Abstract
Rett syndrome (RTT) is a rare disability causing female-oriented pediatric neurodevelopmental unmet medical need. RTT was recognized in 1966. However, over the past 56 years, the United States Food and Drug Administration (USFDA) has authorized no effective treatment for RTT. Recently, Trofinetide was approved by the USFDA on 10 March 2023 as the first RTT treatment. This article underlines the pharmaceutical advancement, patent literature, and prospects of Trofinetide. The data for this study were gathered from the PubMed database, authentic websites (Acadia Pharmaceuticals, Neuren Pharmaceuticals, and USFDA), and free patent databases. Trofinetide was first disclosed by Neuren Pharmaceuticals in 2000 as a methyl group containing analog of the naturally occurring neuroprotective tripeptide called glycine-proline-glutamate (GPE). The joint efforts of Acadia Pharmaceuticals and Neuren Pharmaceuticals have developed Trofinetide. The mechanism of action of Trofinetide is not yet well established. However, it is supposed to improve neuronal morphology and synaptic functioning. The patent literature revealed a handful of inventions related to Trofinetide, providing excellent and unexplored broad research possibilities with Trofinetide. The development of innovative Trofinetide-based molecules, combinations of Trofinetide, patient-compliant drug formulations, and precise MECP2-mutation-related personalized medicines are foreseeable. Trofinetide is in clinical trials for some neurodevelopmental disorders (NDDs), including treating Fragile X syndrome (FXS). It is expected that Trofinetide may be approved for treating FXS in the future. The USFDA-approval of Trofinetide is one of the important milestones for RTT therapy and is the beginning of a new era for the therapy of RTT, FXS, autism spectrum disorder (ASD), brain injury, stroke, and other NDDs.
Keywords:
Trofinetide; Daybue; NNZ-2566; Rett syndrome; rare diseases; development; patent; prospects 1. Introduction
Rett syndrome (RTT), a rare disability causing neurodevelopmental syndrome and an unmet medical need, was first characterized by Andreas Rett in 1966 [1,2]. However, this disorder was first officially recognized in a publication form in 1983 by Bengt Hagberg [3]. In 1999, mutations in the methyl-CpG binding protein 2 (MECP2) gene on the X chromosome was found to be the primary cause of RTT by Zoghbi [2,4,5]. Studies have also highlighted the involvement of forkhead box G1 (FOXG1) and cyclin-dependent kinase-like 5 (CDKL5) genes in some cases of RTT [6]. However, the mutation in the MECP2 gene is found in >90% of the RTT cases. RTT is a female-oriented genetic disorder (X-chromosome-linked disorder) that affects mainly girls and young women at a rate of about 1 in 10,000 to 15,000 births, wherein about 10% of females with significant intellectual disabilities have RTT [1,7,8]. RTT causes profound disabilities that permeate practically every facet of a child’s life (the ability to speak, walk, eat, and even breathe). The sign and symptoms of typical RTT (>95% cases) are commonly diagnosed between the ages of 6 and 18 months, whereas the atypical RTT symptoms may appear before the age of 6 months. Between one and four years of age, RTT patients lose the ability to perform skills (loss of language, communication, motor control, walking, social interaction, etc.) that they previously had and develop stereotypies and ataxia. The loss of communication skills in RTT patients is considered the top concern among caregivers. Later in the disease, patients may develop muscle weakness, joint contractures, scoliosis, and seizures. RTT also affects cholesterol metabolism, general growth, and gastrointestinal and renal systems and may cause other diseases (osteoporosis, aspiration pneumonia, pressure ulcers, and infectious disorders). The life expectancy of an RTT patient is approximately 25 to 50 years of age (Figure 1) [9,10,11,12,13].

Figure 1.
General aspects of RTT [9,10,11,12,13].
RTT is a disability-causing disease that can cause lasting neurological damage or even death in young children. The difficulty in diagnosing RTT, the prevalence of different diseases associated with RTT, the availability of limited treatment options, and high healthcare costs make it a significant unmet medical need [14,15,16]. Therefore, effective treatments for RTT are urgently needed in the clinic. Until 10 March 2023, there were no FDA-approved therapies for RTT, and most treatment options for people with RTT focused on relieving the syndrome’s symptoms and effects (multidisciplinary and interprofessional approach). The USFDA approved Trofinetide as the first RTT treatment on 10 March 2023 (Table 1) [17,18,19,20,21].

Table 1.
Rx data of Trofinetide.
This article aims to spotlight the pharmaceutical development, patent literature, and prospects of Trofinetide. The data for this study were gathered from PubMed (Trofinetide = 15 hits; Daybue = 15 hits; NNZ-2566 = 10 hits), authentic websites (Acadia Pharmaceuticals, Neuren Pharmaceuticals, USFDA, International Rett Syndrome Foundation, Clinicaltrial.gov, PubChem, etc.), and free patent databases such as the United States Patent and Trademark Office (USPTO) (Trofinetide = 13 hits; Daybue = 0 hits; NNZ-2566 = 42 hits) and Espacenet (Trofinetide = 19 hits; Daybue = 0 hit; NNZ-2566 = 50 hits) utilizing different keywords. The duplicate articles were removed, and articles/authentic websites providing relevant information about the subject matter were included in this article. This article’s scientific content will help pharmaceutical, academic, and healthcare scientists plan further Trofinetide-based research and discover better treatments for NDDs, including RTT, FXS, autism spectrum disorder (ASD), brain injury, stroke, and other NDDs.
2. Trofinetide
2.1. General Information
Trofinetide (Figure 2A; Synonyms: Daybue, NNZ-2566, Glycyl-L-2-methylprolyl-L-glutamic acid and G-2-MePE; MF: C13H21N3O6; MW: 315.33; CAS Number: 853400-76-7; Chemical name: (2S)-2-{[(2S)-1-(2-aminoacetyl)-2-methylpyrrolidine-2-carbonyl]amino}pentanedioic acid and Glycyl-L-2-methylprolyl-L-glutamic acid; Class: Peptide) is a peptidase-resistant analog of a naturally occurring neuroprotective tripeptide called glycine-proline-glutamate (GPE) (Figure 2B), which is a N-terminal tripeptide product of the cleavage of insulin-like growth factor 1 (IGF-1) found in the brain [14,22,23,24,25]. Neuren Pharmaceuticals initially identified Trofinetide, which was later developed by Acadia Pharmaceuticals for treating RTT [26].
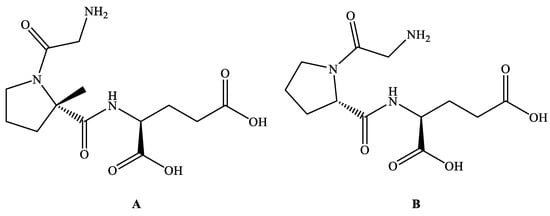
Figure 2.
Chemical structure of Trofinetide (A) and GPE (B).
Trofinetide is soluble in water and can cross the blood–brain barrier. Daybue (shelf life = 18 months at 5 °C) is ready-to-use marketed pink-to-red solution (200 mg/mL) of Trofinetide (Table 1) containing other inactive ingredients (purified water, strawberry flavor, FD&C Red No. 40, sucralose, maltitol, propylparaben sodium, and methylparaben sodium) [22,23].
2.2. Mechanism of Action
The X-linked MECP2 gene produces the MECP2 protein. The MECP2 protein is responsible for various important processes for normal body functions, including brain development and functions. The mutations in the MECP2 gene produce faulty MECP2, affect normal brain development and its function, and cause various disorders, including RTT (Figure 3) [2,5,27,28]. There are about 900 mutations reported in MECP2 genes, most of which arise naturally. To date, the precise means of the pathogenesis of RTT or the exact mutation responsible for RTT is unclear. Studies have suggested various mechanisms of the pathogenesis of RTT via MECP2 gene mutations [12]. One suggested mechanism is that the MECP2 gene mutation retards the normal developments of neurons, axodendritic connections, and the cortex’s synaptic maturation [12]. Another suggested reason for developing RTT is related to the overexpression of IGF-binding protein 3 (IGFBP3) in the brain of RTT patients. It is believed that the MECP2 gene directly regulates the expression of IGFBP3, and a mutation in MECP2 genes can lead to the development of RTT [29].
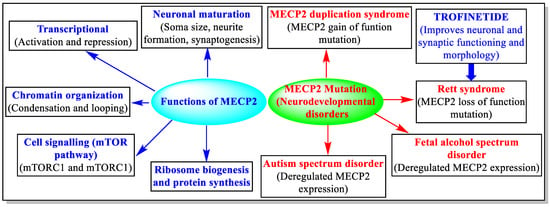
Figure 3.
Functions and disorders associated with MECP2 mutations.
Trofinetide is the first USFDA-approved treatment for RTT. The specific method by which Trofinetide exercises its effect to treat RTT is uncertain. There are many studies indicating that Trofinetide exerts its effect by improving synaptic functions, restoring synaptic structure, reducing the effects of neuro-inflammatory substances in the brain, enhancing antioxidant responses, attenuating injury-induced apoptosis, normalizing the synthesis of essential proteins, restoring normal homeostasis in the brain, and augmenting the concentration of IGF-1 in the CNS [8,24,26,30,31,32,33,34]. According to the sponsor (Acadia Pharmaceuticals and Neuren Pharmaceuticals), Trofinetide improves neuronal and synaptic functioning (synaptic plasticity signals) and morphology (increased branching of dendrites) [35,36,37,38]. Trofinetide is a methyl analog of GPE (Figure 2). At nanomolar doses, GPE protects neurons from excitotoxicity and oxidative stress caused by glutamate. Trofinetide is thought to have similar neuroprotective characteristics (reduces apoptosis, infarct size, inflammation, and excitotoxicity-induced tissue damage to protect neurons) to GPE but with a longer half-life [26].
2.3. Pre-Clinical Studies
Animal studies related to Trofinetide, including its neuroprotective and anti-inflammatory effects in rat brains, are provided in the literature [24,26,30,32]. The USFDA’s non-clinical review document provides non-clinical study data on Trofinetide [36,37]. A short description of the important non-clinical study findings of Trofinetide from USFDA’S documents is provided in Table 2 [36,37].

Table 2.
Summary of important non-clinical study parameters of Trofinetide [36,37].
2.4. Clinical Studies
We searched RTT-based clinical studies on the clinical trial database for Trofinetide utilizing the terms “Trofinetide”, “Daybue”, and “NNZ-2566” [39]. Table 3 summarizes the findings of this search.

Table 3.
Interventional clinical studies on Trofinetide for the treatment of RTT [39].
Our PubMed search revealed the following relevant clinical studies on Trofinetide.
One Phase I study evaluated the influence of food and the evening oral dosing of Trofinetide (60 mL solution, 12 g Trofinetide) on its pharmacokinetic behavior [40]. This study in healthy individuals demonstrated that the bioavailability of Trofinetide was unaffected by diet, showed no diurnal change, and was primarily excreted through urine.
The data of the Phase II study (NCT02715115) have been publicized [8]. Trofinetide (200 mg/kg bid) demonstrated clinical benefits (safety, efficacy, and tolerability) in RTT patients. Another Phase II study also revealed the clinical benefits of Trofinetide (safety, efficacy, and tolerability) at 35 to 70 mg/kg doses [34].
The design and outcome measures of NCT04181723 have been published [14] without results.
A clinical study characterized the population pharmacokinetics of Trofinetide (6–100 mg/kg, oral) among 60 healthy subjects (19–38 years). Trofinetide had a predicted clearance of 10.35 L/h and a center volume of distribution of 20.23 L in the population. There was no treatment-related accumulation, metabolic inhibition, or induction. Because of its short half-life (1.4 h), Trofinetide was suggested to be administered twice or thrice daily [41].
Detailed RTT-based clinical study data of Trofinetide are also provided in the USFDA’s published document [35.42], including the two Phase III clinical studies (NCT04181723 and NCT04279314). The Phase III clinical trial data are utilized to make a drug product’s labeling instructions/information [38]. The important labeling instructions/information of Trofinetide have been discussed in this manuscript’s pharmacology part (Section 2.5). Therefore, to avoid redundancy, the authors did not find it worthy of elaborating on the outcomes of the clinical Phase III data of Trofinetide.
2.5. Pharmacology
The important pharmacological aspects of Trofinetide mentioned in the USFDA’s documents are summarized in Table 4 [22,23,35,36,37,38,39,42].

Table 4.
Pharmacological parameters of Trofinetide.
3. Patents Literature
Trofinetide was explored on the free patent databases, including Espacenet and USPTO [43,44,45,46], on 6 May 2023, utilizing different keywords (Trofinetide; Daybue; NNZ-2566). Table 5 summarizes the most relevant and significant patents and patent applications associated with Trofinetide.

Table 5.
Relevant patents of Trofinetide.
4. Discussion
RTT is a severe burden on patients and their family members because it affects all aspects of the quality of life of RTT patients and requires lifelong medical care and support [14,34,40]. Therefore, the USFDA approval of Trofinetide is one of the important milestones for RTT therapy. Trofinetide was discovered based on the naturally occurring neuroprotective tripeptide called GPE (Figure 2). Before the discovery of Trofinetide, GPE demonstrated modulatory effects on neurons and certain CNS enzymes (ChAT, GAD, NOS, and tyrosine hydroxylase) and was claimed to treat neurodegenerative diseases [47,48,49,50]. However, GPE demonstrated some pharmacokinetic-related concerns [69]. These observations led to the discovery of Trofinetide in 2000 by Neuren Pharmaceuticals [51]. The important milestones for the discovery and development of Trofinetide are provided in Figure 4.
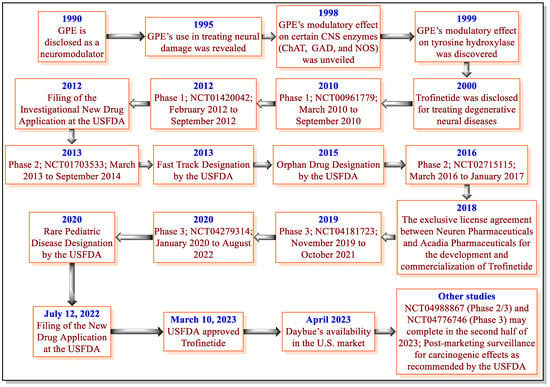
Figure 4.
Discovery and development phases of Trofinetide.
GPE was derivatized to Trofinetide, wherein incorporating the α-methyl group improved the pharmacokinetic profile (half-life, oral bioavailability, and stability against enzymatic degradation) and neuroprotective activity [24,31,41,51,69]. The half-life of Trofinetide is about 1.5 h, necessitating its dosage administration twice a day (about 10 g to 24 g per day, depending on the patient’s weight) (Table 4). The modification of GPE to Trofinetide indicates possibilities of further modifications in GPE or Trofinetide to get another GPE analog, prodrug/conjugate of Trofinetide, having increased half-life, potency, and improved safety profile [70]. This development may provide once-a-day patient-compliant treatment.
IGF-1 is found in the human brain and converts to the neuro-protective tripeptide GPA [14,22,23,24,25]. IGF-1 also has a protective effect in RTT mice and patients [29,50,60,71,72]. Treating MeCP2 mutant mice with IGF-1 has improved locomotor functions, breathing patterns, heart rate irregularities, brain weight and extended life span [71]. Mecasermin (a recombinant human IGF-1) also improved the clinical features of RTT [72]. These findings suggest that IGF-1 or its analogs may be promising candidates for further research for RTT treatment.
Developing a sustained-release dosage form of Trofinetide may also be explored to get a patient-compliant treatment. Treatment of RTT requires more than one drug based on the signs and symptoms of RTT. Trofinetide is an inhibitor of OATP1B1, OATP1B3, and CYP3A4. Accordingly, drug interaction possibilities must be considered before the concomitant administration of any medicine or food item that OATP1B1, OATP1B3, and CYP3A4 metabolize. The mutations in MECP2 have been implicated in cancer development [73]. The carcinogenicity of Trofinetide has not been established yet. The USFDA has asked Acadia Pharmaceutical to assess the carcinogenicity of Trofinetide during the post-marketing surveillance [35,36]. The data of this post-surveillance study will be useful in decision making for RTT patients who have cancer. Trofinetide is water soluble and is mainly excreted in the urine, indicating the kidney is the major organ involved in its excretion from the body (Table 4). This necessitates Trofinetide monitoring among geriatric patients and patients with compromised kidney functions.
The patent literature on a drug provides information about the inventions and innovations related to a drug. This information helps develop other drug inventions [74,75]. Our patent literature search provided different inventions related to Trofinetide (Table 5). The compositions of Trofinetides have been claimed with many CNS-acting drugs for treating RTT, FXS, and brain injuries. However, the patents claiming these compositions are silent about the experimental evidence of such claims. Similarly, different compositions of Trofinetide for administration by different routes have been suggested without experimental evidence. The treatment and invention-related knowledge gaps discussed above allow scientists to investigate the practical effects of unexplored compositions and new treatments to benefit patients (Figure 5).

Figure 5.
Inventions on Trofinetide and drugs of the future for RTT.
A mutation in the MECP2 gene causes RTT. The exact mutation in the MECP2 gene responsible for RTT is unclear [12]. A clear understanding of the exact mutation responsible for RTT will help to develop precision medicine for RTT. Utilizing anti-inflammatory drugs during brain injury is well known [31]. Trofinetide enhances antioxidant responses in the brain [8]. Therefore, the authors recommend assessing the effects of the combinations of Trofinetide with known antioxidants and NSAIDs (such as cyclooxygenase-2 inhibitors) for treating RTT. Trofinetide is also assessed for other neuronal diseases such as FXS (NCT01894958; Phase II completed), traumatic brain injuries (NCT01420042 and NCT00961779), and concussion (NCT02100150; Phase II terminated) [25,33,39]. Being an analog of GPE, Trofinetide can show promising results for certain CNS diseases (Huntington’s disease, Parkinson’s disease, and Alzheimer’s disease). It is expected that Trofinetide may be approved for treating FXS and other neuronal degenerative diseases. Interestingly, many new therapies are also in clinical and pre-clinical trials for RTT (Figure 5). The literature has also cited some innovative treatments for RTT (gene therapy, gene replacement strategies, genome editing, RNA editing, and reactivation of the inactive x chromosome) [1,15]. Accordingly, the authors consider that the USFDA approval of Trofinetide is the beginning of a new era for treating RTT, FXS, ASD, brain injury, stroke, and other neurodevelopmental disorders [24].
5. Conclusions
Trofinetide’s recent USFDA approval marks a significant step forward in treating RTT. Patients and their families affected by RTT would benefit greatly from this approval since they have had few alternatives for alleviating the wide range of symptoms that this condition can bring. The approval of Trofinetide will also speed up the development of new treatments and inventions for RTT and related disorders (FXS, ASD, brain injury, and stroke). Innovative Trofinetide-based molecules, patient-compliant formulations, precise MECP2-mutation-related personalized medicines, and new combinations of Trofinetide with existing RTT-based treatments, CNS-acting drugs, NSAIDs, and immunomodulators (antioxidants) are all likely shortly.
Author Contributions
Conceptualization, S.A.H. and M.I.; methodology, S.A.H., M.I., A., F.E. and A.A.Q.; software, A., A.A. (Adel Alghamdi) and M.A.A.; validation, M.A. (Mazen Almehmadi), A.A.A. and M.A. (Mamdouh Allahyani); formal analysis, A.A. (Abdulelah Aljuaid) and M.S.; writing—original draft preparation, A.A. (Adel Alghamdi), M.A.A., M.A. (Mazen Almehmadi), A.A.A., M.A. (Mamdouh Allahyani), A.A. (Abdulelah Aljuaid) and M.S.; writing—review and editing, S.A.H., M.I., F.E., A. and A.A.Q.; visualization, A.A. (Adel Alghamdi) and M.A.A.; supervision, S.A.H. and M.I.; project administration, S.A.H. and M.I.; funding acquisition, S.A.H., F.E., M.I., M.A. (Mazen Almehmadi) and A.A.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The contents of this review have been obtained from the references cited in this review.
Acknowledgments
All the authors thank their respective institutes for supporting this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Panayotis, N.; Ehinger, Y.; Felix, M.S.; Roux, J.-C. State-of-the-art therapies for Rett syndrome. Dev. Med. Child Neurol. 2023, 65, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.E.; Neul, J.L. Rett syndrome and MECP2 duplication syndrome: Disorders of MeCP2 dosage. Neuropsychiatr. Dis. Treat. 2022, 18, 2813–2835. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, B.; Aicardi, J.; Dias, K.; Ramos, O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Ann. Neurol. 1983, 14, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Percy, A.K. Progress in Rett Syndrome: From discovery to clinical trials. Wien. Med. Wochenschr. 2016, 166, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Tascini, G.; Dell’Isola, G.B.; Mencaroni, E.; Di Cara, G.; Striano, P.; Verrotti, A. Sleep Disorders in Rett Syndrome and Rett-Related Disorders: A Narrative Review. Front. Neurol. 2022, 13, 817195. [Google Scholar] [CrossRef]
- Petriti, U.; Dudman, D.C.; Scosyrev, E.; Lopez-Leon, S. Global prevalence of Rett syndrome: Systematic review and meta-analysis. Syst. Rev. 2023, 12, 5. [Google Scholar] [CrossRef]
- Glaze, D.G.; Neul, J.L.; Kaufmann, W.E.; Berry-Kravis, E.; Condon, S.; Stoms, G.; Oosterholt, S.; Della Pasqua, O.; Glass, L.; Jones, N.E.; et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology 2019, 92, e1912–e1925. [Google Scholar] [CrossRef]
- Banerjee, A.; Miller, M.T.; Li, K.; Sur, M.; Kaufmann, W.E. Towards a better diagnosis and treatment of Rett syndrome: A model synaptic disorder. Brain 2019, 142, 239–248. [Google Scholar] [CrossRef]
- Borloz, E.; Villard, L.; Roux, J.-C. Rett syndrome: Think outside the (skull) box. Fac. Rev. 2021, 10, 59. [Google Scholar] [CrossRef]
- Lyst, M.J.; Bird, A. Rett syndrome: A complex disorder with simple roots. Nat. Rev. Genet. 2015, 16, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Chahil, G.; Bollu, P.C. Rett Syndrome. 2022. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- International Rett Syndrome Foundation. About Rett Syndrome. Available online: https://www.rettsyndrome.org/about-rett-syndrome/ (accessed on 6 May 2023).
- Neul, J.L.; Percy, A.K.; Benke, T.A.; Berry-Kravis, E.M.; Glaze, D.G.; Peters, S.U.; Jones, N.E.; Youakim, J.M. Design and outcome measures of LAVENDER, a phase 3 study of trofinetide for Rett syndrome. Contemp. Clin. Trials. 2022, 114, 106704. [Google Scholar] [CrossRef]
- Gomathi, M.; Padmapriya, S.; Balachandar, V. Drug Studies on Rett Syndrome: From Bench to Bedside. J. Autism. Dev. Disord. 2020, 50, 2740–2764. [Google Scholar] [CrossRef]
- May, D.; Kponee-Shovein, K.; Mahendran, M.; Downes, N.; Sheng, K.; Lefebvre, P.; Cheng, W.Y. Epidemiology and patient journey of Rett syndrome in the United States: A real-world evidence study. BMC Neurol. 2023, 23, 141. [Google Scholar] [CrossRef]
- Harris, E. Trofinetide Receives FDA Approval as First Drug for Rett Syndrome. JAMA 2023, 329, 1142. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. FDA Approves First Treatment for Rett Syndrome. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-first-treatment-rett-syndrome (accessed on 6 May 2023).
- United States Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm (accessed on 6 May 2023).
- Acadia. DAYBUE™ (Trofinetide): The Only FDA-Approved Treatment for Rett Syndrome. Available online: https://acadia.com/our-medicines/daybue/ (accessed on 6 May 2023).
- Neuren Pharmaceuticals. DAYBUE™ (Trofinetide). Available online: https://www.neurenpharma.com/products/daybue-trofinetide (accessed on 6 May 2023).
- Center for Drug Evaluation and Research. Product Quality Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/217026Orig1s000ChemR.pdf (accessed on 6 May 2023).
- Center for Drug Evaluation and Research. Clinical Pharmacology Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/217026Orig1s000ClinPharmR.pdf (accessed on 6 May 2023).
- Lu, X.-C.M.; Chen, R.-W.; Yao, C.; Wei, H.; Yang, X.; Liao, Z.; Dave, J.R.; Tortella, F.C. NNZ-2566, a glypromate analog, improves functional recovery and attenuates apoptosis and inflammation in a rat model of penetrating ballistic-type brain injury. J. Neurotrauma 2009, 26, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.; Glass, L.; Snape, M.; Hurley, M.J.; Altimiras, F.J.; Biekofsky, R.R.; Cogram, P. NNZ-2566, a novel analog of (1–3) IGF-1, as a potential therapeutic agent for fragile X syndrome. Neuromolecular Med. 2015, 17, 71–82. [Google Scholar] [CrossRef]
- Bickerdike, M.J.; Thomas, G.B.; Batchelor, D.C.; Sirimanne, E.S.; Leong, W.; Lin, H.; Sieg, F.; Wen, J.; Brimble, M.A.; Harris, P.W.; et al. NNZ-2566: A Gly-Pro-Glu analogue with neuroprotective efficacy in a rat model of acute focal stroke. J. Neurol. Sci. 2009, 278, 85–90. [Google Scholar] [CrossRef]
- Pejhan, S.; Rastegar, M. Role of DNA Methyl-CpG-Binding Protein MeCP2 in Rett Syndrome Pathobiology and Mechanism of Disease. Biomolecules 2021, 11, 75. [Google Scholar] [CrossRef]
- Tillotson, R.; Bird, A. The Molecular Basis of MeCP2 Function in the Brain. J. Mol. Biol. 2020, 432, 1602–1623. [Google Scholar] [CrossRef]
- Itoh, M.; Ide, S.; Takashima, S.; Kudo, S.; Nomura, Y.; Segawa, M.; Kubota, T.; Mori, H.; Tanaka, S.; Horie, H.; et al. Methyl CpG-binding protein 2 (a mutation of which causes Rett syndrome) directly regulates insulin-like growth factor binding protein 3 in mouse and human brains. J. Neuropathol. Exp. Neurol. 2007, 66, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-C.M.; Si, Y.; Williams, A.J.; Hartings, J.A.; Gryder, D.; Tortella, F.C. NNZ-2566, a glypromate analog, attenuates brain ischemia-induced non-convulsive seizures in rats. J. Cereb. Blood Flow Metab. 2009, 29, 1924–1932. [Google Scholar] [CrossRef]
- Wei, H.H.; Lu, X.-C.M.; Shear, D.A.; Waghray, A.; Yao, C.; Tortella, F.C.; Dave, J.R. NNZ-2566 treatment inhibits neuroinflammation and pro-inflammatory cytokine expression induced by experimental penetrating ballistic-like brain injury in rats. J. Neuroinflamm. 2009, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Cartagena, C.M.; Phillips, K.L.; Williams, G.L.; Konopko, M.; Tortella, F.C.; Dave, J.R.; Schmid, K.E. Mechanism of action for NNZ-2566 anti-inflammatory effects following PBBI involves upregulation of immunomodulator ATF3. Neuromol. Med. 2013, 15, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Horrigan, J.P.; Tartaglia, N.; Hagerman, R.; Kolevzon, A.; Erickson, C.A.; Hatti, S.; Snape, M.; Yaroshinsky, A.; Stoms, G.; et al. A Double-Blind, Randomized, Placebo-Controlled Clinical Study of Trofinetide in the Treatment of Fragile X Syndrome. Pediatr. Neurol. 2020, 110, 30–41. [Google Scholar] [CrossRef]
- Glaze, D.G.; Neul, J.L.; Percy, A.; Feyma, T.; Beisang, A.; Yaroshinsky, A.; Stoms, G.; Zuchero, D.; Horrigan, J.; Glass, L.; et al. A Double-Blind, Randomized, Placebo-Controlled Clinical Study of Trofinetide in the Treatment of Rett Syndrome. Pediatr. Neurol. 2017, 76, 37–46. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Clinical Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/217026Orig1s000MedR.pdf (accessed on 6 May 2023).
- Center for Drug Evaluation and Research. Non-Clinical Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/217026Orig1s000PharmR.pdf (accessed on 6 May 2023).
- Center for Drug Evaluation and Research. Summary Review. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/217026Orig1s000SumR.pdf (accessed on 6 May 2023).
- Center for Drug Evaluation and Research. Labeling. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/217026Orig1s000lbl.pdf (accessed on 6 May 2023).
- United States National Library of Medicine. Available online: https://www.clinicaltrials.gov/ (accessed on 6 May 2023).
- Darwish, M.; Youakim, J.M.; Harlick, J.; DeKarske, D.; Stankovic, S. A Phase 1, Open-Label Study to Evaluate the Effects of Food and Evening Dosing on the Pharmacokinetics of Oral Trofinetide in Healthy Adult Subjects. Clin. Drug Investig. 2022, 42, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Oosterholt, S.P.; Horrigan, J.; Jones, N.; Glass, L.; Della Pasqua, O. Population pharmacokinetics of NNZ-2566 in healthy subjects. Eur. J. Pharm. Sci. 2017, 109, S98–S107. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Risk Assessment and Risk Mitigation Review(s). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/217026Orig1s000RiskR.pdf (accessed on 6 May 2023).
- Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Alshammari, M.K.; Alharbi, A.S.; Hussain, K.H.; Alsubaihi, L.I.; Kamal, M.; Alotaibi, S.S.; Alotaibi, A.N.; et al. A Glance at the Development and Patent Literature of Tecovirimat: The First-in-Class Therapy for Emerging Monkeypox Outbreak. Viruses 2022, 14, 1870. [Google Scholar] [CrossRef]
- Ashour, M.A.; Fatima, W.; Imran, M.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. A Review on the Main Phytoconstituents, Traditional Uses, Inventions, and Patent Literature of Gum Arabic Emphasizing Acacia seyal. Molecules 2022, 27, 1171. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Abida; Alshrari, A.S.; Eltahir Mudawid, M.M.; Alshammari, M.K.; Harshan, A.A.; Alshammari, N.A. Small molecules as kinetoplastid specific proteasome inhibitors for leishmaniasis: A patent review from 1998 to 2021. Expert. Opin. Ther. Pat. 2022, 32, 591–604. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Asdaq, S.M.B.; Almehmadi, M.; Abdulaziz, O.; Kamal, M.; Alshammari, M.K.; Alsubaihi, L.I.; Hussain, K.H.; Alharbi, A.S.; et al. An insight into the discovery, clinical studies, compositions, and patents of macozinone: A drug targeting the DprE1 enzyme of Mycobacterium tuberculosis. J. Infect. Public Health 2022, 15, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Sara, V.R. Neuromodulatory Peptide. European Patent Application Publication Number EP0366638A2, 2 May 1990. [Google Scholar]
- Gluckman, P.D.; Williams, C.E. Composition and Methods to Improve Neural Outcome. PCT Patent Application Publication Number WO9517204A1, 29 June 1995. [Google Scholar]
- Gluckman, P.D.; Williams, C.E.; Guan, J. Regulation of Neural Enzymes. PCT Patent Application Publication Number WO9814202A1, 9 April 1998. [Google Scholar]
- Gluckman, P.D.; Guan, J.; Alexi, T. Regulation of tyrosine hydroxylase. PCT Patent Application Publication Number WO9965509A1, 23 December 1999. [Google Scholar]
- Scheepens, A.; Williams, C.E.; Gluckman, P.D.; Clark, R.G. Neuroprotective Effect of Growth Hormone. United States Patent Number US7304029B1, 4 December 2007. [Google Scholar]
- Gluckman, P.; Alexi, T. GPE Analogs. PCT Patent Application Publication Number WO0216408A2, 28 February 2002. [Google Scholar]
- Abood, N.A.; Brimble, M.A. GPE Analogs and Peptidomimetics. United States Patent Number US7041314B2, 9 May 2006. [Google Scholar]
- Gluckman, P.D.; Thomas, G.B.; Guan, J.; Dragunow, M.; Anand, A.K.; Sieg, F.; Brimble, M.A. Effects of Glycyl-2 Methyl Prolyl Glutamate on Neurodegeneration. United States Patent Number US7605177B2, 20 October 2009. [Google Scholar]
- Gluckman, P.D.; Brimble, M.A.; Wilson, D.; Tortella, F.C.; Williams, A.J.; Lu, X.M.; Hartings, J.A.; Gryder, D. Treatment of Non-Convulsive Seizures in Brain Injury Using G-2-Methyl-Prolyl Glutamate. United States Patent Number US7714020B2, 11 May 2010. [Google Scholar]
- Wen, J.; Thomas, G.B.; Bickerdike, M.J. Oral Formulations of Glycyl-2-Methylprolyl-Glutamate. United States Patent Number US7887839B2, 15 February 2011. [Google Scholar]
- Wen, J.; Thomas, G.B.; Bickerdike, M.J. Oral Formulations of Glycyl-2-Methylprolyl-Glutamate. United States Patent Number US8178125B2, 15 May 2012. [Google Scholar]
- Wen, J.; Thomas, G.B.; Bickerdike, M.J. Oral Formulations of Glycyl-2-Methylprolyl-Glutamate. United States Patent Number US8496963B2, 30 July 2013. [Google Scholar]
- Glass, L.; Bickerdike, M.J.; Snape, M.F. Treatment of Fragile X Syndrome Using Glycyl-L-2-Methylprolyl-L-Glutamate. United States Patent Number US9708366B2, 18 July 2017. [Google Scholar]
- Glass, L.I.; Bickerdike, M.J.; Snape, M.F.; De Cogram, P.P. Treatment of Autism Spectrum Disorders Using Glycyl-l-2-Methylprolyl-l-Glumatic Acid. United States Patent Application Publication Number US2015224164A1, 13 August 2015. [Google Scholar]
- Glass, L.I.; Bickerdike, M.J.; Snape, M.F. Treatment of Rett Syndrome Using Glycyl-L-2-Methylprolyl-L-Glutamic Acid. United States Patent Number US9212204B2, 15 December 2015. [Google Scholar]
- Kole, R.; Marshall, J.; Owen, M. Enhanced Delivery of Antioxidants for Treatment of Central Nervous System Disorders Involving Oxidative Stress. United States Patent Number US11612642B2, 28 March 2023. [Google Scholar]
- Blower, C.; Peterson, M.; Shaw, J.M.; Bonnar, J.A.; Moniotte, E.D.F.P.; Bousmanne, M.B.C.; Betti, C.; Decroos, K.W.L.; Ayoub, M. Compositions of Trofinetide. United States Patent Number US11370755B2, 28 June 2022. [Google Scholar]
- Blower, C.; Peterson, M.; Shaw, J.M.; Bonnar, J.A.; Moniotte, E.D.F.P.; Bousmanne, M.B.C.; Betti, C.; Decroos, K.W.L.; Ayoub, M. Compositions of Trofinetide. United States Patent Application Publication Number US2022324799A1, 13 October 2022. [Google Scholar]
- Peterson, M.; Carlos, M.; Bousmanne, M.B.C.; Betti, C.; Jonaitis, D.T.; Mccracken, L.M.; Grove, L. Crystalline Forms of Trofinetide. United States Patent Application Publication Number US2023023114A1, 26 January 2023. [Google Scholar]
- Darwish, M.; Youakim, J.M.; Glass, L.I.; Jones, N.E.; Oosterholt, S.P.; Pasqua, O.D. Methods and Compositions for Treatment of Rett Syndrome. United States Patent Application Publication Number US2022339138A1, 27 October 2022. [Google Scholar]
- Vigneault, F.; Levin, M.; Ingber, D.; Novak, R. Drugs for Treating Neurodevelopmental Disorders. PCT Patent Application Publication Number WO2022246277A2, 24 November 2022. [Google Scholar]
- Page, D.; Levy, J. Treating Disorders Associated with DYRK1A Dysfunction. PCT Patent Application Publication Number WO2022165250A1, 4 August 2022. [Google Scholar]
- Silva-Reis, S.C.; Sampaio-Dias, I.E.; Costa, V.M.; Correia, X.C.; Costa-Almeida, H.F.; García-Mera, X.; Rodríguez-Borges, J.E. Concise Overview of Glypromate Neuropeptide Research: From Chemistry to Pharmacological Applications in Neurosciences. ACS Chem. Neurosci. 2023, 14, 554–572. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.; Patwari, P.; Pancoast, J. Compounds and Methods for Modulating Pharmacokinetics. United States Patent Number US9790264B2, 17 October 2017. [Google Scholar]
- Hamidi, T.; Singh, A.K.; Chen, T. Genetic alterations of DNA methylation machinery in human diseases. Epigenomics 2015, 7, 247–265. [Google Scholar] [CrossRef]
- Tropea, D.; Giacometti, E.; Wilson, N.R.; Beard, C.; McCurry, C.; Fu, D.D.; Flannery, R.; Jaenisch, R.; Sur, M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl. Acad. Sci. USA 2009, 106, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, O.S.; Ho, E.; Barnes, K.V.; O’Leary, H.M.; Pereira, L.M.; Finkelstein, Y.; Nelson, C.A.; Vogel-Farley, V.; DeGregorio, G.; Holm, I.A.; et al. Safety, pharmacokinetics, and preliminary assessment of efficacy of mecasermin (recombinant human IGF-1) for the treatment of Rett syndrome. Proc. Natl. Acad. Sci. USA 2014, 111, 4596–4601. [Google Scholar] [CrossRef]
- Alshammari, M.K.; Fatima, W.; Alraya, R.A.; Khuzaim Alzahrani, A.; Kamal, M.; Alshammari, R.S.; Alshammari, S.A.; Alharbi, L.M.; Alsubaie, N.S.; Alosaimi, R.B.; et al. Selenium and COVID-19: A spotlight on the clinical trials, inventive compositions, and patent literature. J. Infect. Public Health 2022, 15, 1225–1233. [Google Scholar] [CrossRef]
- Imran, M.; Arora, M.K.; Chaudhary, A.; Khan, S.A.; Kamal, M.; Alshammari, M.M.; Alharbi, R.M.; Althomali, N.A.; Alzimam, I.M.; Alshammari, A.A.; et al. MmpL3 Inhibition as a Promising Approach to Develop Novel Therapies against Tuberculosis: A Spotlight on SQ109, Clinical Studies, and Patents Literature. Biomedicines 2022, 10, 2793. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).