Risk of Ischemic Stroke Associated with Calcium Supplements and Interaction with Oral Bisphosphonates: A Nested Case-Control Study
Abstract
1. Introduction
2. Patients, Materials, and Methods
2.1. Data Source and Study Design
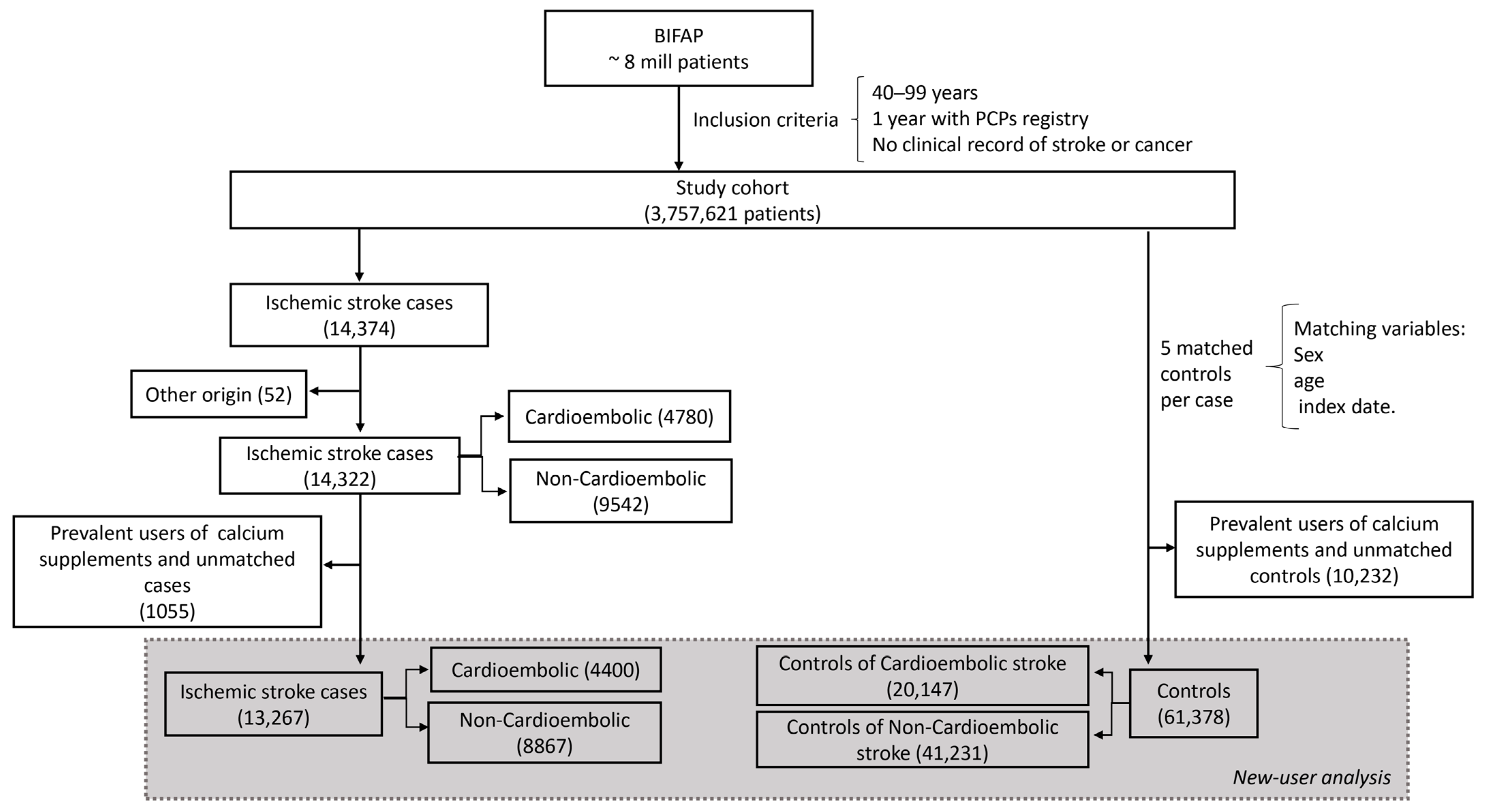
2.2. Cases and Controls Selection
2.3. New-User Design
2.4. Exposure Definition
2.5. Potential Confounding Factors
2.6. Statistical Analysis
2.7. Ethical Aspects
3. Results
3.1. CaM and Risk of Ischemic Stroke Overall and by Pathophysiological Subtype
3.2. CaD and Risk of Ischemic Stroke Overall and by Pathophysiological Subtype
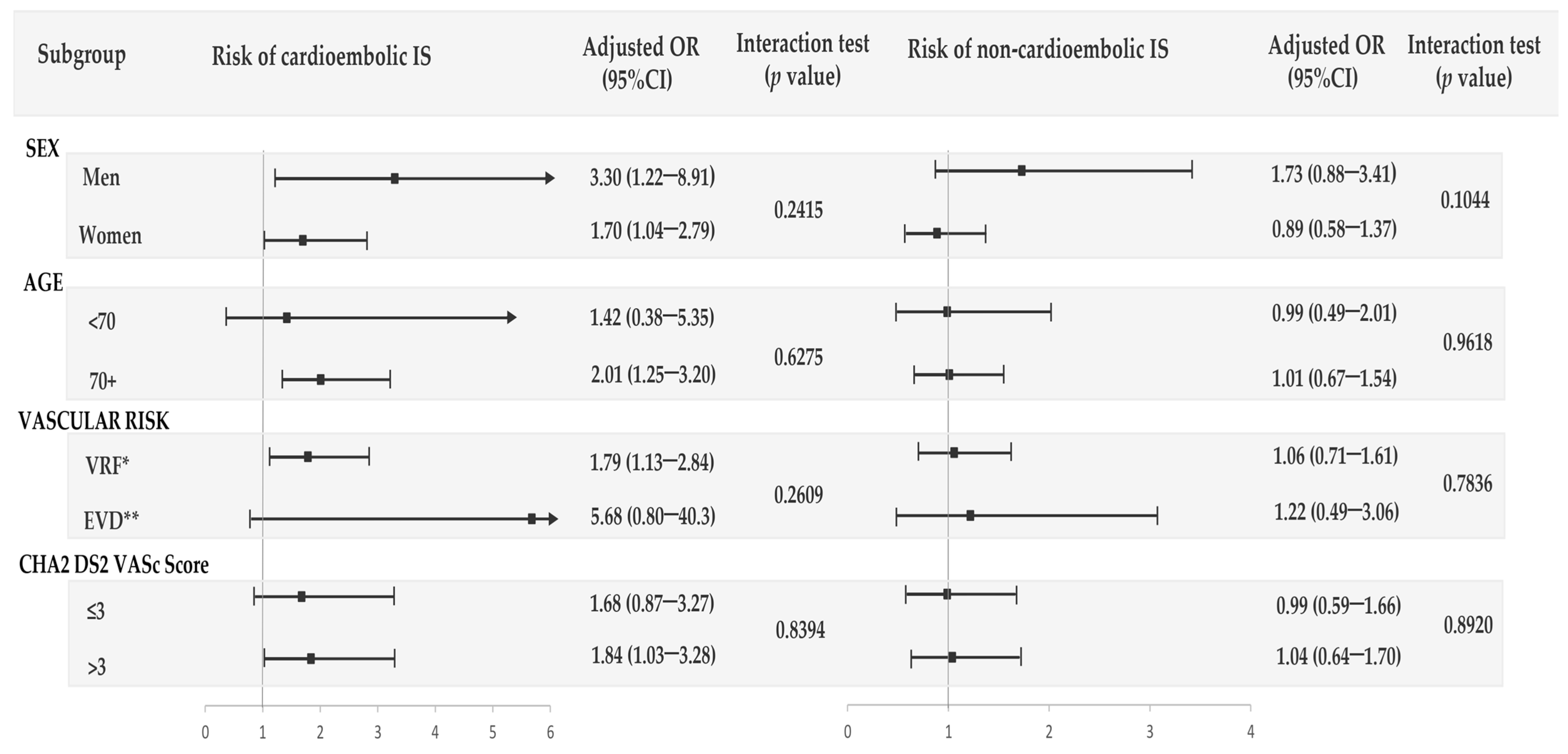
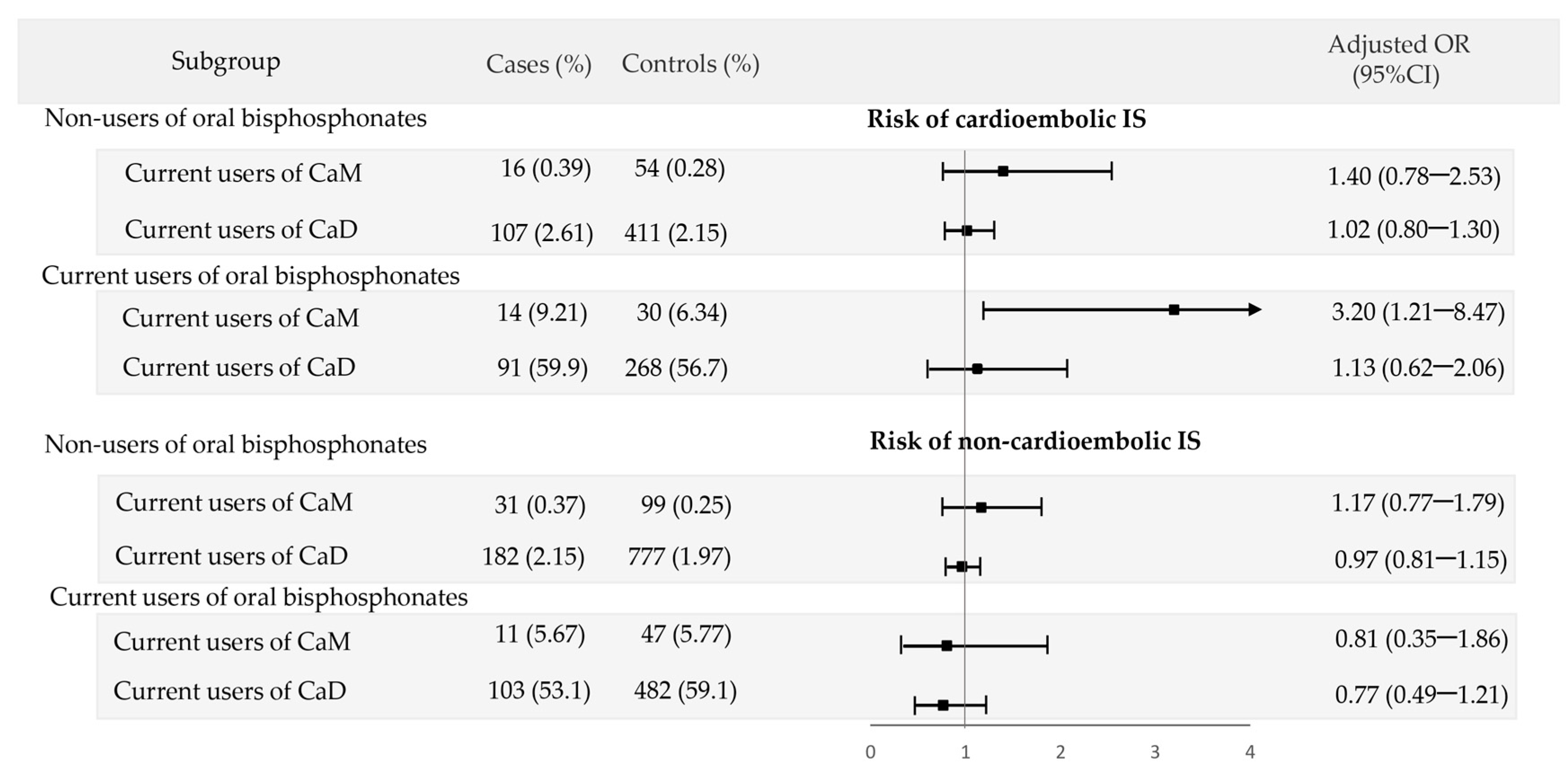
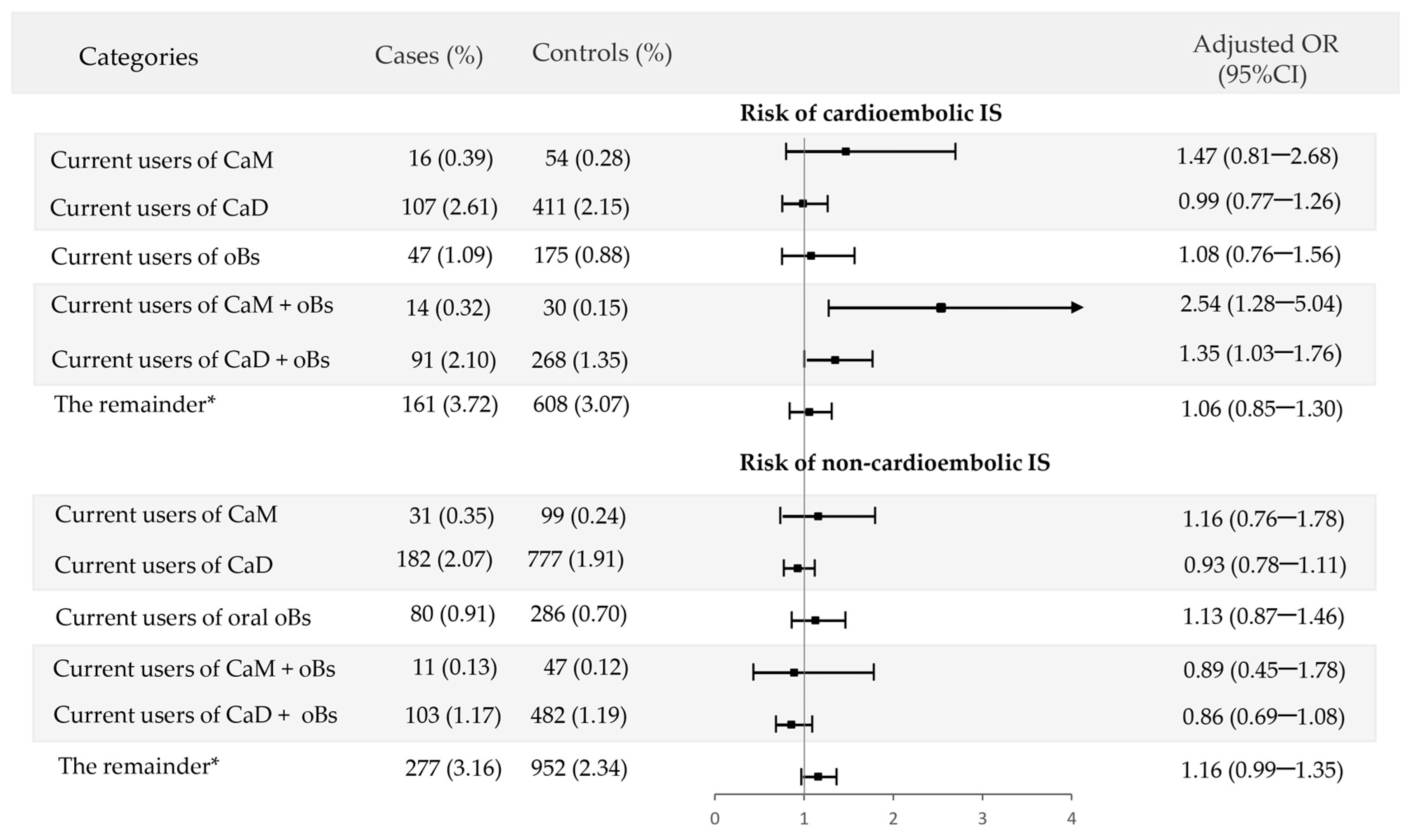
3.3. Interaction of Calcium Supplements with Oral Bisphosphonates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2020 Update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.; Cooper, A.; Cooper, C.; Gittoes, N.; Gregson, C.; Harvey, N.; Hope, S.; Kanis, J.A.; McCloskey, E.V.; Poole, K.E.S.; et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2017, 12, 43. [Google Scholar] [CrossRef]
- Cosman, F.; De Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G.; Chrysant, G.S. Controversy Regarding the Association of High Calcium Intake and Increased Risk for Cardiovascular Disease. J. Clin. Hypertens. 2014, 16, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Danilevicius, C.; Lopes, J.; Pereira, R. Bone metabolism and vascular calcification. Braz. J. Med. Biol. Res. 2007, 40, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Foley, R.N.; Collins, A.J.; Ishani, A.; Kalra, P.A. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 2008, 156, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, W.; Naumnik, B.; Szczepański, M.; Myśliwiec, M. The mechanism of vascular calcification—A systematic review. Med. Sci. Monit. 2012, 18, RA1–RA11. [Google Scholar] [CrossRef]
- Bolland, M.J.; Avenell, A.; Baron, J.A.; Grey, A.; MacLennan, G.S.; Gamble, G.D.; Reid, I.R. Faculty Opinions recommendation of Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ 2010, 341, c3691. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Avenell, A.; Gamble, G.D.; Reid, I.R. Calcium supplements with or without vitamin D and risk of cardiovascular events: Reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ 2011, 342, d2040. [Google Scholar] [CrossRef]
- Chung, M.; Tang, A.M.; Fu, Z.; Wang, D.D.; Newberry, S.J. Calcium Intake and Cardiovascular Disease Risk: An Updated Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 165, 856. [Google Scholar] [CrossRef]
- Tian, D.-Y.; Tian, J.; Shi, C.-H.; Song, B.; Wu, J.; Ji, Y.; Wang, R.-H.; Mao, C.-Y.; Sun, S.-L.; Xu, Y.-M. Calcium intake and the risk of stroke: An up-dated meta-analysis of prospective studies. Asia Pac. J. Clin. Nutr. 2015, 24, 245–252. [Google Scholar] [PubMed]
- de Abajo, F.J.; Rodríguez-Martín, S.; Rodríguez-Miguel, A.; Gil, M.J. Risk of Ischemic Stroke Associated With Calcium Supplements with or without Vitamin D: A Nested Case-Control Study. J. Am. Heart Assoc. 2017, 6, e005795. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Prabhakar, S.; Modi, M.; Bhadada, S.K.; Kalaivani, M.; Lal, V.; Khurana, D. Effect of Vitamin D and calcium supplementation on ischaemic stroke outcome: A randomised controlled open-label trial. Int. J. Clin. Pract. 2016, 70, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martín, S.; Barreira-Hernández, D.; Mazzucchelli, R.; Gil, M.; García-Lledó, A.; Izquierdo-Esteban, L.; Pérez-Gómez, A.; Rodríguez-Miguel, A.; De Abajo, F.J. Association of oral bisphosphonates with cardioembolic ischemic stroke: A nested case-control study. Front. Pharmacol. 2023, 14, 1197238. [Google Scholar] [CrossRef]
- Maciá-Martínez, M.A.; Gil, M.; Huerta, C.; Martín-Merino, E.; Álvarez, A.; Bryant, V.; Montero, D.; BIFAP Team. Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP): A data resource for pharmacoepidemiology in Spain. Pharmacoepidemiol. Drug Saf. 2020, 29, 1236–1245. [Google Scholar]
- Alqdwah-Fattouh, R.; Rodríguez-Martín, S.; Barreira-Hernández, D.; Izquierdo-Esteban, L.; Gil, M.; González-Bermejo, D.; Fernández-Antón, E.; Rodríguez-Miguel, A.; García-Lledó, A.; Bolúmar, F.; et al. Selective Serotonin Reuptake Inhibitors and Risk of Noncardioembolic Ischemic Stroke: A Nested Case-Control Study. Stroke 2022, 53, 1560–1569. [Google Scholar] [CrossRef]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 111–127. [Google Scholar]
- Altman, D.G. Statistics Notes: Interaction revisited: The difference between two estimates. BMJ 2003, 326, 219. [Google Scholar] [CrossRef]
- Rothman, K.J.; Greenland, S. Modern Epidemiology, 2nd ed.; Lippincott-Raven: Philadelphia, PA, USA, 1998; p. 737. [Google Scholar]
- Azur, M.J.; Stuart, E.A.; Frangakis, C.; Leaf, P.J. Multiple imputation by chained equations: What is it and how does it work?: Multiple imputation by chained equations. Int. J. Methods Psychiatr. Res. 2011, 20, 40–49. [Google Scholar]
- Mao, P.-J.; Zhang, C.; Tang, L.; Xian, Y.-Q.; Li, Y.-S.; Wang, W.-D.; Zhu, X.-H.; Qiu, H.-L.; He, J.; Zhou, Y.-H. Effect of calcium or vitamin D supplementation on vascular outcomes: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2013, 169, 106–111. [Google Scholar] [CrossRef]
- Lewis, J.R.; Radavelli-Bagatini, S.; Rejnmark, L.; Chen, J.S.; Simpson, J.M.; Lappe, J.M.; Mosekilde, L.; Prentice, R.L.; Prince, R.L. The Effects of Calcium Supplementation on Verified Coronary Heart Disease Hospitalization and Death in Postmenopausal Women: A Collaborative Meta-Analysis of Randomized Controlled Trials: Calcium Supplementation and CHD in postmenopausal women. J. Bone Miner. Res. 2015, 30, 165–175. [Google Scholar]
- Yang, C.; Shi, X.; Xia, H.; Yang, X.; Liu, H.; Pan, D.; Sun, G. The Evidence and Controversy Between Dietary Calcium Intake and Calcium Supplementation and the Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Cohort Studies and Randomized Controlled Trials. J. Am. Coll. Nutr. 2019, 39, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-K.; Kim, H.-B.; Lee, Y.-J.; Choi, Y.-J.; Oh, S.-W. Calcium Supplements and Risk of Cardiovascular Disease: A Meta-Analysis of Clinical Trials. Nutrients 2021, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Clarke, R.; Halsey, J.; Jackson, R.; Lehman, A.; Prince, R.; Lewis, J.; Baron, J.A.; Kroger, H.; Sund, R.; et al. Calcium Supplements and Risk of CVD: A Meta-Analysis of Randomized Trials. Curr. Dev. Nutr. 2023, 7, 100046. [Google Scholar] [CrossRef]
- Pillai, A.A.; Tadi, P.; Kanmanthareddy, A. Cardioembolic Stroke. In StatPearls [Internet]; [Updated 2023 May 20]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536990/ (accessed on 8 June 2023).
- Kamel, H.; Okin, P.M.; Elkind, M.S.V.; Iadecola, C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke 2016, 47, 895–900. [Google Scholar] [CrossRef]
- Bristow, S.M.; Gamble, G.D.; Stewart, A.; Horne, A.M.; Reid, I.R. Acute effects of calcium supplements on blood pressure and blood coagulation: Secondary analysis of a randomised controlled trial in post-menopausal women. Br. J. Nutr. 2015, 114, 1868–1874. [Google Scholar] [CrossRef]
- Rodríguez-Martín, S.; González-Bermejo, D.; Rodríguez-Miguel, A.; Barreira, D.; García-Lledó, A.; Gil, M.; de Abajo, F.J. Risk of Myocardial Infarction among New Users of Calcium Supplements Alone or Combined with Vitamin D: A Population-Based Case-Control Study. Clin. Pharmacol. Ther. 2020, 107, 359–368. [Google Scholar] [PubMed]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Powell, J.T. Vitamin D and Cardiovascular Disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef]
- Pilz, S.; Verheyen, N.; Grübler, M.R.; Tomaschitz, A.; März, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef]
- Beveridge, L.A.; Witham, M.D. Vitamin D and the cardiovascular system. Osteoporos. Int. 2013, 24, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Gaksch, M.; O’Hartaigh, B.; Tomaschitz, A.; März, W. The role of vitamin D deficiency in cardiovascular disease: Where do we stand in 2013? Arch. Toxicol. 2013, 87, 2083–2103. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.S.F.; Sposito, A.C. Vitamin D for the prevention of cardiovascular disease: Are we ready for that? Atherosclerosis 2015, 241, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Nadir, M.A.; Struthers, A.D. Effect of vitamin D on blood pressure: A systematic review and meta-analysis. J. Hypertens. 2009, 27, 1948–1954. [Google Scholar] [CrossRef]
- Thompson, B.; Waterhouse, M.; English, D.R.; McLeod, D.S.; Armstrong, B.K.; Baxter, C.; Romero, B.D.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; et al. Vitamin D supplementation and major cardiovascular events: D-Health randomised controlled trial. BMJ 2023, 381, e075230. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Lee, I.-M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; MacFadyen, J.G.; Danielson, E.; Lin, J.; et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials 2012, 33, 159–171. [Google Scholar] [CrossRef]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.-T.; Camargo, C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar]
- Virtanen, J.K.; Hantunen, S.; Lamberg-Allardt, C.; Manson, J.E.; Nurmi, T.; Uusitupa, M.; Voutilainen, A.; Tuomainen, T.-P. The effect of vitamin D3 supplementation on atrial fibrillation in generally healthy men and women: The Finnish Vitamin D Trial. Am. Heart J. 2023, in press. [CrossRef]
- Reid, I.R.; Bristow, S.M.; Bolland, M.J. Cardiovascular Complications of Calcium Supplements: Heart Safety of Calcium Supplements. J. Cell Biochem. 2015, 116, 494–501. [Google Scholar]
- Reid, I.R.; Gamble, G.D.; Bolland, M.J. Circulating calcium concentrations, vascular disease and mortality: A systematic review. J. Intern. Med. 2016, 279, 524–540. [Google Scholar] [CrossRef]

| Cardioembolic IS | Non-Cardioembolic IS | |||
|---|---|---|---|---|
| CASES N = 4400 | CONTROLS N = 20,147 | CASES N = 8867 | CONTROLS N = 41,231 | |
| Age, mean (SD), years | 76.5 (±11.39) | 76.3 (±11.56) | 73.2 (±12.85) | 72.8 (±12.97) |
| Men, (%) | 2110 (47.95) | 10,346 (51.35) | 4911 (55.39) | 24,145 (58.56) |
| Number of visits to PCP, (%) | ||||
| <6 | 585 (13.30) | 5290 (26.26) | 1831 (20.65) | 12,771 (30.97) |
| 6–15 | 1459 (33.16) | 7478 (37.12) | 3463 (39.05) | 15,595 (37.82) |
| 16–24 | 1073 (24.39) | 3942 (19.57) | 1987 (22.41) | 7100 (17.22) |
| >24 | 1283 (29.16) | 3437 (17.06) | 1586 (17.89) | 5765 (13.98) |
| BMI, No. (%), kg·m−2 | ||||
| <25 | 642 (14.59) | 2776 (13.78) | 1185 (13.36) | 5314 (12.89) |
| 25–29.9 | 1348 (30.64) | 5947 (29.52) | 2567 (28.95) | 11,913 (28.89) |
| 30–34.9 | 842 (19.14) | 3412 (16.94) | 1529 (17.24) | 6726 (16.31) |
| 35–39.9 | 218 (4.95) | 823 (4.08) | 465 (5.24) | 1774 (4.30) |
| >40 | 88 (2.00) | 222 (1.10) | 140 (1.58) | 455 (1.10) |
| Unknown | 1262 (28.68) | 6967 (34.58) | 2981 (33.62) | 15,049 (36.50) |
| Smoking, (%) | ||||
| Non-smoker | 1630 (37.05) | 6845 (33.98) | 2661 (30.01) | 12,582 (30.52) |
| Current smoker | 532 (12.09) | 2140 (10.62) | 1659 (18.71) | 5297 (12.85) |
| Past smoker | 356 (8.09) | 1129 (5.60) | 574 (6.47) | 2365 (5.74) |
| Unknown | 1882 (42.77) | 10,033 (49.80) | 3973 (44.81) | 20,987 (50.90) |
| Alcohol abuse a | 112 (2.55) | 308 (1.53) | 299 (3.37) | 710 (1.72) |
| Diabetes b | 1154 (26.23) | 3979 (19.75) | 2715 (30.62) | 7728 (18.74) |
| Hyperuricemia | ||||
| Asymptomatic | 429 (9.75) | 1500 (7.45) | 633 (7.14) | 2953 (7.16) |
| Gout | 256 (5.82) | 834 (4.14) | 419 (4.73) | 1727 (4.19) |
| Hypertension | 2889 (65.66) | 11,242 (55.80) | 5355 (60.39) | 20,676 (50.15) |
| Dyslipidemia c | 2031 (46.16) | 8050 (39.96) | 3810 (42.97) | 15,726 (38.14) |
| Peripheral artery disease | 241 (5.48) | 539 (2.68) | 431 (4.86) | 1011 (2.45) |
| Ischemic heart disease | ||||
| Acute myocardial infarction | 375 (8.52) | 746 (3.70) | 445 (5.02) | 1526 (3.70) |
| Angina pectoris d | 515 (11.70) | 1332 (6.61) | 667 (7.52) | 2525 (6.12) |
| TIA | 295 (6.70) | 478 (2.37) | 442 (4.98) | 852 (2.07) |
| Atrial fibrillation | 1903 (43.25) | 1682 (8.35) | 48 (0.54) | 2912 (7.06) |
| Thromboembolic disease | 132 (3.00) | 332 (1.65) | 152 (1.71) | 635 (1.54) |
| Heart failure | 612 (13.91) | 990 (4.91) | 366 (4.13) | 1663 (4.03) |
| Chronic renal failure | 289 (6.57) | 817 (4.06) | 442 (4.98) | 1431 (3.47) |
| Rheumatoid arthritis | 27 (0.61) | 152 (0.75) | 52 (0.59) | 263 (0.64) |
| COPD | 407 (9.25) | 1606 (7.97) | 744 (8.39) | 3053 (7.40) |
| Background vascular risk | ||||
| No risk factors/diseases | 376 (8.55) | 4182 (20.76) | 1399 (15.78) | 9969 (24.18) |
| Risk factors only | 3194 (72.59) | 14,325 (71.10) | 6259 (70.59) | 28,068 (68.07) |
| Established vascular disease | 830 (18.86) | 1640 (8.14) | 1209 (13.63) | 3194 (7.75) |
| CHA2DS2-VASc score | ||||
| Mean (±SD) | 3.39 (±1.63) | 2.90 (±1.50) | 2.89 (±1.62) | 2.51 (±1.59) |
| ≤3 | 2212 (50.27) | 12,727 (63.17) | 5539 (62.47) | 29,239 (70.92) |
| >3 | 2188 (49.73) | 7420 (36.83) | 3328 (37.53) | 11,992 (29.08) |
| Current use of: | ||||
| Antiplatelet drugs | 1178 (26.77) | 3484 (17.29) | 2346 (26.46) | 6487 (15.73) |
| NSAIDs | 334 (7.59) | 2000 (9.93) | 856 (9.65) | 3818 (9.26) |
| Oral anticoagulants drugs | 1013 (23.02) | 1255 (6.23) | 29 (0.33) | 2111 (5.12) |
| Paracetamol (Acetaminophen) | 807 (18.34) | 3533 (17.54) | 1354 (15.27) | 6128 (14.86) |
| Metamizole | 223 (5.07) | 824 (4.09) | 404 (4.56) | 1423 (3.45) |
| Opioids | 231 (5.25) | 891 (4.42) | 394 (4.44) | 1498 (3.63) |
| Proton pump inhibitors | 1608 (36.55) | 5698 (28.28) | 2625 (29.60) | 10,225 (24.80) |
| H2-receptor antagonists | 92 (2.09) | 368 (1.83) | 218 (2.46) | 677 (1.64) |
| Corticosteroids | 107 (2.43) | 310 (1.54) | 161 (1.82) | 633 (1.54) |
| ACEIs | 1028 (23.36) | 3618 (17.96) | 1777 (20.04) | 7007 (16.99) |
| ARBs | 846 (19.23) | 3291 (16.33) | 1485 (16.75) | 5907 (14.33) |
| Calcium channel blockers | 735 (16.70) | 2655 (13.18) | 1317 (14.85) | 4806 (11.66) |
| β-Blockers | 1066 (24.23) | 1935 (9.60) | 967 (10.91) | 3798 (9.21) |
| α-Blockers | 118 (2.68) | 494 (2.45) | 227 (2.56) | 896 (2.17) |
| Diuretics | 1195 (27.16) | 2913 (14.46) | 1172 (13.22) | 5082 (12.33) |
| Active forms of vitamin D | 20 (0.45) | 109 (0.54) | 53 (0.60) | 170 (0.41) |
| Bisphosphonates | 136 (3.09) | 477 (2.37) | 163 (1.84) | 847 (2.05) |
| Hormonal replacement therapy e | 2 (0.05) | 13 (0.06) | 9 (0.10) | 52 (0.13) |
| SERM | 7 (0.16) | 31 (0.15) | 10 (0.11) | 69 (0.17) |
| Strontium ranelate | 10 (0.23) | 35 (0.17) | 14 (0.16) | 43 (0.10) |
| Calcitonin | 3 (0.07) | 15 (0.07) | 5 (0.06) | 47 (0.11) |
| Denosumab | 3 (0.07) | 10 (0.05) | 3 (0.03) | 14 (0.03) |
| Teriparatide | 4 (0.09) | 13 (0.06) | 5 (0.06) | 20 (0.05) |
| Overall IS | Cases (%) N = 13,267 | Controls (%) N = 61,378 | Unadjusted OR * (95% CI) | Adjusted OR † (95% CI) |
|---|---|---|---|---|
| Non-users | 12,225 (92.15) | 57,434 (93.57) | 1 (Ref.) | 1 (Ref.) |
| Recency of use | ||||
| 77 (0.58) | 252 (0.41) | 1.36 (1.05–1.76) | 1.25 (0.95–1.63) |
| 543 (4.09) | 2211 (3.60) | 1.09 (0.99–1.21) | 1.02 (0.91–1.14) |
| 422 (3.18) | 1481 (2.41) | 1.28 (1.14–1.43) | 1.14 (1.00–1.29) |
| Duration | ||||
| ||||
| ≤1 year | 50 (0.38) | 186 (0.30) | 1.19 (0.87–1.63) | 1.08 (0.78–1.51) |
| >1 year | 27 (0.20) | 66 (0.11) | 1.84 (1.17–2.88) | 1.74 (1.08–2.79) |
| ||||
| ≤1 year | 368 (2.70) | 1571 (2.56) | 1.02 (0.91–1.15) | 0.96 (0.84–1.09) |
| >1 year | 185 (1.39) | 640 (1.04) | 1.28 (1.08–1.51) | 1.19 (0.98–1.43) |
| Daily dose of calcium | ||||
| ||||
| Low dose (<1000 mg/d) | 41 (0.31) | 145 (0.24) | 1.24 (0.87–1.76) | 1.22 (0.85–1.76) |
| High dose (≥1000 mg/d) | 23 (0.17) | 58 (0.09) | 1.81 (1.12–2.95) | 1.38 (0.82–2.30) |
| Unknown | 13 (0.10) | 49 (0.08) | 1.19 (0.64–2.19) | 1.13 (0.60–2.12) |
| ||||
| Low dose (<1000 mg/d) | 216 (1.63) | 1022 (1.67) | 0.93 (0.80–1.08) | 0.87 (0.73–1.02) |
| High dose (≥1000 mg/d) | 246 (1.85) | 862 (1.40) | 1.28 (1.11–1.48) | 1.19 (1.01–1.40) |
| Unknown | 81 (0.61) | 327 (0.53) | 1.12 (0.87–1.43) | 1.07 (0.83–1.39) |
| Cardioembolic Stroke | Cases (%) N = 4400 | Controls (%) N = 20,147 | Unadjusted OR * (95% CI) | Adjusted OR † (95% CI) |
|---|---|---|---|---|
| Non-users | 3995 (90.80) | 18,687 (92.75) | 1 (Ref.) | 1 (Ref.) |
| Recency of use | ||||
| 34 (0.77) | 90 (0.45) | 1.67 (1.12–2.49) | 1.88 (1.21–2.90) |
| 224 (5.09) | 788 (3.91) | 1.27 (1.09–1.49) | 1.08 (0.88–1.31) |
| 147 (3.34) | 582 (2.89) | 1.14 (0.94–1.37) | 0.93 (0.74–1.16) |
| Duration | ||||
| ||||
| ≤1 year | 21 (0.48) | 70 (0.35) | 1.32 (0.80–2.15) | 1.60 (0.94–2.70) |
| >1 year | 13 (0.30) | 20 (0.10) | 2.95 (1.46–5.96) | 2.73 (1.25–5.94) |
| ||||
| ≤1 year | 159 (3.61) | 531 (2.64) | 135 (1.12–1.62) | 1.17 (0.94–1.45) |
| >1 year | 65 (1.48) | 257 (1.28) | 1.12 (0.85–1.48) | 0.88 (0.63–1.21) |
| Daily dose of calcium | ||||
| ||||
| Low dose (<1000 mg/d) | 21 (0.48) | 50 (0.25) | 1.80 (1.08–3.01) | 2.28 (1.29–4.00) |
| High dose (≥1000 mg/d) | 9 (0.20) | 20 (0.10) | 2.09 (0.95–4.61) | 1.63 (0.70–3.81) |
| Unknown | 4 (0.09) | 20 (0.10) | 0.89 (0.30–2.63) | 1.18 (0.38–3.62) |
| ||||
| Low dose (<1000 mg/d) | 90 (2.05) | 375 (1.86) | 1.06 (0.83–1.34) | 0.90 (0.69–1.18) |
| High dose (≥1000 mg/d) | 101 (2.30) | 299 (1.48) | 1.53 (1.22–1.93) | 1.30 (0.99–1.71) |
| Unknown | 33 (0.75) | 114 (0.57) | 1.33 (0.90–1.96) | 1.08 (0.70–1.69) |
| Non-Cardioembolic Stroke | Cases (%) N = 8867 | Controls (%) N = 41,231 | Unadjusted OR * (95% CI) | Adjusted OR † (95% CI) |
|---|---|---|---|---|
| Non-users | 8230 (92.82) | 38,747 (93.98) | 1 (Ref.) | 1 (Ref.) |
| Recency of use | ||||
| 43 (0.48) | 162 (0.39) | 1.19 (0.85–1.67) | 1.05 (0.74–1.50) |
| 319 (3.60) | 1423 (3.45) | 0.99 (0.88–1.13) | 0.98 (0.84–1.13) |
| 275 (3.10) | 899 (2.18) | 1.37 (1.19–1.58) | 1.26 (1.07–1.48) |
| Duration | ||||
| ||||
| ≤1 year | 29 (0.33) | 116 (0.28) | 1.12 (0.74–1.69) | 0.96 (0.62–1.47) |
| >1 year | 14 (0.16) | 46 (0.11) | 1.36 (0.74–2.48) | 1.36 (0.73–2.54) |
| ||||
| ≤1 year | 199 (2.24) | 1040 (2.52) | 0.85 (0.73–1.00) | 0.84 (0.71–1.00) |
| >1 year | 120 (1.35) | 383 (0.93) | 1.38 (1.12–1.70) | 1.40 (1.11–1.77) |
| Daily dose of calcium | ||||
| ||||
| Low dose (<1000 mg/d) | 20 (0.23) | 95 (0.23) | 0.94 (0.58–1.52) | 0.88 (0.53–1.45) |
| High dose (≥1000 mg/d) | 14 (0.16) | 38 (0.09) | 1.67 (0.90–3.09) | 1.32 (0.69–2.55) |
| Unknown | 9 (0.10) | 29 (0.07) | 1.39 (0.65–2.94) | 1.23 (0.57–2.68) |
| ||||
| Low dose (<1000 mg/d) | 126 (1.42) | 647 (1.57) | 0.86 (0.71–1.05) | 0.83 (0.68–1.03) |
| High dose (≥1000 mg/d) | 145 (1.64) | 563 (1.37) | 1.14 (0.95–1.38) | 1.12 (0.91–1.38) |
| Unknown | 48 (0.54) | 213 (0.52) | 1.01 (0.74–1.38) | 1.05 (0.76–1.46) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreira-Hernández, D.; Rodríguez-Martín, S.; Gil, M.; Mazzucchelli, R.; Izquierdo-Esteban, L.; García-Lledó, A.; Pérez-Gómez, A.; Rodríguez-Miguel, A.; de Abajo, F.J. Risk of Ischemic Stroke Associated with Calcium Supplements and Interaction with Oral Bisphosphonates: A Nested Case-Control Study. J. Clin. Med. 2023, 12, 5294. https://doi.org/10.3390/jcm12165294
Barreira-Hernández D, Rodríguez-Martín S, Gil M, Mazzucchelli R, Izquierdo-Esteban L, García-Lledó A, Pérez-Gómez A, Rodríguez-Miguel A, de Abajo FJ. Risk of Ischemic Stroke Associated with Calcium Supplements and Interaction with Oral Bisphosphonates: A Nested Case-Control Study. Journal of Clinical Medicine. 2023; 12(16):5294. https://doi.org/10.3390/jcm12165294
Chicago/Turabian StyleBarreira-Hernández, Diana, Sara Rodríguez-Martín, Miguel Gil, Ramón Mazzucchelli, Laura Izquierdo-Esteban, Alberto García-Lledó, Ana Pérez-Gómez, Antonio Rodríguez-Miguel, and Francisco J. de Abajo. 2023. "Risk of Ischemic Stroke Associated with Calcium Supplements and Interaction with Oral Bisphosphonates: A Nested Case-Control Study" Journal of Clinical Medicine 12, no. 16: 5294. https://doi.org/10.3390/jcm12165294
APA StyleBarreira-Hernández, D., Rodríguez-Martín, S., Gil, M., Mazzucchelli, R., Izquierdo-Esteban, L., García-Lledó, A., Pérez-Gómez, A., Rodríguez-Miguel, A., & de Abajo, F. J. (2023). Risk of Ischemic Stroke Associated with Calcium Supplements and Interaction with Oral Bisphosphonates: A Nested Case-Control Study. Journal of Clinical Medicine, 12(16), 5294. https://doi.org/10.3390/jcm12165294








