Associations between Menopausal Hormone Therapy and Colorectal, Lung, or Melanoma Cancer Recurrence and Mortality: A Narrative Review
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Selection of Studies
2.3. Data Extraction, Synthesis, and Risk of Bias Assessment
2.4. Evidence for the Decision Framework and Eligibility Criteria
- Category 1: no restrictions on MHT use;
- Category 2: benefits outweigh the risks;
- Category 3: risks generally outweigh the benefits;
- Category 4: MHT should not be used.
3. Results
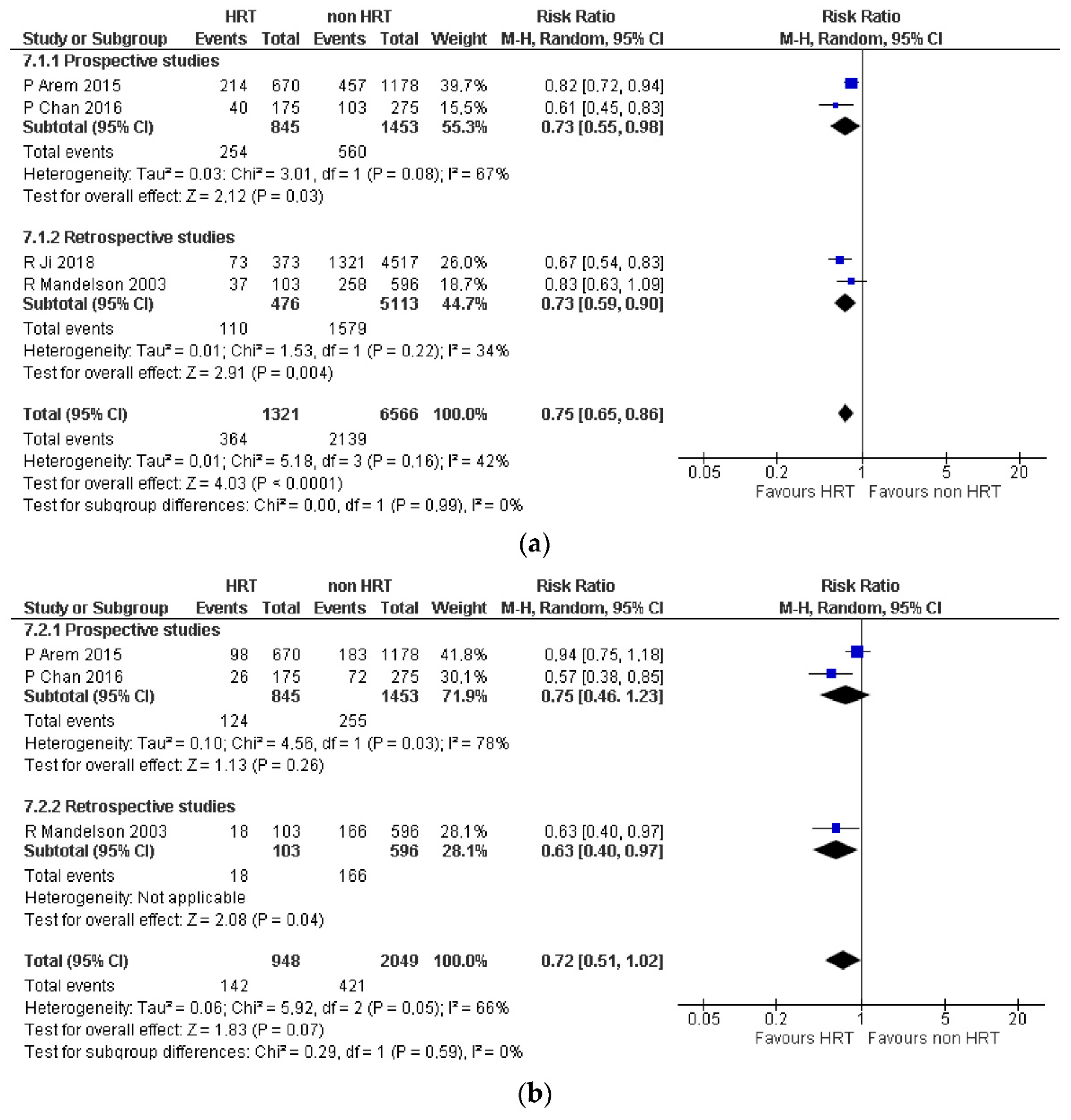
3.1. Colorectal Cancer
3.2. Lung Cancer
3.3. Cutaneous Melanoma
4. Discussion
4.1. Why Is This Report Important?
4.2. Strengths
4.3. Limitations
4.4. Clinical Evidence
4.4.1. Colorectal Cancer
4.4.2. Lung Cancer
4.4.3. Cutaneous Melanoma
4.5. Cancer Risk in Healthy MHT Users
4.5.1. Colorectal Cancer
4.5.2. Lung Cancer
4.5.3. Melanoma
4.6. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Villiers, T.J.; Hall, J.E.; Pinkerton, J.V.; Pérez, S.C.; Rees, M.; Yang, C.; Pierroz, D.D. Revised global consensus statement on menopausal hormone therapy. Maturitas 2016, 91, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Davey, D.A. Menopausal hormone therapy: A better and safer future. Climacteric 2018, 21, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Chester, R.C.; Kling, J.M.; Manson, J.E. What the Women’s Health Initiative has taught us about menopausal hormone therapy? Clin. Cardiol. 2018, 41, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Stepan, J.J.; Hruskova, H.; Kverka, M. Update on Menopausal Hormone Therapy for Fracture Prevention. Curr. Osteoporos. Rep. 2019, 17, 465–473. [Google Scholar] [CrossRef]
- Baber, R.J.; Panay, N.; Fenton, A.; IMS Writing Group. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150. [Google Scholar] [CrossRef]
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2018, 25, 1362–1387. [Google Scholar] [CrossRef]
- WHO. Medical Eligibility Criteria for Contraceptive Use: A WHO Family Planning Cornerstone, 4th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Mendoza, N.; Juliá, M.D.; Galliano, D.; Coronado, P.; Díaz, B.; Fontes, J.; Gallo, J.L.; García, A.; Guinot, M.; Munnamy, M.; et al. Spanish consensus on premature menopause. Maturitas 2015, 80, 220–225. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer 2023. Globocan 2020—Global Cancer Observatory. Available online: http://gco.iarc.fr/ (accessed on 4 June 2023).
- Rossouw, J.J.; Anderson, G.G.; Prentice, R.R.; LaCroix, A.A.; Kooperberg, C.; Stefanick, M.M.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Matthews, J.; Wihlen, B.; Tujague, M.; Wan, J.; Strom, A.; Gustafsson, J.-A. Estrogen Receptor (ER) β Modulates ERα-Mediated Transcriptional Activation by Altering the Recruitment of c-Fos and c-Jun to Estrogen-Responsive Promoters. Mol. Endocrinol. 2006, 20, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.; Toth, C.; Hoffmeister, M.; Roth, W.; Herpel, E.; Jansen, L.; Marx, A.; Brenner, H.; Chang-Claude, J. Expression of oestrogen receptor β and prognosis of colorectal cancer. Br. J. Cancer 2012, 107, 831–839. [Google Scholar] [CrossRef]
- Looijer-van Langen, M.; Hotte, N.; Dieleman, L.A.; Albert, E.; Mulder, C.; Madsen, K.L. Estrogen receptor-β signaling modulates epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G621–G626. [Google Scholar] [CrossRef] [PubMed]
- Schleipen, B.; Hertrampf, T.; Fritzemeier, K.H.; Kluxen, F.M.; Lorenz, A.; Molzberger, A.; Velders, M.; Diel, P. ERβ-specific agonists and genistein inhibit proliferation and induce apoptosis in the large and small intestine. Carcinogenesis 2011, 32, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Saha, J.; Pillai, S.; Lam, A.K.Y.; Gopalan, V.; Islam, F. Implications of estrogen and its receptors in colorectal carcinoma. Cancer Med. 2023, 12, 4367–4379. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Meireles, S.I.; Xu, X.; Smith, W.E.; Slifker, M.J.; Riel, S.L.; Zhai, S.; Zhang, G.; Ma, X.; Kurzer, M.S.; et al. Estrogen metabolism in the human lung: Impact of tumorigenesis, smoke, sex and race/ethnicity. Oncotarget 2017, 8, 106778–106789. [Google Scholar] [CrossRef]
- Fuentes, N.; Rodriguez, M.S.; Silveyra, P. Role of sex hormones in lung cancer. Exp. Biol. Med. 2021, 246, 2098–2110. [Google Scholar] [CrossRef]
- Stabile, L.P.; Davis, A.L.; Gubish, C.T.; Hopkins, T.M.; Luketich, J.D.; Christie, N.; Finkelstein, S.; Siegfried, J.M. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological re-sponses to estrogen. Cancer Res. 2002, 62, 2141–2150. [Google Scholar]
- Burns, T.F.; Stabile, L.P.; Maskarinec, G.; Beckford, F.; Morimoto, Y.; Franke, A.A.; Stanczyk, F.Z.; Knowlton, A.A.; Hertz, D.L.; Henry, N.L.; et al. Targeting the estrogen pathway for the treatment and prevention of lung cancer. Lung Cancer Manag. 2014, 3, 43–52. [Google Scholar] [CrossRef]
- Dika, E.; Patrizi, A.; Lambertini, M.; Manuelpillai, N.; Fiorentino, M.; Altimari, A.; Ferracin, M.; Lauriola, M.; Fabbri, E.; Campione, E.; et al. Estrogen Receptors and Melanoma: A Review. Cells 2019, 8, 1463. [Google Scholar] [CrossRef]
- Hannaford, P.; Villard-Mackintosh, L.; Vessey, M.; Kay, C. Oral contraceptives and malignant melanoma. Br. J. Cancer 1991, 63, 430–433. [Google Scholar] [CrossRef][Green Version]
- Palmer, J.R.; Rosenberg, L.; Strom, B.L.; Harlap, S.; Zauber, A.G.; Warshauer, M.E.; Shapiro, S. Oral contraceptive use and risk of cutaneous malignant melanoma. Cancer Causes Control. 1992, 3, 547–554. [Google Scholar] [CrossRef]
- Botteri, E.; Støer, N.C.; Sakshaug, S.; Graff-Iversen, S.; Vangen, S.; Hofvind, S.; Ursin, G.; Weiderpass, E. Menopausal hormone therapy and risk of melanoma: Do estrogens and progestins have a different role? Int. J. Cancer 2017, 141, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Manager (RevMan) [Computer Program], version 5.4. The Cochrane Collaboration, 2020. Available online: https://training.cochrane.org/system/files/uploads/protected_file/RevMan5.4_user_guide.pdf (accessed on 28 April 2020).
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Updated October 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 28 April 2020).
- Alonso-Coello, P.; Schünemann, H.J.; Moberg, J.; Brignardello-Petersen, R.; Akl, E.A.; Davoli, M.; Treweek, S.; Mustafa, R.A.; Rada, G.; Rosenbaum, S.; et al. GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016, 353, i2016. [Google Scholar] [CrossRef]
- Chan, J.A.; Meyerhardt, J.A.; Chan, A.T.; Giovannucci, E.L.; Colditz, G.A.; Fuchs, C.S. Hormone Replacement Therapy and Survival After Colorectal Cancer Diagnosis. J. Clin. Oncol. 2006, 24, 5680–5686. [Google Scholar] [CrossRef]
- Arem, H.; Park, Y.; Felix, A.S.; Zervoudakis, A.; Brinton, L.A.; Matthews, C.E.; Gunter, M.J. Reproductive and hormonal factors and mortality among women with colorectal cancer in the NIH-AARP Diet and Health Study. Br. J. Cancer 2015, 113, 562–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mandelson, M.T.; Miglioretti, D.; Newcomb, P.A.; Harrison, R.; Potter, J.D. Hormone replacement therapy in relation to survival in women diagnosed with colon cancer. Cancer Causes Control 2003, 14, 979–984. [Google Scholar] [CrossRef]
- Ji, J.; Sundquist, J.; Sundquist, K. Use of hormone replacement therapy improves the prognosis in patients with colorectal cancer: A population-based study in Sweden. Int. J. Cancer 2018, 142, 2003–2010. [Google Scholar] [CrossRef]
- Slattery, M.L.; Anderson, K.; Samowitz, W.; Edwards, S.L.; Curtin, K.; Caan, B.; Potter, J.D. Hormone replacement therapy and improved survival among postmenopausal women diagnosed with colon cancer (USA). Cancer Causes Control 1999, 10, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ganti, A.K.; Sahmoun, A.E.; Panwalkar, A.W.; Tendulkar, K.K.; Potti, A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J. Clin. Oncol. 2006, 24, 59–63. [Google Scholar] [CrossRef]
- Ayeni, O.; Robinson, A. Hormone replacement therapy and outcomes for women with non-small-cell lung cancer: Can an association be confirmed? Curr. Oncol. 2009, 16, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Katcoff, H.; Wenzlaff, A.S.; Schwartz, A.G. Survival in Women with NSCLC: The Role of Reproductive History and Hormone Use. J. Thorac. Oncol. 2014, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Carloss, H.; Wyatt, S.W.; Riley, E. Hormone replacement therapy and survival in lung cancer in postmenopausal women in a rural population. Cancer 2009, 115, 4167–4175. [Google Scholar] [CrossRef]
- Clague, J.; Reynolds, P.; Henderson, K.D.; Sullivan-Halley, J.; Ma, H.; Lacey, J.V., Jr.; Chang, S.; Delclos, G.L.; Du, X.L.; Forman, M.R.; et al. Menopausal Hormone Therapy and Lung Cancer-Specific Mortality Following Diagnosis: The California Teachers Study. PLoS ONE 2014, 9, e103735. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, L.; Huang, Y.; Luan, X. Meta-analysis for the effect of hormone replacement therapy on survival rate in female with lung cancer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 372–377. [Google Scholar]
- MacKie, R.M.; Bray, C.A. Hormone replacement therapy after surgery for stage 1 or 2 cutaneous melanoma. Br. J. Cancer 2004, 90, 770–772. [Google Scholar] [CrossRef]
- Symer, M.M.; Wong, N.N.; Abelson, J.J.; Milsom, J.J.; Yeo, H.H. Hormone Replacement Therapy and Colorectal Cancer Inci-dence and Mortality in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Clin. Colorectal. Cancer 2018, 17, e281–e288. [Google Scholar] [CrossRef]
- Manson, J.E.; Aragaki, A.K.; Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Chlebowski, R.T.; Howard, B.V.; Thomson, C.A.; Margolis, K.L.; et al. Menopausal Hormone Therapy and Long-term All-Cause and Cause-Specific Mortality: The Women’s Health Initiative Randomized Trials. JAMA 2017, 318, 927–938. [Google Scholar] [CrossRef]
- Titan, A.L.; He, H.; Lui, N.; Liou, D.; Berry, M.; Shrager, J.B.; Backhus, L.M. The influence of hormone replacement therapy on lung cancer incidence and mortality. J. Thorac. Cardiovasc. Surg. 2020, 159, 1546–1556.e4. [Google Scholar] [CrossRef]
- Jin, C.; Lang, B. Hormone replacement therapy and lung cancer risk in women: A meta-analysis of cohort studies: Hormone replacement therapy and lung cancer risk. Medicine 2019, 98, e17532. [Google Scholar] [CrossRef]
- Hammouz, R.Y.; Orzechowska, M.; Anusewicz, D.; Bednarek, A.K. X or Y Cancer: An Extensive Analysis of Sex Differences in Lung Adenocarcinoma. Curr. Oncol. 2023, 30, 1395–1415. [Google Scholar] [CrossRef] [PubMed]
- Hicks, B.M.; Kristensen, K.B.; Pedersen, S.A.; Hölmich, L.R.; Pottegård, A. Hormone replacement therapy and the risk of melanoma in post-menopausal women. Hum. Reprod. 2019, 34, 2418–2429. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Støer, N.C.; Weiderpass, E.; Pukkala, E.; Ylikorkala, O.; Lyytinen, H. Menopausal Hormone Therapy and Risk of Melanoma: A Nationwide Register-Based Study in Finland. Epidemiol. Biomarkers Prev. 2019, 28, 1857–1860. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, I.; de la Viuda, E.; Calaf, J.; Baquedano, L.; Coronado, P.; Llaneza, P.; Nieto, V.; Otero, B.; Sánchez, S.; Mendoza, N. Criterios de Elegibilidad de la Terapia Hormonal de la Menopausia; Universidad de Granada: Granada, Spain, 2021. [Google Scholar]
- Mendoza, N.; Ramírez, I.; de la Viuda, E.; Coronado, P.; Baquedano, L.; Llaneza, P.; Nieto, V.; Otero, B.; Sánchez-Méndez, S.; de Frutos, V.Á.; et al. Eligibility criteria for Menopausal Hormone Therapy (MHT): A position statement from a consortium of scientific societies for the use of MHT in women with medical conditions. MHT Eligibility Criteria Group. Maturitas 2022, 166, 65–85. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow , C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Study | Study Period | Country | Age | Number of Participants | Stage | Grade | MHT Type | MHT Recency | CRC Death MHT User vs. Non User | All-Cause Mean Death MHT User vs. Non User | Mean Follow-Up Year |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective cohort | |||||||||||
| Chan et al. (2006) [30] | 1976–2004 | USA | 62.2–65.7 | 834 | 22.3% stage I, 26.1% stage II, 25.5% stage III, 15.6% stage IV, 10.5% unknown | 22.3% stage I, 26.1% stage II, 25.5% stage III, 15.6% stage IV, 10.5% unknown | E, E + P | Current former | Current 0.64 (0.47–0.88) former 1.05 (0.79–1.40) | Current 0.74 (0.56–0.97) former1.00 (0.78–1.30) | 5–10 |
| Arem et al. (2015) [31] | 1995–2001 | USA | 50–71 | 2053 | 30.5% localized, 31.3% regional or distant, 38.2% unknown | 12.0% well-differentiated, 57.5%; moderately-differentiated, 0.9%; undifferentiated, 29.6% unknown | E, E + P | Current former | Current 0.76 (0.59, 0.97) former 1.03 (0.72, 1.47) | Current 0.79 (0.66, 0.94) former1.13 (0.89, 1.43) | 7.7 |
| Retrospective cohort | |||||||||||
| Mandelson et al. (2003) [32] | 1980–1998 | USA | 50–79 | 699 | NR | NR | E, E + P | Current | 0.59 (0.35–0.97) | 0.77 (0.54–1.09) | 5.33 |
| Ji et al. (2018) [33] | 2006–2015 | Sweden | 45–69 | 5626 | 23.7% stage I, 27.8% stage II, 36.2% stage III, 12.3% stage IV | NR | E, E + P | Current | 0.74 (0.62–0.88); p = 0.0006 | 0.70 (0.60–0.82); p < 0.0001 | 5.4 |
| Slattery et al. (1999) [34] | 1991–1998 | USA | 50–79 | 801 | 35.4% local, 53.2% regional, 11.4% distant | NR | E, E + P | Current to stop less than 5 years | 504 (62.9) 297 (37.1) 0.6 (0.4 ± 0.9) | 0.7 (0.5 ± 0.9) | 4 |
| Study | Study Period | Country | Age | Number of Participants | Stage | Smokers | MHT Type | Median Overall Survival | Median Overall Survival with MHT Smokers/Non-Smokers |
|---|---|---|---|---|---|---|---|---|---|
| Retrospective cohort | |||||||||
| Ganti et al. (2006) [35] | 1994–1999 | USA | 31–93 | 498 | I: 26% II: 21% IIIA: 11% IIIB: 8% IV: 28% | 86% | All types | Never used MHT 79 months; 95% CI: 65–95 months MHT 39 months; 95% CI: 35–77 months p < 0.02 | Smoker and MHT smoker; and used/smoke and no MHT 39 vs. 73 months; p < 0.03). Non-smoker and MHT/Non-smoker and not MHT; 92 vs. 98 NS |
| Ayeni et al. (2009) [36] | |||||||||
| Katkoff et al. (2014) [37] | 2001–2005 | USA | 17–74 | 485 | Local: 33.6% Regional: 33.4% Distant: 33.0 | Current or former 92.3% | Estrogen only: 99 Estrogen plus progesterone: 85 | Median survival time, MHT 80.0 m No MHT 37.5 m p < 0.001 | Never smoked and MHT vs. no MHT: 17 (7.4)/ 20 (7.9) Current smokers and MHT vs. no MHT: 126 (54.8)/165 (65.2) NS |
| Huang et al. (2009) [38] | 1995–2005 | USA | 37–90 | 648 |
I: 20.8% II: 4.8% III: 30.1% IV: 37.4% Unknown stage: 6.9% | 61.9% | All types |
MHT/no MHT 16.4 vs. 10.5 NS | Smoker and MHT/Non-smoker and MHT 11.3 vs. 16.9 months p < 0.03 Smoker and MHT/Non-smoker and MHT NS |
| Prospective cohort | |||||||||
| Clague et al. (2014) [39] | 1995–1996 | USA | NR | 727 | Localized: 153 Regional: 51 Lymph nodes: 73 Regional and lymph nodes: 33 Distant: 365 Unknown: 52 | 543 (74.69%) | Estrogen: 188 Estrogen + progesterone: 176 | MHT: 21.4 m No MHT: 15.6 m p = 0.002 | Used MHT vs. never used MHT (HR) Never smoked: 1.23 (0.58–2.63) Former smokers: 0.74 (0.50–1.10) Current smokers: 0.44 (0.26–0.75) |
| Meta-analysis | |||||||||
| Li et al. (2020) [40] | No HRT: 1054 HRT: 1528 | With MHT, survival increased time for 5 years (ES = 0.346; 95% CI 0.216–0.476; p < 0.001) |
| Study | Study Period | Country | Age | Number of Participants | Type | HRT Type | CRC Death/HRT User | All-Cause Mean Death/MHT User | Mean Follow-Up Year |
|---|---|---|---|---|---|---|---|---|---|
| Prospective cohort | |||||||||
| MacKie et al. (2004) [41] | 1990–1995 | Scotland | 46–59 | 206 | Ulceration: Yes, 5 (6.2); 21 (17.8) 0.017 patients with tumors 1 mm thick: 42 (50.6); 58 (47.2); 0.627 Patients with superficially spreading melanoma: 60 (73.2); 84 (69.4); 0.846 Nodular/polypoid melanoma: 15 (18.3); 25 (20.7); Lentigo maligna melanoma: 4 (4.9); 4 (4.3); Acral/mucosal melanoma: 1 (1.2); 2 (1.7); Other and unspecified melanomas: 2 (2.4); 6 (5.0). | 21 oestrogen 62 oestrogen/progesrterone | MHT: 1 No MHT: 22 | MHT: 0 No MHT: 4 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiol, G.; Lete, I.; Nieto, L.; Santaballa, A.; Pla, M.J.; Baquedano, L.; Calaf, J.; Coronado, P.; de la Viuda, E.; Llaneza, P.; et al. Associations between Menopausal Hormone Therapy and Colorectal, Lung, or Melanoma Cancer Recurrence and Mortality: A Narrative Review. J. Clin. Med. 2023, 12, 5263. https://doi.org/10.3390/jcm12165263
Fiol G, Lete I, Nieto L, Santaballa A, Pla MJ, Baquedano L, Calaf J, Coronado P, de la Viuda E, Llaneza P, et al. Associations between Menopausal Hormone Therapy and Colorectal, Lung, or Melanoma Cancer Recurrence and Mortality: A Narrative Review. Journal of Clinical Medicine. 2023; 12(16):5263. https://doi.org/10.3390/jcm12165263
Chicago/Turabian StyleFiol, Gabriel, Iñaki Lete, Laura Nieto, Ana Santaballa, María Jesús Pla, Laura Baquedano, Joaquín Calaf, Pluvio Coronado, Esther de la Viuda, Plácido Llaneza, and et al. 2023. "Associations between Menopausal Hormone Therapy and Colorectal, Lung, or Melanoma Cancer Recurrence and Mortality: A Narrative Review" Journal of Clinical Medicine 12, no. 16: 5263. https://doi.org/10.3390/jcm12165263
APA StyleFiol, G., Lete, I., Nieto, L., Santaballa, A., Pla, M. J., Baquedano, L., Calaf, J., Coronado, P., de la Viuda, E., Llaneza, P., Otero, B., Sánchez-Méndez, S., Ramírez, I., & Mendoza, N., on behalf of the “HMT Elegibility Criteria Group”. (2023). Associations between Menopausal Hormone Therapy and Colorectal, Lung, or Melanoma Cancer Recurrence and Mortality: A Narrative Review. Journal of Clinical Medicine, 12(16), 5263. https://doi.org/10.3390/jcm12165263





