Increased Cerebello-Prefrontal Connectivity Predicts Poor Executive Function in Congenital Heart Disease
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Brain Imaging Procedure
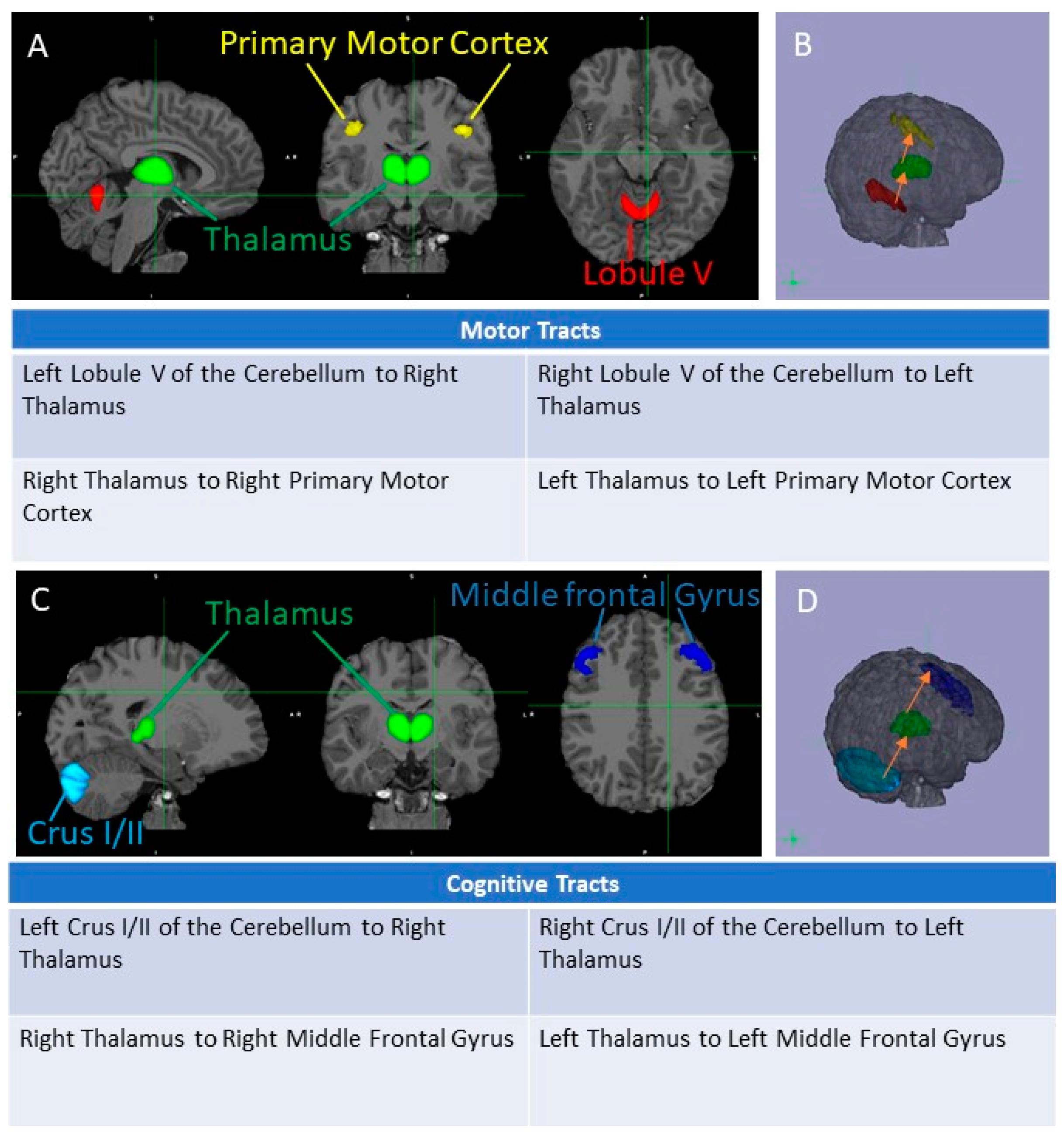
2.3. Image Processing
2.4. Neuropsychological Evaluation
2.5. Statistical Analysis
3. Results
3.1. Clinical and Neurocognitive Characterization of the Cohort
3.2. Cognitive and Motor Tract Imaging Data
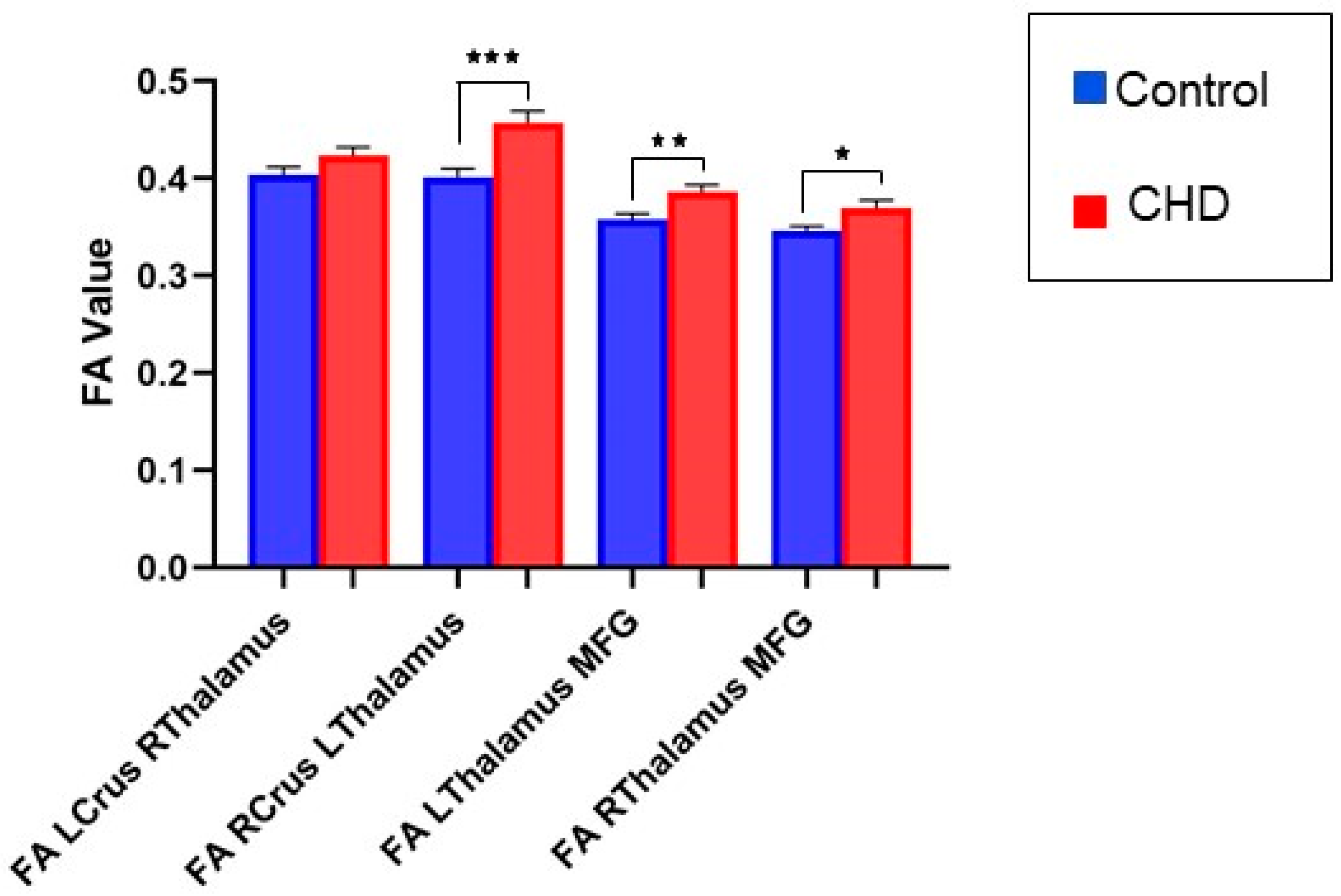
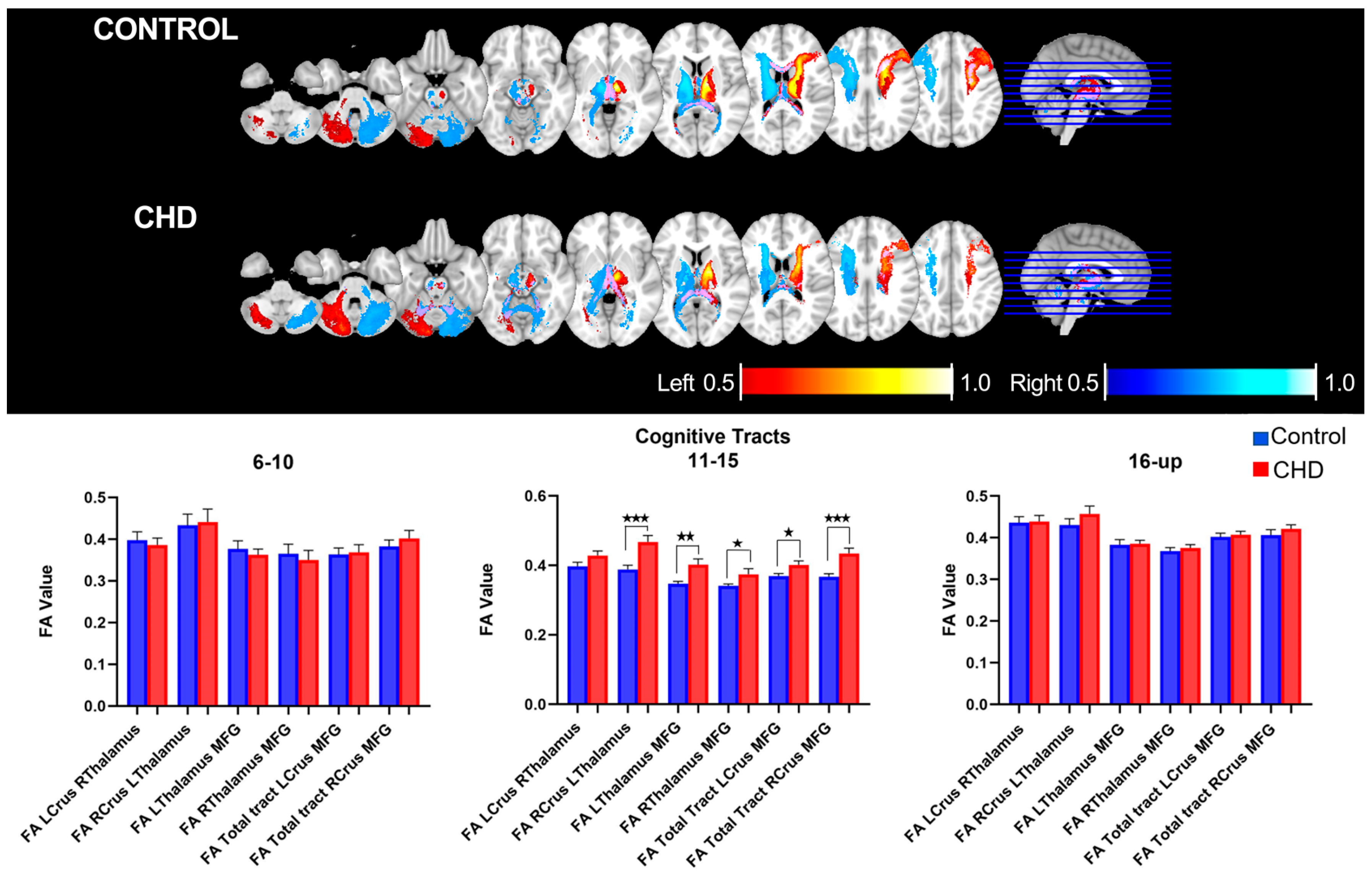
3.2.1. FA Measures in the Cognitive Loop: White Matter Tracts
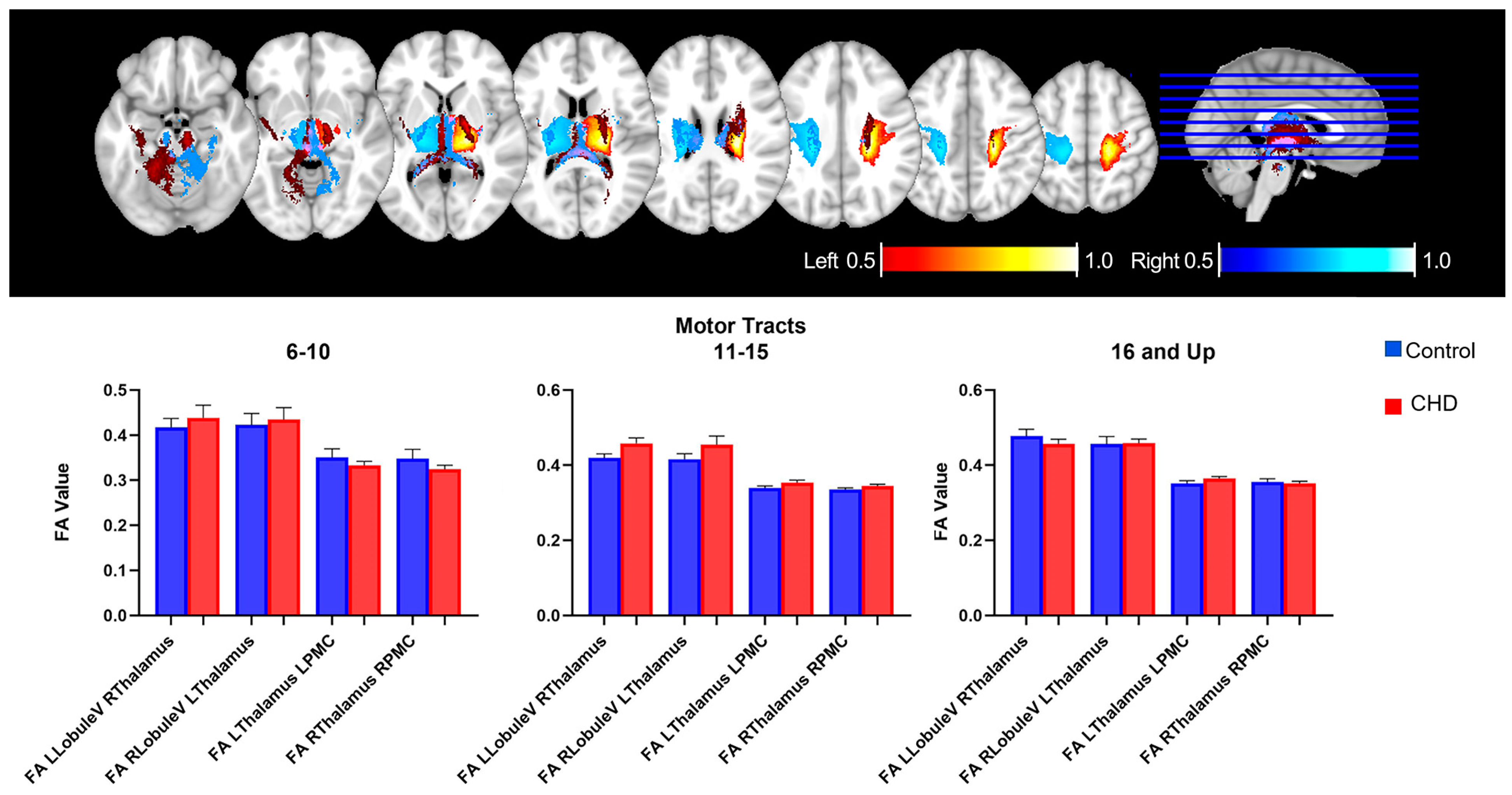
3.2.2. FA Measures in the Motor Loop: White Matter Tracts
3.3. Neurocognitive Assessment and Cerebello-Cerebral Cognitive Loop
3.4. Age-Related Neuro-Cognitive and FA Measures in the Cognitive Loop
4. Discussion
4.1. Adolescence: A Critical and Exclusive Period
4.2. Increase in Connectivity Inversely Correlate the Decrease in NIHTB Performance: The Relevance of Networks in Cognitive Outcome
4.3. Cognitive Performance in the Adolescent and Young Adult Population
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wray, J. Congenital heart disease and cardiac surgery in childhood: Effects on cognitive function and academic ability. Heart 2001, 85, 687–691. [Google Scholar] [CrossRef]
- Cassidy, A.R.; White, M.T.; DeMaso, D.R.; Newburger, J.W.; Bellinger, D.C. Executive function in children and adolescents with critical cyanotic congenital heart disease. J. Int. Neuropsychol. Soc. 2015, 21, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Gerstle, M.; Beebe, D.W.; Drotar, D.; Cassedy, A.; Marino, B.S. Executive Functioning and School Performance Among Pediatric Survivors of Complex Congenital Heart Disease. J. Pediatr. 2016, 173, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C.; Newburger, J.W. Late neurodevelopmental outcomes in children with congenital heart disease. In Pediatric Neuropsychology: Medical Advances and Lifespan Outcomes; Baron, I.S., Rey-Casserly, C., Eds.; Oxford University Press: Oxford, UK, 2013; pp. 91–112. [Google Scholar]
- Majnemer, A.; Limperopoulos, C.; Shevell, M.; Rohlicek, C.; Rosenblatt, B.; Tchervenkov, C. Developmental and Functional Outcomes at School Entry in Children with Congenital Heart Defects. J. Pediatr. 2008, 153, 55–60.e1. [Google Scholar] [CrossRef]
- Snookes, S.H.; Gunn, J.K.; Eldridge, B.J.; Donath, S.M.; Hunt, R.W.; Galea, M.P.; Shekerdemian, L. A Systematic Review of Motor and Cognitive Outcomes After Early Surgery for Congenital Heart Disease. Pediatrics 2010, 125, e818–e827. [Google Scholar] [CrossRef]
- von Rhein, M.; Scheer, I.; Loenneker, T.; Huber, R.; Knirsch, W.; Latal, B. Structural Brain Lesions in Adolescents with Congenital Heart Disease. J. Pediatr. 2011, 158, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C.; Wypij, D.; Duplessis, A.J.; Rappaport, L.A.; Jonas, R.A.; Wernovsky, G.; Newburger, J.W. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1385–1396. [Google Scholar] [CrossRef]
- Massaro, A.N.; Evangelou, I.; Fatemi, A.; Vezina, G.; Mccarter, R.; Glass, P.; Limperopoulos, C. White matter tract integrity and developmental outcome in newborn infants with hypoxic ischemic encephalopathy (HIE) treated with hypothermia. Dev. Med. Child Neurol. 2015, 57, 441–448. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsui, M.; Matsuzawa, J.; Tanaka, C.; Noguchi, K.; Yoshimura, N.; Hongo, K.; Ishiguro, M.; Wanatabe, S.; Hirono, K.; et al. Impaired neuroanatomic development in infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2009, 137, 146–153. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Wypij, D.; Rivkin, M.J.; DeMaso, D.R.; Robertson, R.L., Jr.; Dunbar-Masterson, C.; Rappaport, L.A.; Wernowsky, G.; Jonas, R.A.; Newburger, J.W. Adolescents with d-Transposition of the Great Arteries Corrected with the Arterial Switch Procedure: Neuropsychological Assessment and Structural Brain Imaging. Circulation 2011, 124, 1361–1369. [Google Scholar] [CrossRef]
- Von Rhein, M.; Buchmann, A.; Hagmann, C.; Huber, R.; Klaver, P.; Knirsch, W.; Latal, B. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain 2013, 137, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, M.E.; Du Plessis, A.J.; Sullivan, N.; Guizard, N.; Zhang, X.; Robertson, R.L.; Limperopoulos, C. Regional Cerebellar Volumes Predict Functional Outcome in Children with Cerebellar Malformations. Cerebellum 2012, 11, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, M.E.; Du Plessis, A.J.; Sullivan, N.; Guizard, N.; Zhang, X.; Robertson, R.L.; Limperopoulos, C. The complex genetics of hypoplastic left heart syndrome. Nat. Genet. 2017, 49, 1152–1159. [Google Scholar]
- Wu, Y.; Kapse, K.; Jacobs, M.; Niforatos-Andescavage, N.; Donofrio, M.T.; Krishnan, A.; Vezina, G.; Wessel, D.; du Plessis, A.; Limperopoulos, C. Association of Maternal Psychological Distress with in Utero Brain Development in Fetuses with Congenital Heart Disease. JAMA Pediatr. 2020, 174, e195316. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, A.; Lee, V.; Ceschin, R.; Zuccoli, G.; Beluk, N.; Khalifa, O.; Votava-Smith, J.K.; DeBrunner, M.; Munoz, R.; Domnina, Y.; et al. Brain Dysplasia Associated with Ciliary Dysfunction in Infants with Congenital Heart Disease. J. Pediatr. 2016, 178, 141–148.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Klena, N.T.; Gabriel, G.C.; Liu, X.; Kim, A.J.; Lemke, K.; Chen, Y.; Chatterjee, B.; Devine, W.; Damerla, R.R.; et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature 2015, 521, 520–524. [Google Scholar] [CrossRef]
- Ceschin, R.; Zahner, A.; Reynolds, W.; Gaesser, J.; Zuccoli, G.; Lo, C.W.; Gopalakrishnan, V.; Panigrahy, A. A computational framework for the detection of subcortical brain dysmaturation in neonatal MRI using 3D Convolutional Neural Networks. Neuroimage 2018, 178, 183–197. [Google Scholar] [CrossRef]
- Rollins, C.K.; Newburger, J.W. Neurodevelopmental Outcomes in Congenital Heart Disease. Circulation 2014, 130, e124–e126. [Google Scholar] [CrossRef]
- Watson, C.G.; Stopp, C.; Wypij, D.; Bellinger, D.C.; Newburger, J.W.; Rivkin, M.J. Altered White Matter Microstructure Correlates with IQ and Processing Speed in Children and Adolescents Post-Fontan. J. Pediatr. 2018, 200, 140–149.e4. [Google Scholar] [CrossRef]
- Brewster, R.C.; King, T.Z.; Burns, T.G.; Drossner, D.M.; Mahle, W.T. White Matter Integrity Dissociates Verbal Memory and Auditory Attention Span in Emerging Adults with Congenital Heart Disease. J. Int. Neuropsychol. Soc. 2015, 21, 22–33. [Google Scholar] [CrossRef]
- Panigrahy, A.; Schmithorst, V.J.; Wisnowski, J.L.; Watson, C.G.; Bellinger, D.C.; Newburger, J.W.; Rivkin, M.J. Relationship of white matter network topology and cognitive outcome in adolescents with d-transposition of the great arteries. NeuroImage Clin. 2015, 7, 438–448. [Google Scholar] [CrossRef]
- Badaly, D.; Beers, S.R.; Ceschin, R.; Lee, V.K.; Sulaiman, S.; Zahner, A.; Wallace, J.; Berdaa-Sahel, A.; Burns, C.; Panigrahy, A. Cerebellar and Prefrontal Structures Associated with Executive Functioning in Pediatric Patients with Congenital Heart Defects. Front. Neurol. 2022, 13, 827780. [Google Scholar] [CrossRef]
- Schmidt, E.L.; Burge, W.; Visscher, K.M.; Ross, L.A. Cortical Thickness in Fronto-parietal and Cingulo-opercular Networks Predicts Executive Function Performance in Older Adults. Neuropsychology 2016, 30, 322–331. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Schmahmann, J.D. The cerebellum and language: Evidence from patients with cerebellar degeneration. Brain Lang. 2009, 110, 149–153. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Guell, X.; Stoodley, C.J.; Halko, M.A. The Theory and Neuroscience of Cerebellar Cognition. Annu. Rev. Neurosci. 2019, 42, 337–364. [Google Scholar] [CrossRef] [PubMed]
- Diedrichsen, J. A spatially unbiased atlas template of the human cerebellum. NeuroImage 2006, 33, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Diedrichsen, J.; Balsters, J.H.; Flavell, J.; Cussans, E.; Ramnani, N. A probabilistic MR atlas of the human cerebellum. Neuroimage 2009, 46, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Johansen-Berg, H.; Behrens, T.E.; Sillery, E.; Ciccarelli, O.; Thompson, A.J.; Smith, S.M.; Matthews, P.M. Functional–Anatomical Validation and Individual Variation of Diffusion Tractography-based Segmentation of the Human Thalamus. Cereb. Cortex 2005, 15, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Geyer, S.; Ledberg, A.; Schleicher, A.; Kinomura, S.; Schormann, T.; Bürgel, U.; Klingberg, T.; Larsson, J.; Zilles, K.; Roland, P.E. Two different areas within the primary motor cortex of man. Nature 1996, 382, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Behrens, T.E.; Berg, H.J.; Jbabdi, S.; Rushworth, M.F.; Woolrich, M.W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage 2007, 34, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.A.; Orr, J.M.; Mittal, V.A. Differential Motor and Prefrontal Cerebello-Cortical Network Development: Evidence from Multimodal Neuroimaging. NeuroImage 2016, 124, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Salmi, J.; Pallesen, K.J.; Neuvonen, T.; Brattico, E.; Korvenoja, A.; Salonen, O.; Carlson, S. Cognitive and Motor Loops of the Human Cerebro-cerebellar System. J. Cogn. Neurosci. 2010, 22, 2663–2676. [Google Scholar] [CrossRef]
- Gershon, R.C.; Wagster, M.V.; Hendrie, H.C.; Fox, N.A.; Cook, K.F.; Nowinski, C.J. NIH Toolbox for Assessment of Neurological and Behavioral Function. Neurology 2013, 80, S2–S6. [Google Scholar] [CrossRef]
- Akshoomoff, N.; Beaumont, J.L.; Bauer, P.J.; Dikmen, S.S.; Gershon, R.C.; Mungas, D.; Slotkin, J.; Tulsky, D.; Weintraub, S.; Zelazo, P.D.; et al. NIH Toolbox Cognitive Function Battery (CFB): Composite Scores of Crystallized, Fluid, and Overall Cognition. Monogr. Soc. Res. Child Dev. 2013, 78, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Schmithorst, V.J.; Votava-Smith, J.K.; Tran, N.; Kim, R.; Lee, V.; Ceschin, R.; Lai, H.; Johnson, J.A.; Sanchez De Toledo, J.; Blüml, S.; et al. Structural Network Topology Correlates of Microstructural Brain Dysmaturation in Term Infants with Congenital Heart Disease. Hum. Brain Mapp. 2018, 39, 4593–4610. [Google Scholar] [CrossRef]
- Li, Y.; Yin, S.; Fang, J.; Hua, Y.; Wang, C.; Mu, D.; Zhou, K. Neurodevelopmental delay with critical congenital heart disease is mainly from prenatal injury not infant cardiac surgery: Current evidence based on a meta-analysis of functional magnetic resonance imaging. Ultrasound Obstet. Gynecol. 2015, 45, 639–648. [Google Scholar]
- Schmithorst, V.J.; Yuan, W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010, 72, 16–25. [Google Scholar] [CrossRef]
- Degnan, A.J.; Wisnowski, J.L.; Choi, S.; Ceschin, R.; Bhushan, C.; Leahy, R.M.; Corby, P.; Schmithorst, V.J.; Panigrahy, A. Altered Structural and Functional Connectivity in Late Preterm Preadolescence: An Anatomic Seed-Based Study of Resting State Networks Related to the Posteromedial and Lateral Parietal Cortex. PLoS ONE 2015, 10, e0130686. [Google Scholar] [CrossRef]
- Blakemore, S.-J.; Choudhury, S. Development of the adolescent brain: Implications for executive function and social cognition. J. Child Psychol. Psychiatry 2006, 47, 296–312. [Google Scholar] [CrossRef]
- Hutsler, J.J.; Zhang, H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010, 1309, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Fiala, J.C.; Spacek, J.; Harris, K.M. Dendritic Spine Pathology: Cause or Consequence of Neurological Disorders? Brain Res. Rev. 2002, 39, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, T.; Silberberg, G.; Markram, H. Hyperconnectivity of Local Neocortical Microcircuitry Induced by Prenatal Exposure to Valproic Acid. Cereb. Cortex 2008, 18, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Bostan, A.C.; Strick, P.L. The basal ganglia and the cerebellum: Nodes in an integrated network. Nat. Reviews. Neurosci. 2018, 19, 338–350. [Google Scholar]
- Habas, C.; Kamdar, N.; Nguyen, D.; Prater, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct Cerebellar Contributions to Intrinsic Connectivity Networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef]
- Karsdorp, P.A.; Everaerd, W.; Kindt, M.; Mulder, B.J. Psychological and Cognitive Functioning in Children and Adolescents with Congenital Heart Disease: A Meta-Analysis. J. Pediatr. Psychol. 2007, 32, 527–541. [Google Scholar] [CrossRef]
| Controls | CHD | p | |
|---|---|---|---|
| n | 73 | 53 | |
| Age | 13.23 | 14.88 | 0.06726 |
| Maternal Education | 5.6 | 4.4 | 0.7523 |
| Mean Household Income | 3.644 | 3.04 | 0.10118 |
| Total Cognition Composite | 113.7 | 101.7 | 0.0023 * |
| Crystallized Cognition Composite | 114.9 | 105.6 | 0.0004 * |
| Fluid Cognition Composite | 106.7 | 97.88 | 0.0025 * |
| Dimensional Card Change Sort (DCCS) | 102.1 | 96.45 | 0.0478 * |
| Flanker Inhibitory Control and Attention | 100.2 | 98.79 | 0.5506 |
| List Sorting | 106.1 | 102.3 | 0.0999 |
| Picture Sequence Memory | 107.5 | 98.02 | 0.0028 * |
| Oral Reading | 117 | 106 | 0.0003 |
| Picture Vocabulary | 107.8 | 103 | 0.0221 * |
| Pattern Comparison | 99.35 | 97.95 | 0.7206 |
| 9-Hole Pegboard Dexterity | 106.9 | 104.8 | 0.4128 |
| Estimate | Standard Error | 95% CI | p Value | R2 | |
|---|---|---|---|---|---|
| Cognitive Tracts | |||||
| LCrus_RThalamus | 0.01471 | 0.01273 | −0.01053 to 0.03995 | 0.2503 | 0.05678 |
| RCrus_LThalamus | 0.05415 | 0.01509 | 0.02422 to 0.08409 | 0.0005 * | 0.1252 |
| LThalamus_MFG | 0.02672 | 0.009964 | 0.006952 to 0.04648 | 0.0086 * | 0.1747 |
| RThalamus_MFG | 0.02113 | 0.009381 | 0.002522 to 0.03974 | 0.0264 * | 0.1702 |
| Motor Tracts | |||||
| LLobuleV_RThalamus | 0.01867 | 0.01384 | −0.008759 to 0.04610 | 0.1801 | 0.2675 |
| RLobuleV_LThalamus | 0.02038 | 0.01644 | −0.01226 to 0.05301 | 0.2182 | 0.3336 |
| LThalamus_LPMC | 0.006271 | 0.007993 | −0.009550 to 0.02209 | 0.4342 | 0.1442 |
| RThalamus_RPMC | −0.002758 | 0.008069 | −0.01873 to 0.01321 | 0.733 | 0.07514 |
| NIHTB Fluid Score | Estimate | Standard Error | 95% CI | p Value | R2 |
|---|---|---|---|---|---|
| LCrus_RThalamus | −8.756 | 31.86 | −71.92 to 54.41 | 0.784 | 0.07693 |
| RCrus_LThalamus | −3.288 | 24.41 | −51.70 to 45.12 | 0.8931 | 0.09260 |
| LThalamus_MFG | 44.47 | 34.36 | −23.61 to 112.6 | 0.1982 | 0.0765 |
| RThalamus_MFG | 98.57 | 35.69 | 27.84 to 169.3 | 0.0067 * | 0.1186 |
| NIHTB Crystallized Score | |||||
| LCrus_RThalamus | 2.44 | 20.97 | −39.14 to 44.02 | 0.9076 | 0.1390 |
| RCrus_LThalamus | 6.195 | 16.36 | −26.25 to 38.64 | 0.7056 | 0.1594 |
| LThalamus_MFG | 24.64 | 22.55 | −20.03 to 69.32 | 0.2767 | 0.1507 |
| RThalamus_MFG | 60.05 | 23.41 | 13.66 to 106.4 | 0.0117 * | 0.1841 |
| NIHTB Composite Score | |||||
| LCrus_RThalamus | −7.976 | 31.36 | −70.17 to 54.22 | 0.7998 | 0.1368 |
| RCrus_LThalamus | −0.5671 | 23.92 | −48.02 to 46.88 | 0.9811 | 0.1618 |
| LThalamus_MFG | 45.54 | 33.7 | −21.23 to 112.3 | 0.1793 | 0.1423 |
| RThalamus_MFG | 109.4 | 34.55 | 40.91 to 177.8 | 0.002 * | 0.1932 |
| NIHTB DCCS | |||||
| LCrus_RThalamus | −0.589 | 25.18 | −50.51 to 49.33 | 0.9814 | 0.0617 |
| RCrus_LThalamus | −0.5211 | 19.62 | −39.42 to 38.38 | 0.9789 | 0.0540 |
| LThalamus_MFG | 34.89 | 28.41 | −21.40 to 91.17 | 0.222 | 0.05001 |
| RThalamus_MFG | 53.37 | 29.49 | −5.050 to 111.8 | 0.073 | 0.0562 |
| NIHTB Flanker Inhibitory Test | |||||
| LCrus_RThalamus | −19.24 | 21.91 | −62.69 to 24.21 | 0.382 | 0.0929 |
| RCrus_LThalamus | −3.257 | 17.32 | −37.60 to 31.09 | 0.8512 | 0.0610 |
| LThalamus_MFG | 44.21 | 23.93 | −3.211 to 91.63 | 0.0674 | 0.3414 |
| RThalamus_MFG | 79.22 | 24.51 | 30.66 to 127.8 | 0.0016 * | 0.0904 |
| Cognitive Tracts | Estimate | Standard Error | 95% CI | p Value | R2 | |
|---|---|---|---|---|---|---|
| Age 6–10 | ||||||
| LCrus- RThalamus | 0.01867 | 0.01384 | −0.008759 to 0.04610 | 0.9148 | 0.07885 | |
| RCrus- LThalamus | 0.03746 | 0.03626 | −0.03775 to 0.1127 | 0.3129 | 0.05057 | |
| LThalamus-MFG | 0.003297 | 0.02032 | −0.03885 to 0.04545 | 0.8726 | 0.07358 | |
| RThalamus-MFG | 0.01341 | 0.02664 | −0.04184 to 0.06865 | 0.6198 | 0.1565 | |
| Age 11−15 | ||||||
| LCrus- RThalamus | 0.03114 | 0.0188 | −0.006716 to 0.06900 | 0.1045 | 0.05812 | |
| RCrus- LThalamus | 0.07916 | 0.02133 | 0.03620 to 0.1221 | 0.0006 | 0.2349 * | |
| LThalamus-MFG | 0.05516 | 0.01567 | 0.02359 to 0.08673 | 0.0010 | 0.2161 * | |
| RThalamus-MFG | 0.03261 | 0.0128 | 0.006831 to 0.05838 | 0.0143 | 0.1532 * | |
| Age 16 and Up | ||||||
| LCrus- RThalamus | 0.0029 | 0.02286 | −0.04385 to 0.04965 | 0.8999 | 0.06234 | |
| RCrus- LThalamus | 0.02731 | 0.02748 | −0.02890 to 0.08352 | 0.3286 | 0.03437 | |
| LThalamus-MFG | 0.008608 | 0.01293 | −0.01783 to 0.03505 | 0.5108 | 0.1528 | |
| RThalamus-MFG | 0.005372 | 0.01537 | −0.02606 to 0.03680 | 0.01940 | 0.7292 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahel, A.; Ceschin, R.; Badaly, D.; Lewis, M.; Lee, V.K.; Wallace, J.; Weinberg, J.; Schmithorst, V.; Lo, C.; Panigrahy, A. Increased Cerebello-Prefrontal Connectivity Predicts Poor Executive Function in Congenital Heart Disease. J. Clin. Med. 2023, 12, 5264. https://doi.org/10.3390/jcm12165264
Sahel A, Ceschin R, Badaly D, Lewis M, Lee VK, Wallace J, Weinberg J, Schmithorst V, Lo C, Panigrahy A. Increased Cerebello-Prefrontal Connectivity Predicts Poor Executive Function in Congenital Heart Disease. Journal of Clinical Medicine. 2023; 12(16):5264. https://doi.org/10.3390/jcm12165264
Chicago/Turabian StyleSahel, Aurelia, Rafael Ceschin, Daryaneh Badaly, Madison Lewis, Vince K. Lee, Julia Wallace, Jacqueline Weinberg, Vanessa Schmithorst, Cecilia Lo, and Ashok Panigrahy. 2023. "Increased Cerebello-Prefrontal Connectivity Predicts Poor Executive Function in Congenital Heart Disease" Journal of Clinical Medicine 12, no. 16: 5264. https://doi.org/10.3390/jcm12165264
APA StyleSahel, A., Ceschin, R., Badaly, D., Lewis, M., Lee, V. K., Wallace, J., Weinberg, J., Schmithorst, V., Lo, C., & Panigrahy, A. (2023). Increased Cerebello-Prefrontal Connectivity Predicts Poor Executive Function in Congenital Heart Disease. Journal of Clinical Medicine, 12(16), 5264. https://doi.org/10.3390/jcm12165264






