Measuring Quality of Life in Adults with Scoliosis: A Cross-Sectional Study Comparing SRS-22 and ISYQOL Questionnaires
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Characteristics
2.2. Data Collection
2.3. Participants
2.4. Sample Size Calculation
2.5. Health-Related Quality-of-Life Questionnaires: SRS-22 and ISYQOL
2.6. Statistical Analysis
2.6.1. Categories’ Functioning
2.6.2. Fit the Model
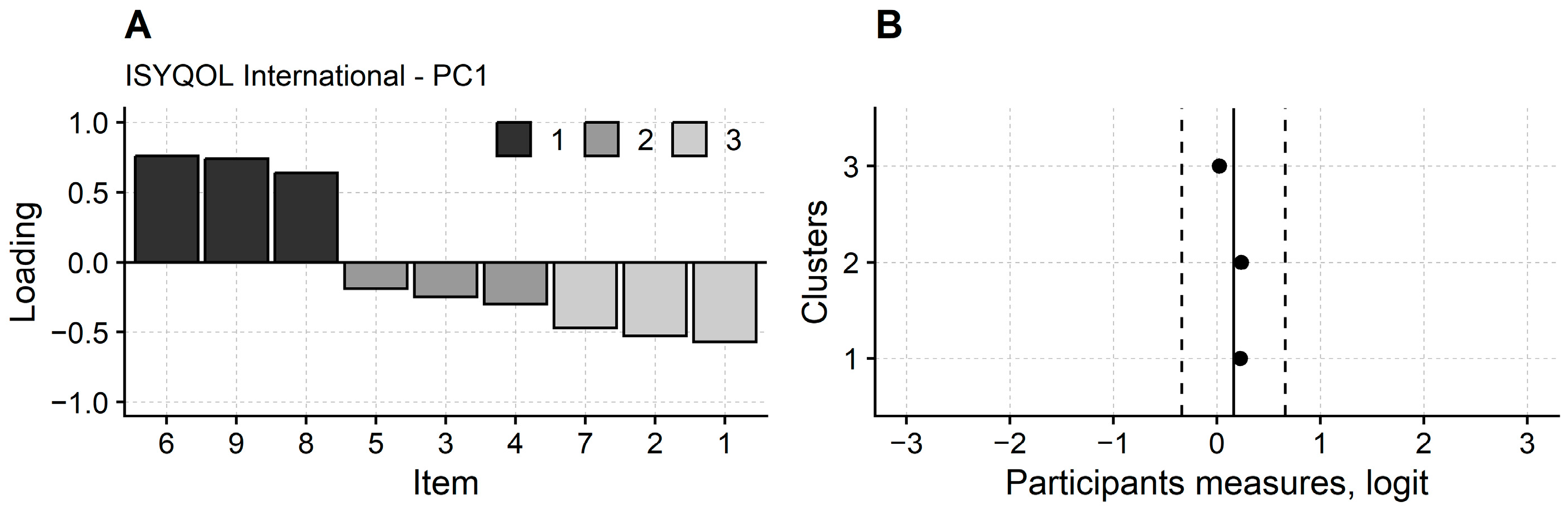
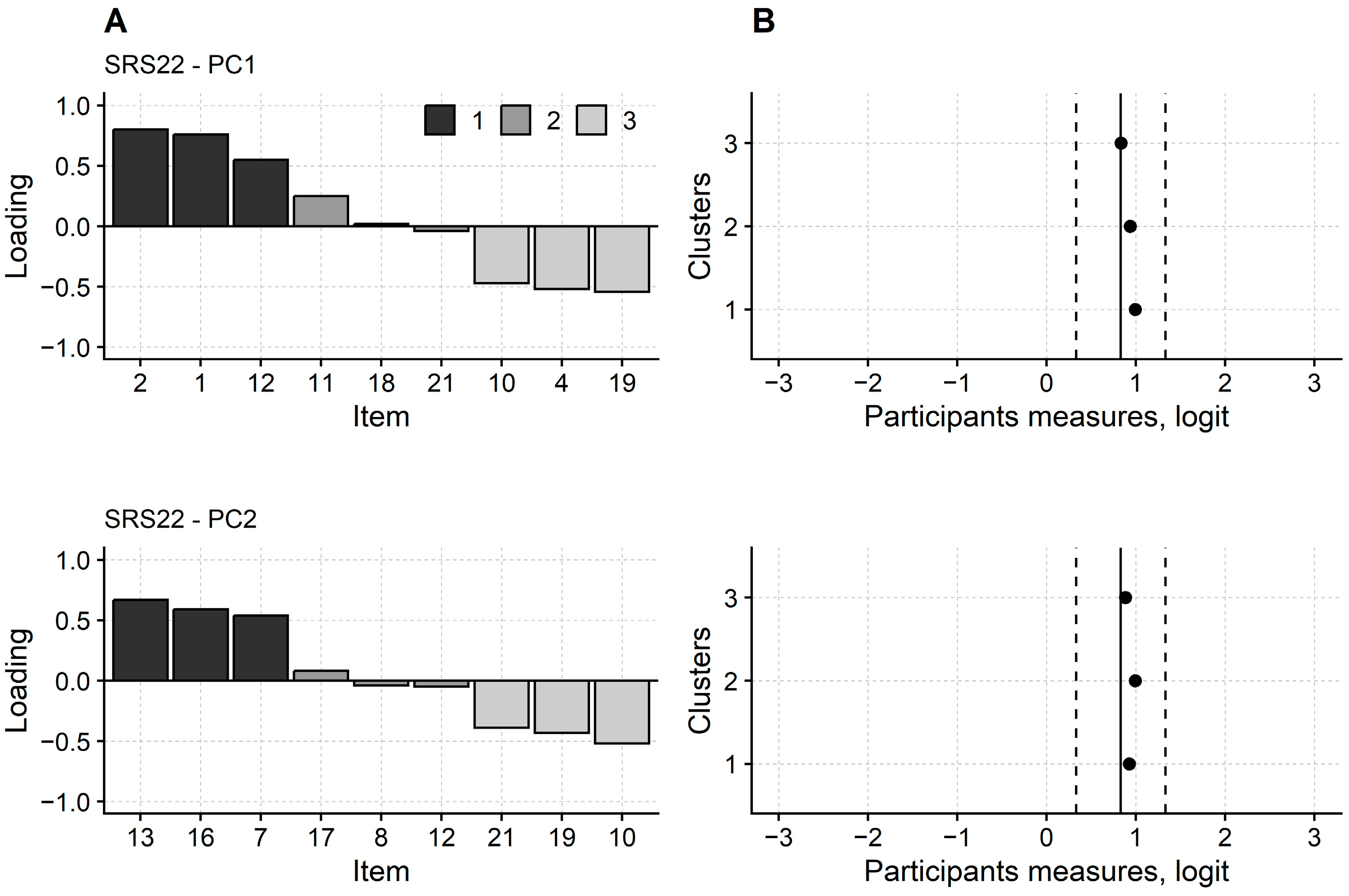
2.6.3. Dimensionality
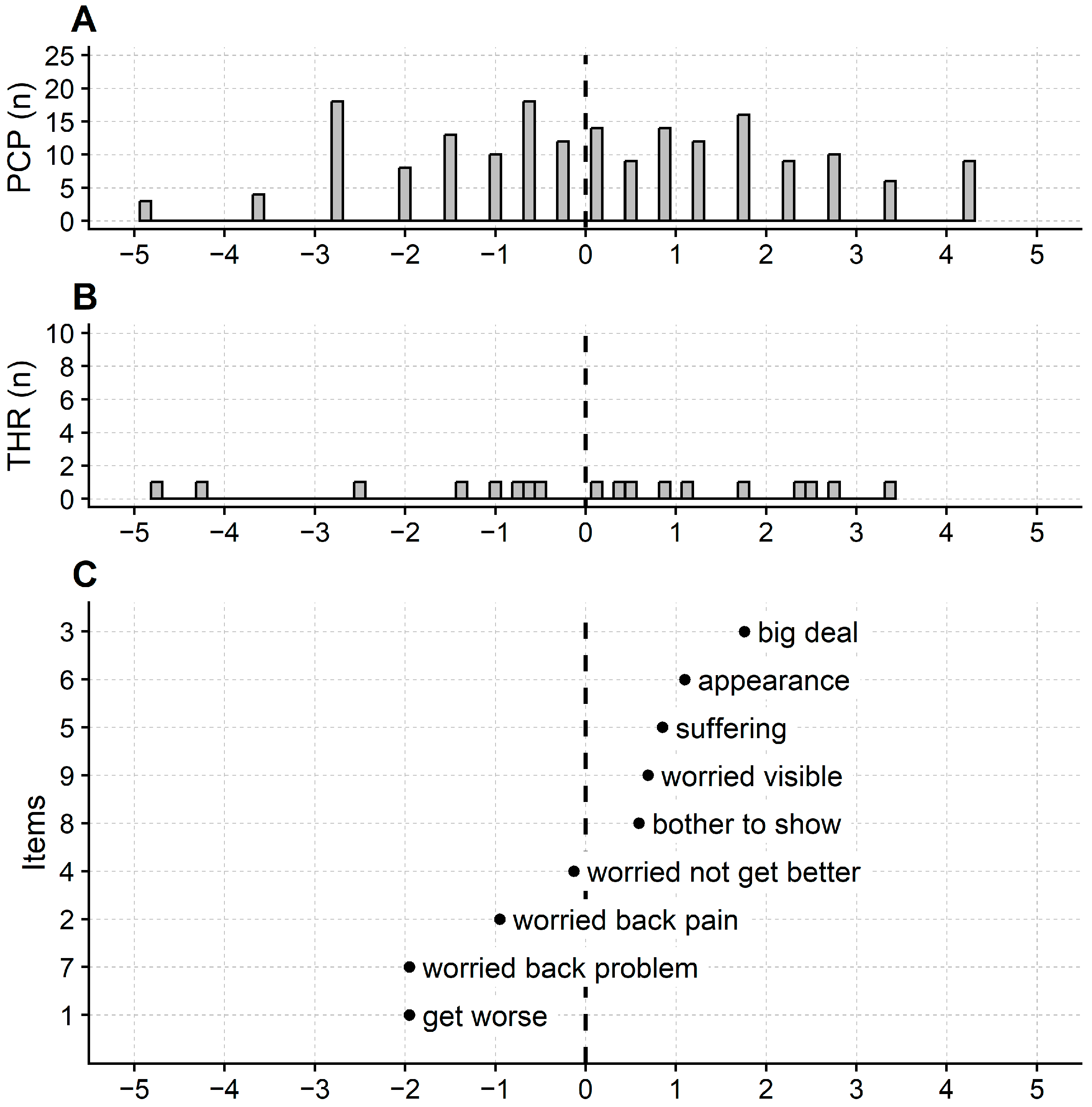
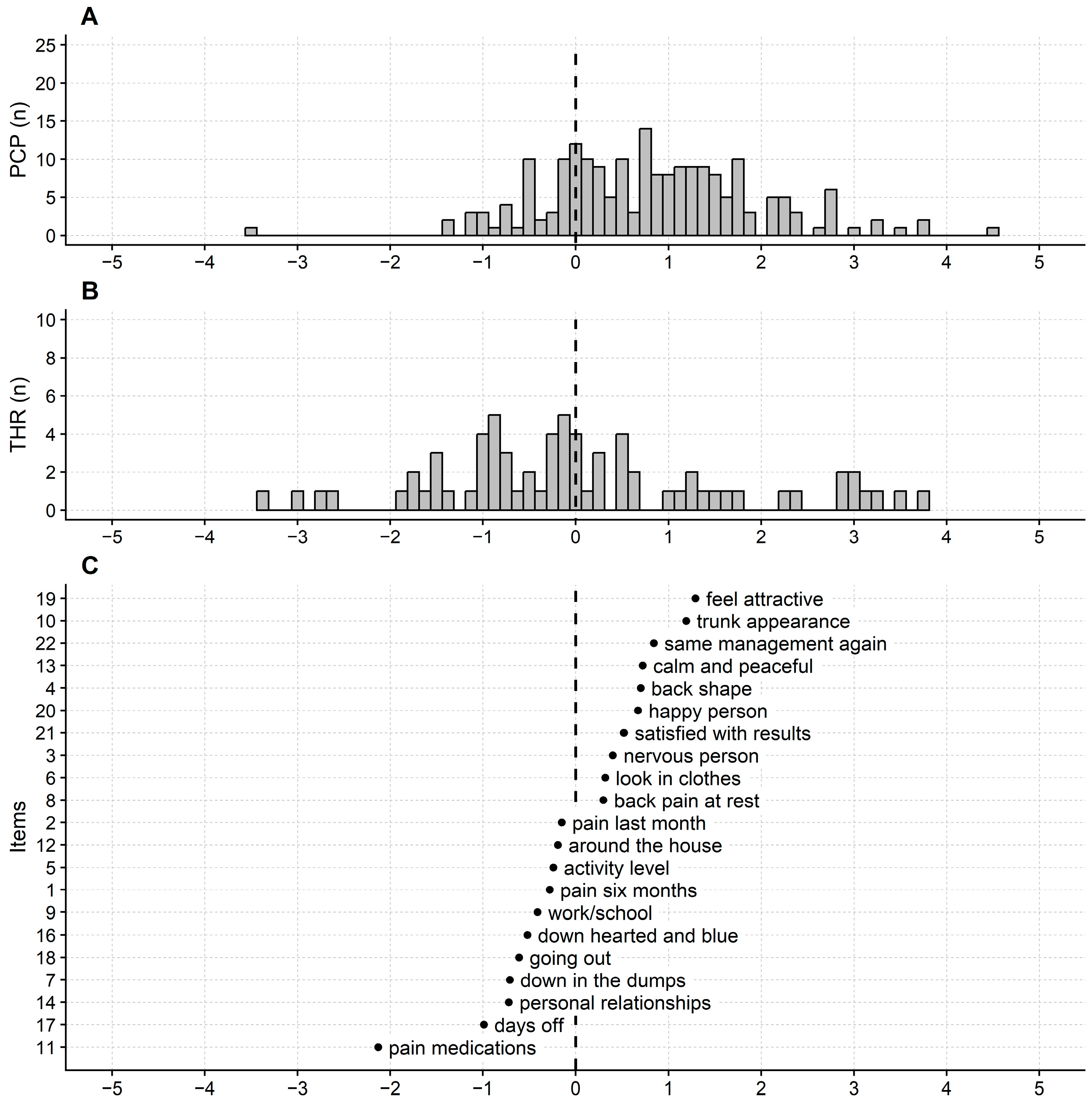
2.6.4. Differential Item Functioning
2.6.5. Questionnaire Reliability and Targeting
2.7. Ethical Approval
3. Results
3.1. Rasch Analysis of ISYQOL International
3.2. Rasch Analysis of SRS-22
4. Discussion
Study Limitations and Further Developments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Methods: The Rasch Analysis of the SRS-22 and ISYQOL International Questionnaires
- 1.
- Categories’ functioning
- 2.
- Fit to the model
- 3.
- Dimensionality
- 4.
- Differential Item Functioning
- 5.
- Targeting and reliability
Appendix B. Supplementary Results
| Item | Score | Count Used | Average Measure | Andrich Threshold | |
|---|---|---|---|---|---|
| Calibration | SE | ||||
| 1, get worse | 0 | 9 | −1.36 | - | - |
| 1 | 88 | 1.13 | −2.27 | 0.39 | |
| 2 | 88 | 3.45 | 2.27 | 0.19 | |
| 2, worried back pain | 0 | 28 | −0.91 | - | - |
| 1 | 77 | 0.30 | −1.49 | 0.26 | |
| 2 | 80 | 2.60 | 1.49 | 0.20 | |
| 3, big deal | 0 | 89 | −3.03 | - | - |
| 1 | 77 | −0.50 | −1.64 | 0.20 | |
| 2 | 19 | 0.71 | 1.64 | 0.29 | |
| 4, worried not get better | 0 | 47 | −1.82 | - | - |
| 1 | 76 | 0.21 | −1.30 | 0.22 | |
| 2 | 62 | 2.01 | 1.30 | 0.21 | |
| 5, suffering | 0 | 65 | −2.54 | - | - |
| 1 | 86 | −0.17 | −1.61 | 0.20 | |
| 2 | 34 | 1.57 | 1.61 | 0.24 | |
| 6, appearance | 0 | 71 | −2.66 | - | - |
| 1 | 85 | −0.41 | −1.64 | 0.20 | |
| 2 | 29 | 1.76 | 1.64 | 0.25 | |
| 7, worried back problem | 0 | 6 | −0.65 | - | - |
| 1 | 106 | 1.08 | −2.84 | 0.46 | |
| 2 | 73 | 3.85 | 2.84 | 0.20 | |
| 8, bother to show | 0 | 67 | −1.94 | - | - |
| 1 | 72 | −0.29 | −1.20 | 0.21 | |
| 2 | 46 | 1.55 | 1.20 | 0.22 | |
| 9, worried visible | 0 | 58 | −2.35 | - | - |
| 1 | 92 | −0.18 | −1.74 | 0.21 | |
| 2 | 35 | 1.60 | 1.74 | 0.24 | |
| Item | Score | Count Used | Average Measure | Andrich Threshold | |
|---|---|---|---|---|---|
| Calibration | SE | ||||
| 1, pain six months | 1 | 3 | −1.69 | - | - |
| 2 | 37 | 0.08 | −2.74 | 0.63 | |
| 3 | 50 | 0.53 | 0.13 | 0.21 | |
| 4 | 56 | 1.44 | 0.88 | 0.18 | |
| 5 | 53 | 2.21 | 1.73 | 0.19 | |
| 2, pain last month | 1 | 6 | −0.91 | - | - |
| 2 | 26 | −0.10 | −1.78 | 0.46 | |
| 3 | 51 | 0.37 | −0.42 | 0.22 | |
| 4 | 60 | 1.15 | 0.65 | 0.18 | |
| 5 | 56 | 2.06 | 1.56 | 0.19 | |
| 3, nervous person | 1 | 4 | −1.96 | - | - |
| 2 | 46 | −0.21 | −3.19 | 0.55 | |
| 3 | 88 | 0.34 | −0.67 | 0.19 | |
| 4 | 45 | 1.09 | 1.34 | 0.18 | |
| 5 | 16 | 1.47 | 2.51 | 0.30 | |
| 4, back shape | 1 | 18 | −1.15 | - | - |
| 2 | 36 | −0.55 | −1.54 | 0.28 | |
| 3 | 77 | 0.07 | −1.04 | 0.19 | |
| 4 | 44 | 0.63 | 0.90 | 0.18 | |
| 5 | 24 | 1.39 | 1.67 | 0.26 | |
| 5, activity level | 1 | 26 | 0.10 | - | - |
| 2 | 63 | 0.40 | −0.54 | 0.23 | |
| 3 | 110 | 1.68 | 0.54 | 0.16 | |
| 6, look in clothes | 1 | 5 | −0.89 | - | - |
| 2 | 19 | −0.67 | −2.12 | 0.50 | |
| 3 | 94 | 0.28 | −1.69 | 0.24 | |
| 4 | 65 | 1.02 | 0.97 | 0.17 | |
| 5 | 16 | 1.73 | 2.85 | 0.29 | |
| 7, down in the dumps | 1 | 8 | 0.20 | - | - |
| 2 | 30 | 0.54 | −1.00 | 0.40 | |
| 3 | 35 | 1.04 | 0.73 | 0.21 | |
| 4 | 126 | 2.01 | 0.27 (*) | 0.17 | |
| 8, back pain at rest | 1 | 28 | −0.30 | - | - |
| 2 | 49 | 0.02 | −0.86 | 0.23 | |
| 3 | 53 | 0.70 | 0.19 | 0.18 | |
| 4 | 69 | 1.09 | 0.68 | 0.18 | |
| 9, work/school | 1 | 15 | −0.07 | - | - |
| 2 | 26 | 0.47 | −0.43 | 0.31 | |
| 3 | 37 | 0.77 | 0.30 | 0.21 | |
| 4 | 120 | 1.73 | 0.12 (*) | 0.17 | |
| 10, trunk appearance | 1 | 13 | −1.89 | - | - |
| 2 | 56 | −0.88 | −2.73 | 0.31 | |
| 3 | 100 | −0.20 | −1.17 | 0.17 | |
| 4 | 23 | 0.54 | 1.64 | 0.23 | |
| 5 | 7 | 1.39 | 2.26 | 0.44 | |
| 11, pain medications | 1 | 2 | 1.97 | - | - |
| 2 | 38 | 2.01 | −1.25 | 0.77 | |
| 3 | 159 | 3.20 | 1.25 | 0.19 | |
| 12, around the house | 1 | 11 | −0.84 | - | - |
| 2 | 20 | −0.05 | −0.82 | 0.35 | |
| 3 | 42 | 0.41 | −0.50 | 0.23 | |
| 4 | 38 | 1.04 | 0.82 | 0.19 | |
| 5 | 88 | 1.78 | 0.50 (*) | 0.18 | |
| 13, calm and peaceful | 1 | 21 | −0.89 | - | - |
| 2 | 57 | −0.32 | −1.70 | 0.26 | |
| 3 | 98 | 0.36 | −0.56 | 0.17 | |
| 4 | 23 | 1.04 | 2.26 | 0.25 | |
| 14, personal relationships | 1 | 5 | −0.38 | - | - |
| 2 | 11 | 0.05 | −0.78 | 0.52 | |
| 3 | 26 | 0.67 | −0.34 | 0.31 | |
| 4 | 42 | 1.15 | 0.51 | 0.21 | |
| 5 | 115 | 2.12 | 0.62 | 0.17 | |
| 16, down hearted and blue | 1 | 6 | −0.43 | - | - |
| 2 | 8 | −0.05 | −0.41 | 0.48 | |
| 3 | 38 | 0.79 | −1.16 (*) | 0.32 | |
| 4 | 55 | 1.15 | 0.53 | 0.19 | |
| 5 | 92 | 1.95 | 1.05 | 0.17 | |
| 17, days off | 1 | 10 | 0.31 | - | - |
| 2 | 6 | 0.83 | 0.92 | 0.39 | |
| 3 | 7 | 1.52 | 0.75 (*) | 0.32 | |
| 4 | 176 | 1.95 | −1.67 (*) | 0.26 | |
| 18, going out | 1 | 18 | 0.27 | - | - |
| 2 | 52 | 0.80 | −0.45 | 0.27 | |
| 3 | 129 | 1.87 | 0.45 | 0.17 | |
| 19, feel attractive | 1 | 26 | −1.70 | - | - |
| 2 | 25 | −1.22 | −1.31 | 0.24 | |
| 3 | 104 | −0.33 | −2.18 (*) | 0.19 | |
| 4 | 37 | 0.14 | 0.99 | 0.20 | |
| 5 | 7 | 1.81 | 2.51 | 0.43 | |
| 20, happy person | 1 | 15 | −0.84 | - | - |
| 2 | 65 | −0.38 | −2.16 | 0.29 | |
| 3 | 100 | 0.40 | −0.40 | 0.17 | |
| 4 | 19 | 1.52 | 2.56 | 0.27 | |
| 21, satisfied with results | 1 | 11 | −1.09 | - | - |
| 2 | 26 | −0.62 | −1.68 | 0.35 | |
| 3 | 76 | −0.01 | −1.32 | 0.21 | |
| 4 | 63 | 0.83 | 0.57 | 0.17 | |
| 5 | 18 | 1.68 | 2.43 | 0.28 | |
| 22, same management again | 1 | 67 | −0.71 | - | - |
| 2 | 64 | 0.10 | −0.37 | 0.18 | |
| 3 | 62 | 0.55 | 0.37 | 0.18 | |
References
- Negrini, S.; Donzelli, S.; Aulisa, A.G.; Czaprowski, D.; Schreiber, S.; de Mauroy, J.C.; Diers, H.; Grivas, T.B.; Knott, P.; Kotwicki, T.; et al. 2016 SOSORT Guidelines: Orthopaedic and Rehabilitation Treatment of Idiopathic Scoliosis during Growth. Scoliosis Spinal Disord. 2018, 13, 3. [Google Scholar] [CrossRef]
- Sanders, A.E.; Andras, L.M.; Iantorno, S.E.; Hamilton, A.; Choi, P.D.; Skaggs, D.L. Clinically Significant Psychological and Emotional Distress in 32% of Adolescent Idiopathic Scoliosis Patients. Spine Deform. 2018, 6, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Tones, M.; Moss, N.; Polly, D.W. A Review of Quality of Life and Psychosocial Issues in Scoliosis. Spine 2006, 31, 3027–3038. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.L.; Dolan, L.A.; Spratt, K.F.; Peterson, K.K.; Spoonamore, M.J.; Ponseti, I.V. Health and Function of Patients with Untreated Idiopathic Scoliosis: A 50-Year Natural History Study. JAMA 2003, 289, 559–567. [Google Scholar] [CrossRef]
- Asher, M.; Min Lai, S.; Burton, D.; Manna, B. Discrimination Validity of the Scoliosis Research Society-22 Patient Questionnaire: Relationship to Idiopathic Scoliosis Curve Pattern and Curve Size. Spine 2003, 28, 74–78. [Google Scholar] [CrossRef]
- Asher, M.; Min Lai, S.; Burton, D.; Manna, B. Scoliosis Research Society-22 Patient Questionnaire: Responsiveness to Change Associated with Surgical Treatment. Spine 2003, 28, 70–73. [Google Scholar] [CrossRef]
- Bridwell, K.H.; Berven, S.; Glassman, S.; Hamill, C.; Horton, W.C.; Lenke, L.G.; Schwab, F.; Baldus, C.; Shainline, M. Is the SRS-22 Instrument Responsive to Change in Adult Scoliosis Patients Having Primary Spinal Deformity Surgery? Spine 2007, 32, 2220–2225. [Google Scholar] [CrossRef]
- Bridwell, K.H.; Cats-Baril, W.; Harrast, J.; Berven, S.; Glassman, S.; Farcy, J.-P.; Horton, W.C.; Lenke, L.G.; Baldus, C.; Radake, T. The Validity of the SRS-22 Instrument in an Adult Spinal Deformity Population Compared with the Oswestry and SF-12: A Study of Response Distribution, Concurrent Validity, Internal Consistency, and Reliability. Spine 2005, 30, 455–461. [Google Scholar] [CrossRef]
- Berven, S.; Deviren, V.; Demir-Deviren, S.; Hu, S.S.; Bradford, D.S. Studies in the Modified Scoliosis Research Society Outcomes Instrument in Adults: Validation, Reliability, and Discriminatory Capacity. Spine 2003, 28, 2164–2169, discussion 2169. [Google Scholar] [CrossRef]
- Mannion, A.F.; Elfering, A.; Bago, J.; Pellise, F.; Vila-Casademunt, A.; Richner-Wunderlin, S.; Domingo-Sàbat, M.; Obeid, I.; Acaroglu, E.; Alanay, A.; et al. Factor Analysis of the SRS-22 Outcome Assessment Instrument in Patients with Adult Spinal Deformity. Eur. Spine J. 2017, 27, 685–699. [Google Scholar] [CrossRef]
- Tesio, L.; Scarano, S.; Hassan, S.; Kumbhare, D.; Caronni, A. Why Questionnaire Scores Are Not Measures: A Question-Raising Article. Am. J. Phys. Med. Rehabil. 2023, 102, 75–82. [Google Scholar] [CrossRef]
- Caronni, A.; Zaina, F.; Negrini, S. Improving the Measurement of Health-Related Quality of Life in Adolescent with Idiopathic Scoliosis: The SRS-7, a Rasch-Developed Short Form of the SRS-22 Questionnaire. Res. Dev. Disabil. 2014, 35, 784–799. [Google Scholar] [CrossRef]
- Caronni, A.; Sciumè, L.; Donzelli, S.; Zaina, F.; Negrini, S. ISYQOL: A Rasch-Consistent Questionnaire for Measuring Health-Related Quality of Life in Adolescents with Spinal Deformities. Spine J. 2017, 17, 1364–1372. [Google Scholar] [CrossRef]
- Caronni, A.; Donzelli, S.; Zaina, F.; Negrini, S. The Italian Spine Youth Quality of Life Questionnaire Measures Health-Related Quality of Life of Adolescents with Spinal Deformities Better than the Reference Standard, the Scoliosis Research Society 22 Questionnaire. Clin. Rehabil. 2019, 33, 1404–1415. [Google Scholar] [CrossRef]
- Negrini, S.; Zaina, F.; Buyukaslan, A.; Fortin, C.; Karavidas, N.; Kotwicki, T.; Korbel, K.; Parent, E.; Sanchez-Raya, J.; Shearer, K.; et al. Cross-Cultural Validation of the Italian Spine Youth Quality of Life Questionnaire: The ISYQOL International. Eur. J. Phys. Rehabil. Med. 2023, 59, 364. [Google Scholar] [CrossRef]
- McAviney, J.; Roberts, C.; Sullivan, B.; Alevras, A.J.; Graham, P.L.; Brown, B.T. The Prevalence of Adult de Novo Scoliosis: A Systematic Review and Meta-Analysis. Eur. Spine J. 2020, 29, 2960–2969. [Google Scholar] [CrossRef]
- Linacre, J.M. Sample Size and Item Calibration (or Person Measure) Stability. Rasch Meas. Trans. 1994, 7, 328. [Google Scholar]
- Mallinson, T.; Kozlowski, A.J.; Johnston, M.V.; Weaver, J.; Terhorst, L.; Grampurohit, N.; Juengst, S.; Ehrlich-Jones, L.; Heinemann, A.W.; Melvin, J.; et al. Rasch Reporting Guideline for Rehabilitation Research (RULER): The RULER Statement. Arch. Phys. Med. Rehabil. 2022, 103, 1477–1486. [Google Scholar] [CrossRef]
- Van de Winckel, A.; Kozlowski, A.J.; Johnston, M.V.; Weaver, J.; Grampurohit, N.; Terhorst, L.; Juengst, S.; Ehrlich-Jones, L.; Heinemann, A.W.; Melvin, J.; et al. Reporting Guideline for RULER: Rasch Reporting Guideline for Rehabilitation Research: Explanation and Elaboration. Arch. Phys. Med. Rehabil. 2022, 103, 1487–1498. [Google Scholar] [CrossRef]
- Monticone, M.; Nava, C.; Leggero, V.; Rocca, B.; Salvaderi, S.; Ferrante, S.; Ambrosini, E. Measurement Properties of Translated Versions of the Scoliosis Research Society-22 Patient Questionnaire, SRS-22: A Systematic Review. Qual. Life Res. 2015, 24, 1981–1998. [Google Scholar] [CrossRef]
- Aulisa, A.G.; Guzzanti, V.; Perisano, C.; Marzetti, E.; Specchia, A.; Galli, M.; Giordano, M.; Aulisa, L. Determination of Quality of Life in Adolescents with Idiopathic Scoliosis Subjected to Conservative Treatment. Scoliosis 2010, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Linacre, J. Category Disordering (Disordered Categories) vs. Threshold Disordering (Disordered Thresholds). Rasch Meas. Trans. 1999, 13, 675. [Google Scholar]
- Andrich, D. An Expanded Derivation of the Threshold Structure of the Polytomous Rasch Model That Dispels Any “Threshold Disorder Controversy”. Educ. Psychol. Meas. 2013, 73, 78–124. [Google Scholar] [CrossRef]
- Masters, G.N. A Rasch Model for Partial Credit Scoring. Psychometrika 1982, 47, 149–174. [Google Scholar] [CrossRef]
- Linacre, J.M. Facets Computer Program for Many-Facet Rasch Measurement. 2022. Available online: https://www.winsteps.com/facets.htm (accessed on 29 June 2023).
- Caronni, A.; Picardi, M.; Redaelli, V.; Antoniotti, P.; Pintavalle, G.; Aristidou, E.; Gilardone, G.; Carpinella, I.; Lencioni, T.; Arcuri, P.; et al. The Falls Efficacy Scale International Is a Valid Measure to Assess the Concern about Falling and Its Changes Induced by Treatments. Clin. Rehabil. 2021, 36, 558–570. [Google Scholar] [CrossRef]
- Lange, R.; Irwin, H.J.; Houran, J. Top-down Purification of Tobacyk’s Revised Paranormal Belief Scale. Personal. Individ. Differ. 2000, 29, 131–156. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2953. [Google Scholar] [CrossRef]
- Zaina, F.; Poggio, M.; Di Felice, F.; Donzelli, S.; Negrini, S. Bracing Adults with Chronic Low Back Pain Secondary to Severe Scoliosis: Six Months Results of a Prospective Pilot Study. Eur. Spine J. 2021, 30, 2962–2966. [Google Scholar] [CrossRef]
- Archer, J.E.; Baird, C.; Gardner, A.; Rushton, A.B.; Heneghan, N.R. Evaluating Measures of Quality of Life in Adult Scoliosis: A Systematic Review and Narrative Synthesis. Spine Deform 2022, 10, 991–1002. [Google Scholar] [CrossRef]
- Luce, R.D.; Tukey, J.W. Simultaneous Conjoint Measurement: A New Type of Fundamental Measurement. J. Math. Psychol. 1964, 1, 1–27. [Google Scholar] [CrossRef]
- Caronni, A.; Picardi, M.; Scarano, S.; Tropea, P.; Gilardone, G.; Bolognini, N.; Redaelli, V.; Pintavalle, G.; Aristidou, E.; Antoniotti, P.; et al. Differential Item Functioning of the Mini-BESTest Balance Measure: A Rasch Analysis Study. Int. J. Environ. Res. Public Health 2023, 20, 5166. [Google Scholar] [CrossRef]
| Adults | Adolescents | |
|---|---|---|
| Males vs. females, N | 30 vs. 120 | 10 vs. 40 |
| Mean age (SD), years | 49 (17.8) | 16 (1.4) |
| Mean disease severity (SD), °Cobb | 46.2 (16.6) | 24.7 (9) |
| Median TRACE score (IQR) | 7 (4) | 5 (3) |
| Items | Calibration | SE | Infit | Outfit | ||
|---|---|---|---|---|---|---|
| MnSq | Z-Std | MnSq | Z-Std | |||
| 1 (1), get worse | −1.95 | 0.17 | 0.97 | −0.30 | 0.99 | 0.00 |
| 2 (2), worried back pain | −0.95 | 0.15 | 1.12 | 1.17 | 1.54 | 3.11 |
| 3 (3), big deal | 1.76 | 0.16 | 1.00 | 0.00 | 0.95 | −0.23 |
| 4 (4), worried not get better | −0.13 | 0.14 | 1.01 | 0.10 | 1.08 | 0.61 |
| 5 (7), suffering | 0.85 | 0.15 | 0.93 | −0.64 | 0.84 | −1.22 |
| 6 (8), appearance | 1.10 | 0.15 | 0.82 | −1.92 | 0.75 | −1.81 |
| 7 (9), worried back problem | −1.95 | 0.18 | 0.83 | −1.68 | 0.75 | −1.72 |
| 8 (11), bother to show | 0.59 | 0.14 | 1.25 | 2.31 | 1.24 | 1.53 |
| 9 (12), worried visible | 0.69 | 0.15 | 1.07 | 0.69 | 1.02 | 0.23 |
| Item | Group | Obs − Exp | Bias | SE | p Value |
|---|---|---|---|---|---|
| ISYQOL International | |||||
| 8 (11), bother to show | Adolescents | 0.19 | 0.83 | 0.28 | 0.006 |
| SRS-22 | |||||
| 3, nervous person | Older | 0.31 | 0.61 | 0.20 | 0.003 |
| 4, back shape | Adolescents | −0.46 | 0.68 | 0.17 | 0.000 |
| 8, back pain at rest | Older | 0.36 | 0.51 | 0.17 | 0.004 |
| 12, around the house | Adolescents | 0.39 | 1.19 | 0.35 | 0.002 |
| Score | Burden of Disease | HRQOL | ||
|---|---|---|---|---|
| Measure, Logit | SE, Logit | Measure, % | SE, % | |
| 0 | −6.44 | 1.95 | 100.0 | 16.2 |
| 1 | −4.89 | 1.24 | 87.1 | 10.3 |
| 2 | −3.64 | 1.03 | 76.7 | 8.6 |
| 3 | −2.73 | 0.89 | 69.1 | 7.4 |
| 4 | −2.04 | 0.78 | 63.3 | 6.5 |
| 5 | −1.49 | 0.71 | 58.8 | 5.9 |
| 6 | −1.03 | 0.66 | 54.9 | 5.5 |
| 7 | −0.61 | 0.63 | 51.4 | 5.3 |
| 8 | −0.22 | 0.62 | 48.1 | 5.2 |
| 9 | 0.16 | 0.61 | 45.0 | 5.1 |
| 10 | 0.54 | 0.61 | 41.9 | 5.1 |
| 11 | 0.92 | 0.62 | 38.7 | 5.2 |
| 12 | 1.32 | 0.64 | 35.4 | 5.3 |
| 13 | 1.73 | 0.66 | 31.9 | 5.5 |
| 14 | 2.19 | 0.69 | 28.1 | 5.8 |
| 15 | 2.70 | 0.74 | 23.9 | 6.2 |
| 16 | 3.32 | 0.84 | 18.7 | 7.0 |
| 17 | 4.22 | 1.10 | 11.2 | 9.1 |
| 18 | 5.56 | 1.88 | 0.0 | 15.7 |
| Items | Calibration | SE | Infit | Outfit | ||
|---|---|---|---|---|---|---|
| MnSq | Z-Std | MnSq | Z-Std | |||
| 1, pain six months | −0.28 | 0.09 | 0.69 | −3.72 | 0.66 | −3.67 |
| 2, pain last month | −0.15 | 0.09 | 0.75 | −2.83 | 0.71 | −3.06 |
| 3, nervous person | 0.40 | 0.10 | 1.14 | 1.41 | 1.21 | 2.02 |
| 4, back shape | 0.70 | 0.09 | 1.09 | 0.96 | 1.10 | 1.00 |
| 5, activity level | −0.24 | 0.12 | 0.87 | −1.46 | 0.85 | −1.13 |
| 6, look in clothes | 0.32 | 0.10 | 1.04 | 0.41 | 1.04 | 0.45 |
| 7, down in the dumps | −0.71 | 0.10 | 0.97 | −0.27 | 1.13 | 0.68 |
| 8, back pain at rest | 0.30 | 0.09 | 1.42 | 4.07 | 1.52 | 3.90 |
| 9, work/school | −0.41 | 0.09 | 1.09 | 0.79 | 1.08 | 0.44 |
| 10, trunk appearance | 1.19 | 0.10 | 1.00 | 0.03 | 1.01 | 0.10 |
| 11, pain medications | −2.13 | 0.18 | 0.96 | −0.24 | 1.00 | 0.06 |
| 12, around the house | −0.19 | 0.08 | 0.74 | −2.73 | 0.81 | −1.30 |
| 13, calm and peaceful | 0.72 | 0.11 | 1.17 | 1.73 | 1.18 | 1.72 |
| 14, personal relationships | −0.72 | 0.09 | 0.80 | −1.82 | 0.72 | −1.63 |
| 16, downhearted and blue | −0.52 | 0.09 | 1.09 | 0.83 | 1.08 | 0.64 |
| 17, days off | −0.99 | 0.12 | 1.35 | 1.67 | 1.80 | 1.21 |
| 18, going out | −0.61 | 0.13 | 0.89 | −1.10 | 0.98 | −0.07 |
| 19, feel attractive | 1.29 | 0.09 | 0.97 | −0.22 | 0.91 | −0.84 |
| 20, happy person | 0.67 | 0.11 | 1.03 | 0.37 | 1.05 | 0.48 |
| 21, satisfied with results | 0.52 | 0.09 | 0.95 | −0.52 | 0.93 | −0.65 |
| 22, same management again | 0.84 | 0.11 | 1.20 | 2.26 | 1.24 | 2.01 |
| Score | HRQOL | HRQOL | ||
|---|---|---|---|---|
| Measure, Logit | SE, Logit | Measure, % | SE, % | |
| 21 | −5.99 | 1.85 | 0.0 | 14.7 |
| 22 | −4.73 | 1.04 | 10.1 | 8.3 |
| 23 | −3.96 | 0.75 | 16.2 | 6.0 |
| 24 | −3.49 | 0.63 | 19.9 | 5.0 |
| 25 | −3.15 | 0.55 | 22.7 | 4.4 |
| 26 | −2.88 | 0.50 | 24.8 | 4.0 |
| 27 | −2.65 | 0.46 | 26.6 | 3.6 |
| 28 | −2.46 | 0.42 | 28.2 | 3.4 |
| 29 | −2.29 | 0.40 | 29.5 | 3.2 |
| 30 | −2.14 | 0.37 | 30.7 | 3.0 |
| 31 | −2.01 | 0.36 | 31.7 | 2.8 |
| 32 | −1.89 | 0.34 | 32.7 | 2.7 |
| 33 | −1.78 | 0.33 | 33.6 | 2.6 |
| 34 | −1.67 | 0.32 | 34.4 | 2.5 |
| 35 | −1.58 | 0.31 | 35.2 | 2.4 |
| 36 | −1.49 | 0.30 | 35.9 | 2.4 |
| 37 | −1.40 | 0.29 | 36.6 | 2.3 |
| 38 | −1.32 | 0.28 | 37.2 | 2.3 |
| 39 | −1.24 | 0.28 | 37.8 | 2.2 |
| 40 | −1.17 | 0.27 | 38.4 | 2.2 |
| 41 | −1.10 | 0.27 | 39.0 | 2.1 |
| 42 | −1.03 | 0.26 | 39.6 | 2.1 |
| 43 | −0.96 | 0.26 | 40.1 | 2.1 |
| 44 | −0.89 | 0.26 | 40.7 | 2.1 |
| 45 | −0.82 | 0.26 | 41.2 | 2.1 |
| 46 | −0.76 | 0.26 | 41.7 | 2.0 |
| 47 | −0.69 | 0.25 | 42.2 | 2.0 |
| 48 | −0.63 | 0.25 | 42.7 | 2.0 |
| 49 | −0.56 | 0.25 | 43.3 | 2.0 |
| 50 | −0.50 | 0.25 | 43.8 | 2.0 |
| 51 | −0.44 | 0.25 | 44.3 | 2.0 |
| 52 | −0.37 | 0.25 | 44.8 | 2.0 |
| 53 | −0.31 | 0.26 | 45.3 | 2.0 |
| 54 | −0.24 | 0.26 | 45.8 | 2.0 |
| 55 | −0.18 | 0.26 | 46.4 | 2.1 |
| 56 | −0.11 | 0.26 | 46.9 | 2.1 |
| 57 | −0.04 | 0.26 | 47.4 | 2.1 |
| 58 | 0.03 | 0.26 | 48.0 | 2.1 |
| 59 | 0.10 | 0.26 | 48.5 | 2.1 |
| 60 | 0.17 | 0.27 | 49.1 | 2.1 |
| 61 | 0.24 | 0.27 | 49.7 | 2.2 |
| 62 | 0.31 | 0.27 | 50.2 | 2.2 |
| 63 | 0.39 | 0.28 | 50.8 | 2.2 |
| 64 | 0.47 | 0.28 | 51.5 | 2.2 |
| 65 | 0.54 | 0.28 | 52.1 | 2.3 |
| 66 | 0.63 | 0.29 | 52.7 | 2.3 |
| 67 | 0.71 | 0.29 | 53.4 | 2.3 |
| 68 | 0.80 | 0.30 | 54.1 | 2.4 |
| 69 | 0.89 | 0.30 | 54.8 | 2.4 |
| 70 | 0.98 | 0.31 | 55.6 | 2.5 |
| 71 | 1.08 | 0.32 | 56.4 | 2.5 |
| 72 | 1.18 | 0.32 | 57.2 | 2.6 |
| 73 | 1.29 | 0.33 | 58.0 | 2.6 |
| 74 | 1.40 | 0.34 | 58.9 | 2.7 |
| 75 | 1.52 | 0.35 | 59.9 | 2.8 |
| 76 | 1.65 | 0.36 | 60.9 | 2.9 |
| 77 | 1.78 | 0.37 | 61.9 | 2.9 |
| 78 | 1.92 | 0.38 | 63.0 | 3.0 |
| 79 | 2.07 | 0.39 | 64.2 | 3.1 |
| 80 | 2.22 | 0.40 | 65.5 | 3.2 |
| 81 | 2.39 | 0.41 | 66.8 | 3.3 |
| 82 | 2.57 | 0.43 | 68.2 | 3.4 |
| 83 | 2.76 | 0.44 | 69.7 | 3.5 |
| 84 | 2.96 | 0.46 | 71.4 | 3.7 |
| 85 | 3.19 | 0.49 | 73.2 | 3.9 |
| 86 | 3.44 | 0.52 | 75.2 | 4.1 |
| 87 | 3.73 | 0.56 | 77.5 | 4.5 |
| 88 | 4.09 | 0.63 | 80.3 | 5.0 |
| 89 | 4.56 | 0.75 | 84.1 | 6.0 |
| 90 | 5.31 | 1.03 | 90.1 | 8.2 |
| 91 | 6.56 | 1.84 | 100.0 | 14.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaina, F.; Ferrario, I.; Caronni, A.; Scarano, S.; Donzelli, S.; Negrini, S. Measuring Quality of Life in Adults with Scoliosis: A Cross-Sectional Study Comparing SRS-22 and ISYQOL Questionnaires. J. Clin. Med. 2023, 12, 5071. https://doi.org/10.3390/jcm12155071
Zaina F, Ferrario I, Caronni A, Scarano S, Donzelli S, Negrini S. Measuring Quality of Life in Adults with Scoliosis: A Cross-Sectional Study Comparing SRS-22 and ISYQOL Questionnaires. Journal of Clinical Medicine. 2023; 12(15):5071. https://doi.org/10.3390/jcm12155071
Chicago/Turabian StyleZaina, Fabio, Irene Ferrario, Antonio Caronni, Stefano Scarano, Sabrina Donzelli, and Stefano Negrini. 2023. "Measuring Quality of Life in Adults with Scoliosis: A Cross-Sectional Study Comparing SRS-22 and ISYQOL Questionnaires" Journal of Clinical Medicine 12, no. 15: 5071. https://doi.org/10.3390/jcm12155071
APA StyleZaina, F., Ferrario, I., Caronni, A., Scarano, S., Donzelli, S., & Negrini, S. (2023). Measuring Quality of Life in Adults with Scoliosis: A Cross-Sectional Study Comparing SRS-22 and ISYQOL Questionnaires. Journal of Clinical Medicine, 12(15), 5071. https://doi.org/10.3390/jcm12155071









