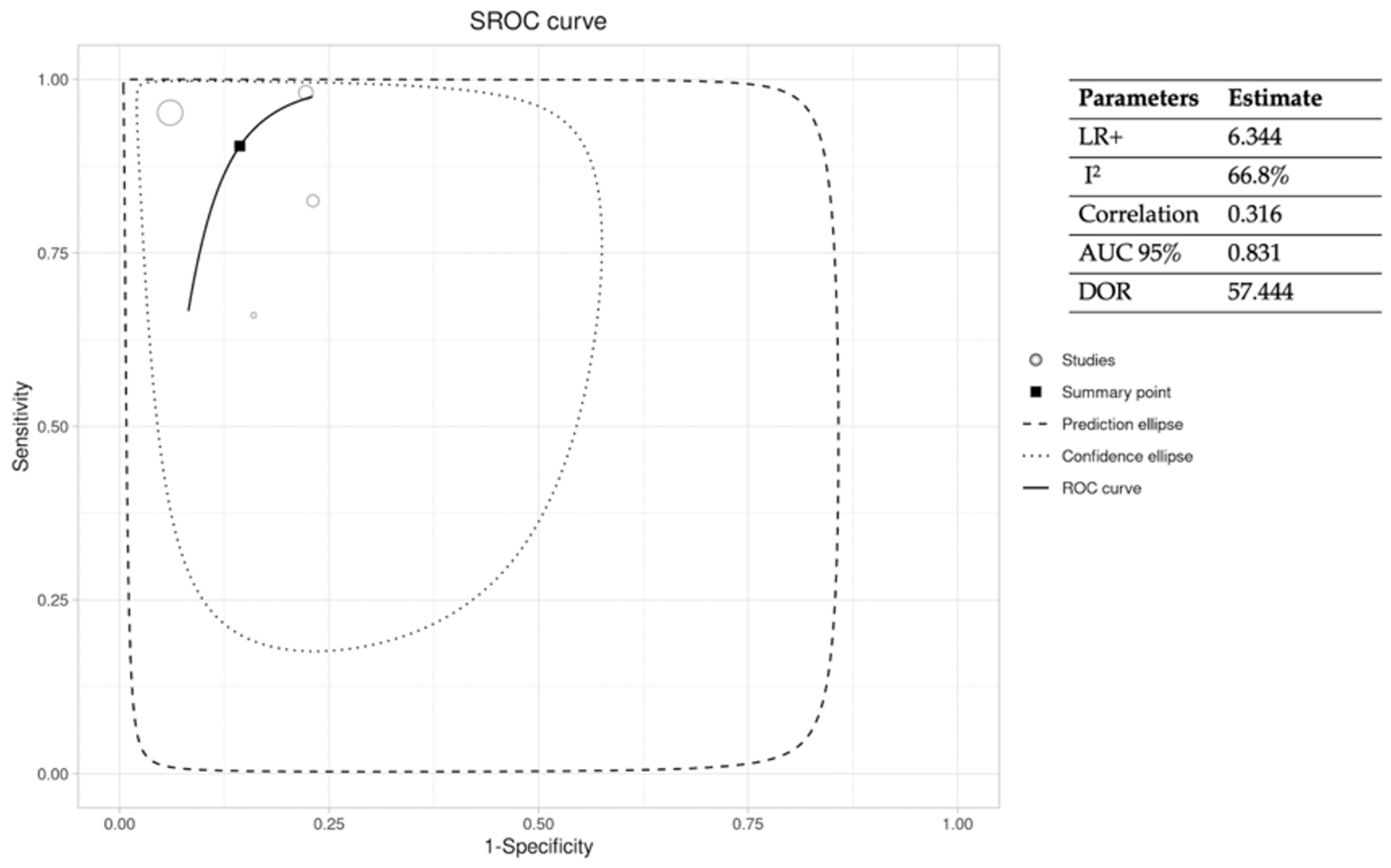
3.3.1. PTB
3.3.2. Plain X-ray
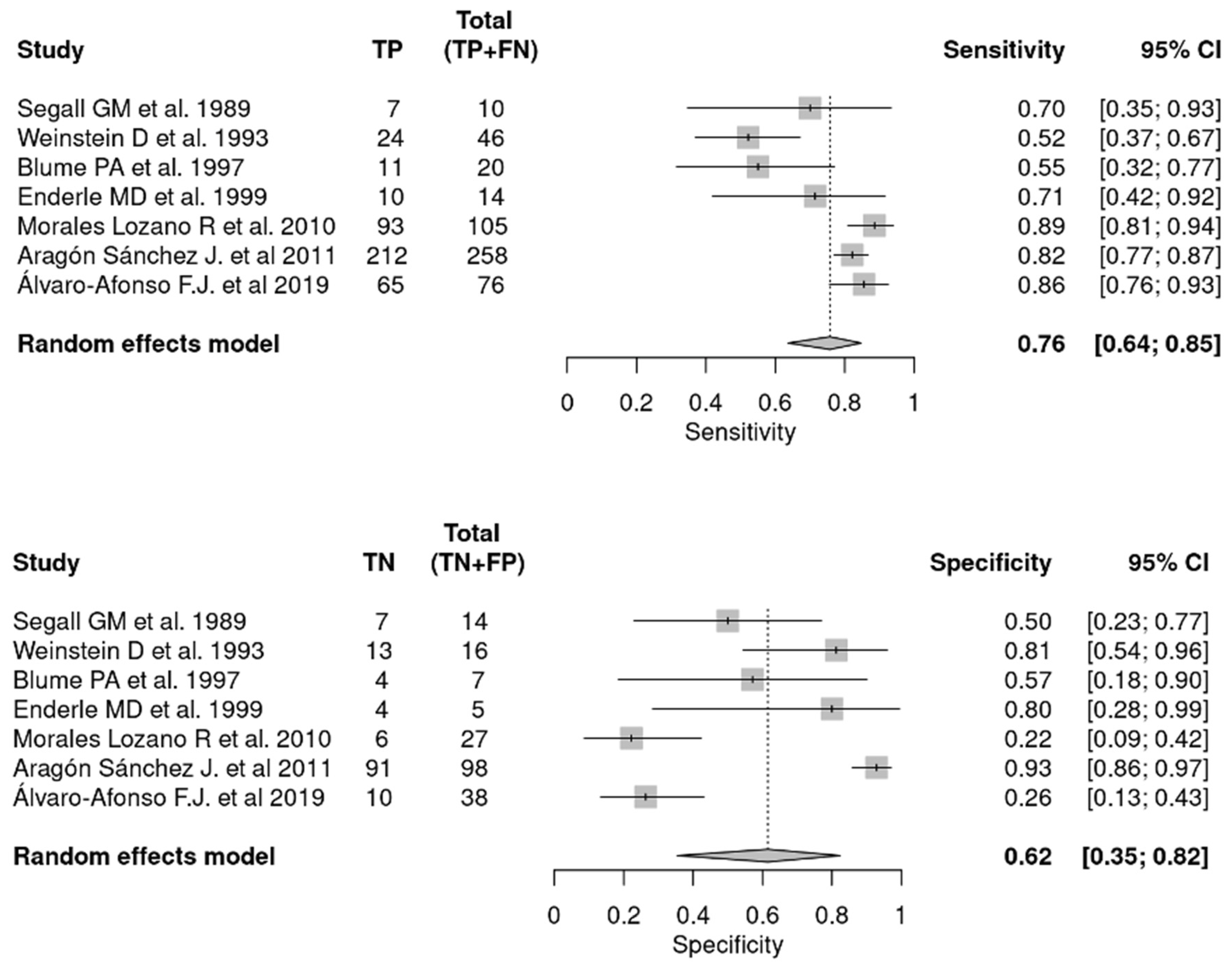
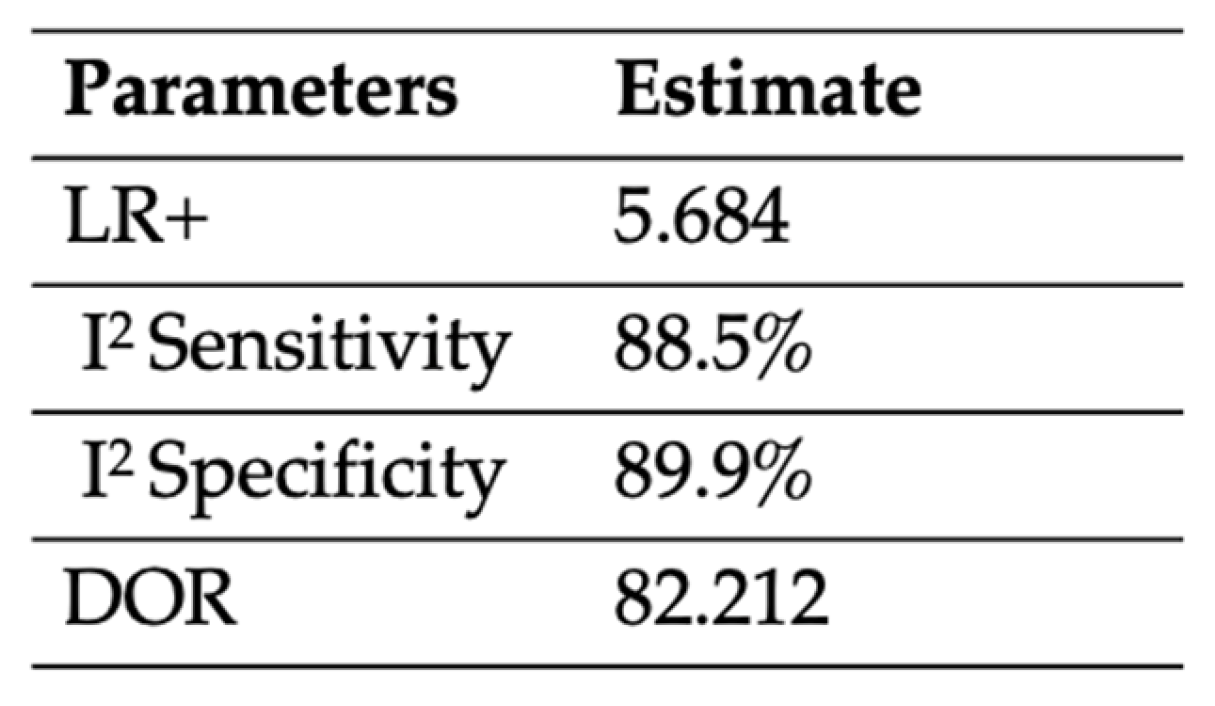
3.3.3. Combination of PTB and Plain X-ray
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lazzarini, P.A.; Pacella, R.E.; Armstrong, D.G.; Van Netten, J.J. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabet. Med. 2018, 35, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Prompers, L.; Huijberts, M.; Apelqvist, J.; Jude, E.; Piaggesi, A.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovska, A.; Mauricio, D.; et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007, 50, 18–25. [Google Scholar] [CrossRef]
- Raspovic, K.M.; Wukich, D.K. Self-reported quality of life in patients with diabetes: A comparison of patients with and without Charcot neuroarthropathy. Foot Ankle Int. 2014, 35, 195–200. [Google Scholar] [CrossRef]
- Lázaro Martínez, J.L.; García Álvarez, Y.; Tardáguila-García, A.; García Morales, E. Optimal management of diabetic foot osteomyelitis: Challenges and solutions. Diabetes Metab. Syndr. Obes. 2019, 12, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.; Karchmer, A.; et al. 2012 infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, 132–173. [Google Scholar] [CrossRef]
- Shahbazian, H.; Yazdanpanah, L.; Latifi, S.M. Risk assessment of patients with diabetes for foot ulcers according to risk classification consensus of International Working Group on Diabetic Foot (IWGDF). Pak. J. Med. Sci. 2013, 29, 730–734. [Google Scholar] [CrossRef]
- Lipsky, B.A. Bone of contention: Diagnosing diabetic foot osteomyelitis. Clin. Infect. Dis. 2008, 47, 528–530. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; Van Asten, S.A.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, e3280. [Google Scholar] [CrossRef]
- Dinh, M.T.; Abad, C.L.; Safdar, N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: Meta-analysis. Clin. Infect. Dis. 2008, 47, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Butalia, S.; Palda, V.A.; Sargeant, R.J.; Detsky, A.S.; Mourad, O. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA 2008, 299, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Berendt, A.R.; Peters, E.J.; Bakker, K.; Embil, J.M.; Eneroth, M.; Hinchliffe, R.J.; Jeffcoate, W.J.; Lipsky, B.A.; Senneville, E.; Teh, J.; et al. Diabetic foot osteomyelitis: A progress report on diagnosis and a systematic review of treatment. Diabetes Metab. Res. Rev. 2008, 24 (Suppl. S1), S145–S161. [Google Scholar] [CrossRef] [PubMed]
- Grayson, M.L.; Gibbons, G.W.; Balogh, K.; Levin, E.; Karchmer, A.W. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA 1995, 273, 721–723. [Google Scholar] [CrossRef]
- Aragon-Sanchez, J.; Lipsky, B.A.; Lazaro-Martinez, J.L. Diagnosing diabetic foot osteomyelitis: Is the combination of probe-to-bone test and plain radiography sufficient for high-risk inpatients? Diabet. Med. 2011, 28, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Shone, A.; Burnside, J.; Chipchase, S.; Game, F.; Jeffcoate, W. Probing the validity of the probe-to-bone test in the diagnosis of osteomyelitis of the foot in diabetes. Diabetes Care 2006, 29, 945. [Google Scholar] [CrossRef]
- Lavery, L.A.; Armstrong, D.G.; Peters, E.J.; Lipsky, B.A. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: Reliable or relic? Diabetes Care 2007, 30, 270–274. [Google Scholar] [CrossRef]
- Morales Lozano, R.; González Fernández, M.L.; Martinez Hernández, D.; Beneit Montesinos, J.V.; Guisado Jiménez, S.; Gonzalez Jurado, M.A. Validating the probe-to-bone test and other tests for diagnosing chronic osteomyelitis in the diabetic foot. Diabetes Care 2010, 33, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Mutluoglu, M.; Uzun, G.; Sildiroglu, O.; Turhan, V.; Mutlu, H.; Yildiz, S. Performance of the probe-to-bone test in a population suspected of having osteomyelitis of the foot in diabetes. J. Am. Podiatr. Med. Assoc. 2012, 102, 369–373. [Google Scholar] [PubMed]
- Meyr, A.J.; Seo, K.; Khurana, J.S.; Choksi, R.; Chakraborty, B. Level of Agreement with a Multi-Test Approach to the Diagnosis of Diabetic Foot Osteomyelitis. J. Foot Ankle Surg. 2018, 57, 1137–1139. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S31–S34. [Google Scholar] [CrossRef]
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.S.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006, 6, 31. [Google Scholar] [CrossRef]
- Malone, M.; Bowling, F.L.; Gannass, A.; Jude, E.B.; Boulton, A.J. Deep wound cultures correlate well with bone biopsy culture in diabetic foot osteomyelitis. Diabetes Metab. Res. Rev. 2013, 29, 546–550. [Google Scholar] [PubMed]
- Zaiton, F.; Samir, A.M.; Elkamash, T.H.; Tawfik, A.M.; Hadhoud, K.M. Evaluation of diabetic foot osteomyelitis using probe to bone test and magnetic resonance imaging and their impact on surgical intervention. Egypt. J. Radiol. Nucl. Med. 2014, 45, 795–802. [Google Scholar] [CrossRef]
- Yuh, W.T.; Corson, J.D.; Baraniewski, H.M.; Rezai, K.; Shamma, A.R.; Kathol, M.H.; Sato, Y.; El-Khoury, G.Y.; Hawes, D.R.; Platz, C.E.; et al. Osteomyelitis of the foot in diabetic patients: Evaluation with plain film, 99mTc-MDP bone scintigraphy, and MR imaging. AJR Am. J. Roentgenol. 1989, 152, 795–800. [Google Scholar] [CrossRef]
- Segall, G.M.; Nino-Murcia, M.; Jacobs, T.; Chang, K. The role of bone scan and radiography in the diagnostic evaluation of suspected pedal osteomyelitis. Clin. Nucl. Med. 1989, 14, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Larcos, G.; Brown, M.L.; Sutton, R.T. Diagnosis of osteomyelitis of the foot in diabetic patients: Value of 111In-leukocyte scintigraphy. AJR Am. J. Roentgenol. 1991, 157, 527–531. [Google Scholar] [CrossRef]
- Weinstein, D.; Wang, A.; Chambers, R.; Stewart, C.A.; Motz, H.A. Evaluation of magnetic resonance imaging in the diagnosis of osteomyelitis in diabetic foot infections. Foot Ankle 1993, 14, 18–22. [Google Scholar] [CrossRef]
- Levine, S.E.; Neagle, C.E.; Esterhai, J.L.; Wright, D.G.; Dalinka, M.K. Magnetic resonance imaging for the diagnosis of osteomyelitis in the diabetic patient with a foot ulcer. Foot Ankle Int. 1994, 15, 151–156. [Google Scholar] [CrossRef]
- Croll, S.D.; Nicholas, G.G.; Osborne, M.A.; Wasser, T.E.; Jones, S. Role of magnetic resonance imaging in the diagnosis of osteomyelitis in diabetic foot infections. J. Vasc. Surg. 1996, 24, 266–270. [Google Scholar] [CrossRef]
- Blume, P.A.; Dey, H.M.; Daley, L.J.; Arrighi, J.A.; Soufer, R.; Gorecki, G.A. Diagnosis of pedal osteomyelitis with Tc-99m HMPAO labeled leukocytes. J. Foot Ankle Surg. 1997, 36, 120–126. [Google Scholar] [CrossRef]
- Enderle, M.D.; Coerper, S.; Schweizer, H.P.; Kopp, A.E.; Thelen, M.H.; Meisner, C.; Pressler, H.; Becker, H.D.; Claussen, C.; Häring, H.U.; et al. Correlation of imaging techniques to histopathology in patients with diabetic foot syndrome and clinical suspicion of chronic osteomyelitis. The role of high-resolution ultrasound. Diabetes Care 1999, 22, 294–299. [Google Scholar] [CrossRef]
- Nawaz, A.; Torigian, D.A.; Siegelman, E.S.; Basu, S.; Chryssikos, T.; Alavi, A. Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol. Imaging Biol. 2010, 12, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Afonso, F.J.; Lázaro-Martínez, J.L.; García-Morales, E.; García-Álvarez, Y.; Sanz-Corbalán, I.; Molines-Barroso, R.J. Cortical disruption is the most reliable and accurate plain radiographic sign in the diagnosis of diabetic foot osteomyelitis. Diabet. Med. 2019, 36, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, A.; Kraft, J.; Holton, C.; Harden, M.; Simmonds, M. Imaging for detection of osteomyelitis in people with diabetic foot ulcers: A systematic review and meta-analysis. Eur. J. Radiol. 2020, 131, 109215. [Google Scholar] [CrossRef] [PubMed]
- García Morales, E.; Lázaro-Martínez, J.L.; Aragón-Sánchez, F.J.; Cecilia-Matilla, A.; Beneit-Montesinos, J.V.; González Jurado, M.A. Inter-observer reproducibility of probing to bone in the diagnosis of diabetic foot osteomyelitis. Diabet. Med. 2011, 28, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Afonso, F.J.; Lázaro-Martínez, J.L.; Aragón-Sánchez, F.J.; García-Morales, E.; Carabantes-Alarcón, D.; Molines-Barroso, R.J. Does the location of the ulcer affect the interpretation of the probe-to-bone test in the diagnosis of osteomyelitis in diabetic foot ulcers? Diabet. Med. 2014, 31, 112–113. [Google Scholar] [CrossRef]
- Álvaro-Afonso, F.J.; Lázaro-Martínez, J.L.; Aragón-Sánchez, J.; García-Morales, E.; Cecilia-Matilla, A.; Beneit-Montesinos, J.V. Interobserver and intraobserver reproducibility of plain X-rays in the diagnosis of diabetic foot osteomyelitis. Int. J. Low. Extrem. Wounds 2013, 12, 12–15. [Google Scholar] [CrossRef]
- Álvaro-Afonso, F.J.; Lázaro-Martínez, J.L.; Aragón-Sánchez, J.; García-Morales, E.; García-Álvarez, Y.; Molines-Barroso, R.J. Inter-observer reproducibility of diagnosis of diabetic foot osteomyelitis based on a combination of probe-to-bone test and simple radiography. Diabetes Res. Clin. Pract. 2014, 105, e3–e5. [Google Scholar] [CrossRef]
- Lam, K.; Van Asten, S.A.; Nguyen, T.; La Fontaine, J.; Lavery, L.A. Diagnostic Accuracy of Probe to Bone to Detect Osteomyelitis in the Diabetic Foot: A Systematic Review. Clin. Infect. Dis. 2016, 63, 944–948. [Google Scholar] [CrossRef]


| Item Number–STROBE Guidelines | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (a) | 1 (b) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | |
| Grayson M. 1995, [12] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Shone A. 2006, [14] | Yes | No | No | No | No | Yes | No | Yes | No | No | No | No | No | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes |
| Lavery LA. 2007, [15] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Morales Lozano R. 2010, [16] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Aragón Sánchez J. 2011, [13] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Mutluoglu M. 2012, [17] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Malone M. 2013, [22] | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Zaiton F. 2014, [23] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Yuh WT. 1989, [24] | No | Yes | No | Yes | No | Yes | No | Yes | Yes | No | No | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Segall GM. 1989, [25] | No | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Largos G 1991, [26] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Weinstein D. 1993, [27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | No |
| Levine SE. 1994, [28] | No | Yes | Yes | No | Yes | No | No | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes |
| Croll SD. 1996, [29] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| Blume PA. 1997, [30] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Enderle MD. 1999, [31] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Nawaz A. 2009, [32] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Álvaro Afonso F.J. 2019, [33] | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Wright, M.d.M.; Álvaro-Afonso, F.J.; López-Moral, M.; García-Álvarez, Y.; García-Morales, E.; Lázaro-Martínez, J.L. Is the Combination of Plain X-ray and Probe-to-Bone Test Useful for Diagnosing Diabetic Foot Osteomyelitis? A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 5369. https://doi.org/10.3390/jcm12165369
Calvo-Wright MdM, Álvaro-Afonso FJ, López-Moral M, García-Álvarez Y, García-Morales E, Lázaro-Martínez JL. Is the Combination of Plain X-ray and Probe-to-Bone Test Useful for Diagnosing Diabetic Foot Osteomyelitis? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(16):5369. https://doi.org/10.3390/jcm12165369
Chicago/Turabian StyleCalvo-Wright, María del Mar, Francisco Javier Álvaro-Afonso, Mateo López-Moral, Yolanda García-Álvarez, Esther García-Morales, and José Luis Lázaro-Martínez. 2023. "Is the Combination of Plain X-ray and Probe-to-Bone Test Useful for Diagnosing Diabetic Foot Osteomyelitis? A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 16: 5369. https://doi.org/10.3390/jcm12165369
APA StyleCalvo-Wright, M. d. M., Álvaro-Afonso, F. J., López-Moral, M., García-Álvarez, Y., García-Morales, E., & Lázaro-Martínez, J. L. (2023). Is the Combination of Plain X-ray and Probe-to-Bone Test Useful for Diagnosing Diabetic Foot Osteomyelitis? A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(16), 5369. https://doi.org/10.3390/jcm12165369










