Is Thoracic Kyphosis Relevant to Pain, Autonomic Nervous System Function, Disability, and Cervical Sensorimotor Control in Patients with Chronic Nonspecific Neck Pain?
Abstract
1. Introduction
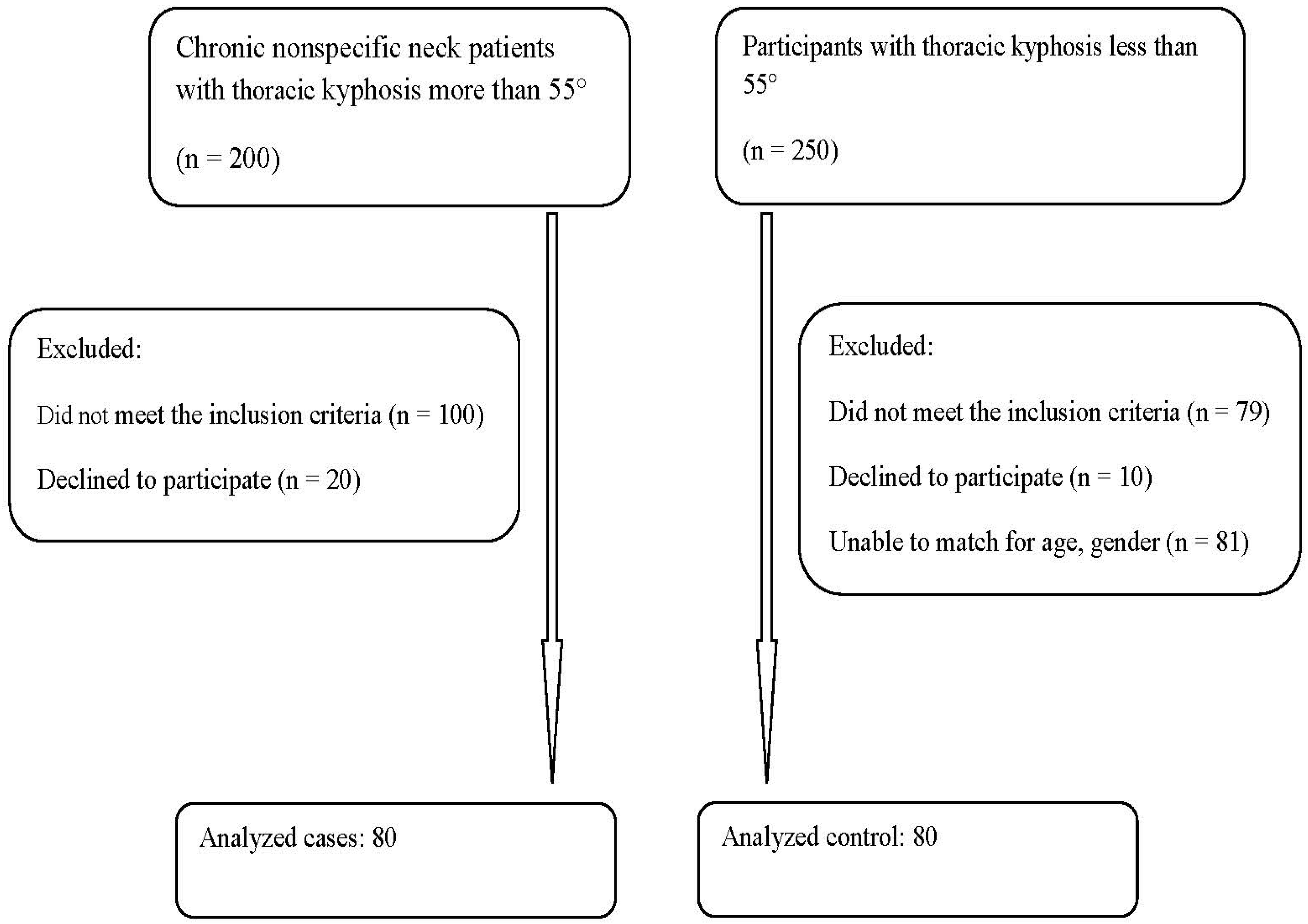
2. Materials and Methods
2.1. Participant Inclusion and Exclusion Criteria
2.1.1. Inclusion
2.1.2. Exclusion
- Neck pain associated with whiplash injury;
- Neck pain with bilateral cervical radiculopathy;
- Fibromyalgia syndrome;
- Surgery in the neck area, regardless of the cause;
- Neck pain accompanied by vertigo caused by vertebra-basilar insufficiency or accompanied with non-cervicogenic headaches;
- Recent or recurrent middle ear infections or any hearing impairment requiring the use of a hearing aid;
- Visual impairment not corrected by glasses;
- Any disorder of the central nervous system.
2.2. Measurement Procedures
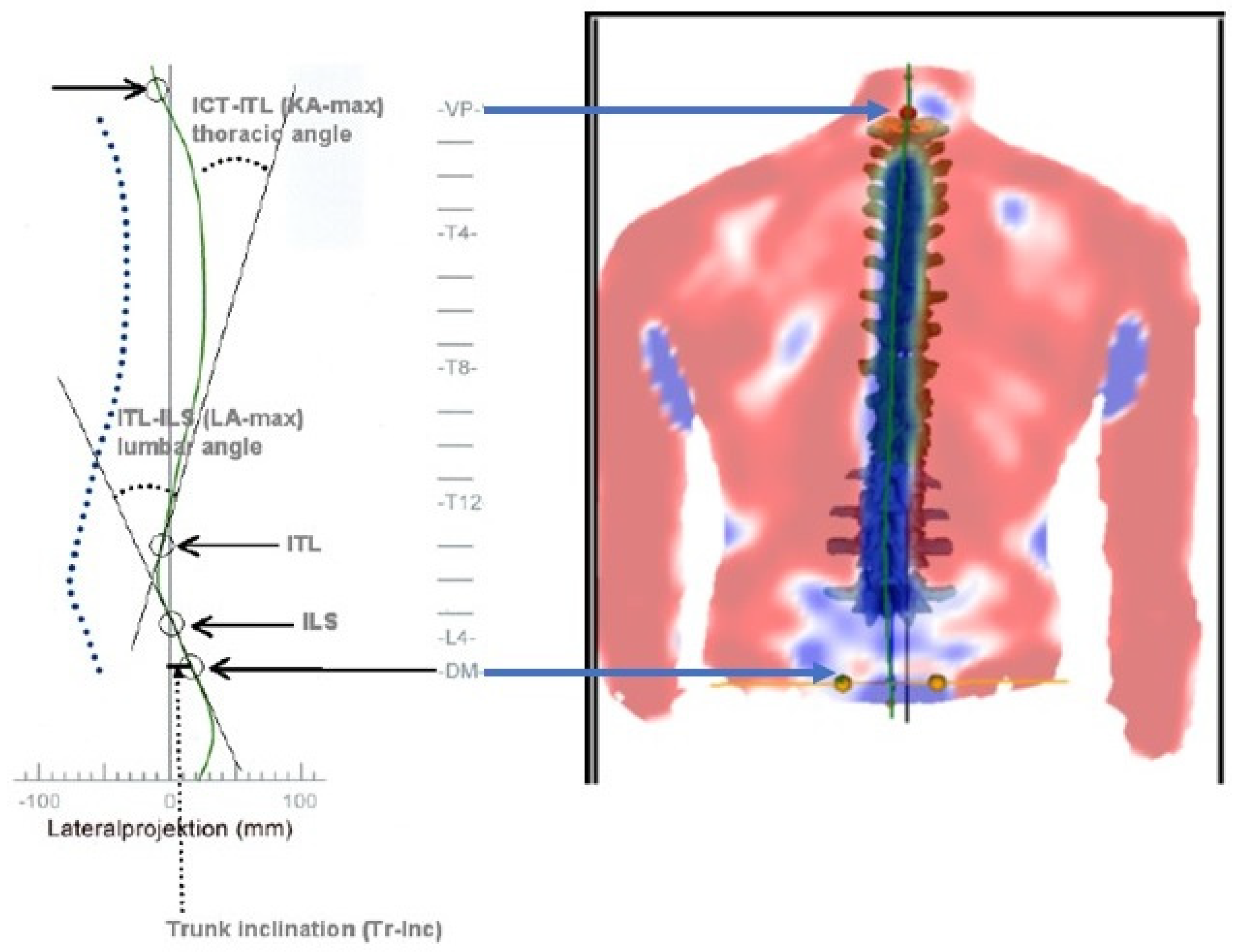
2.2.1. ICT-ITL (Max)
- look straight ahead in a relaxed breathing state with their head in a neutral position, not being twisted or bent;
- relax their shoulders, do not hunch them or rotate them forward;
- keep their upper arms, elbows and hands comfortably at their sides;
- stand with their legs straight, but with knees relaxed, not locked back (preventing hyperextension).
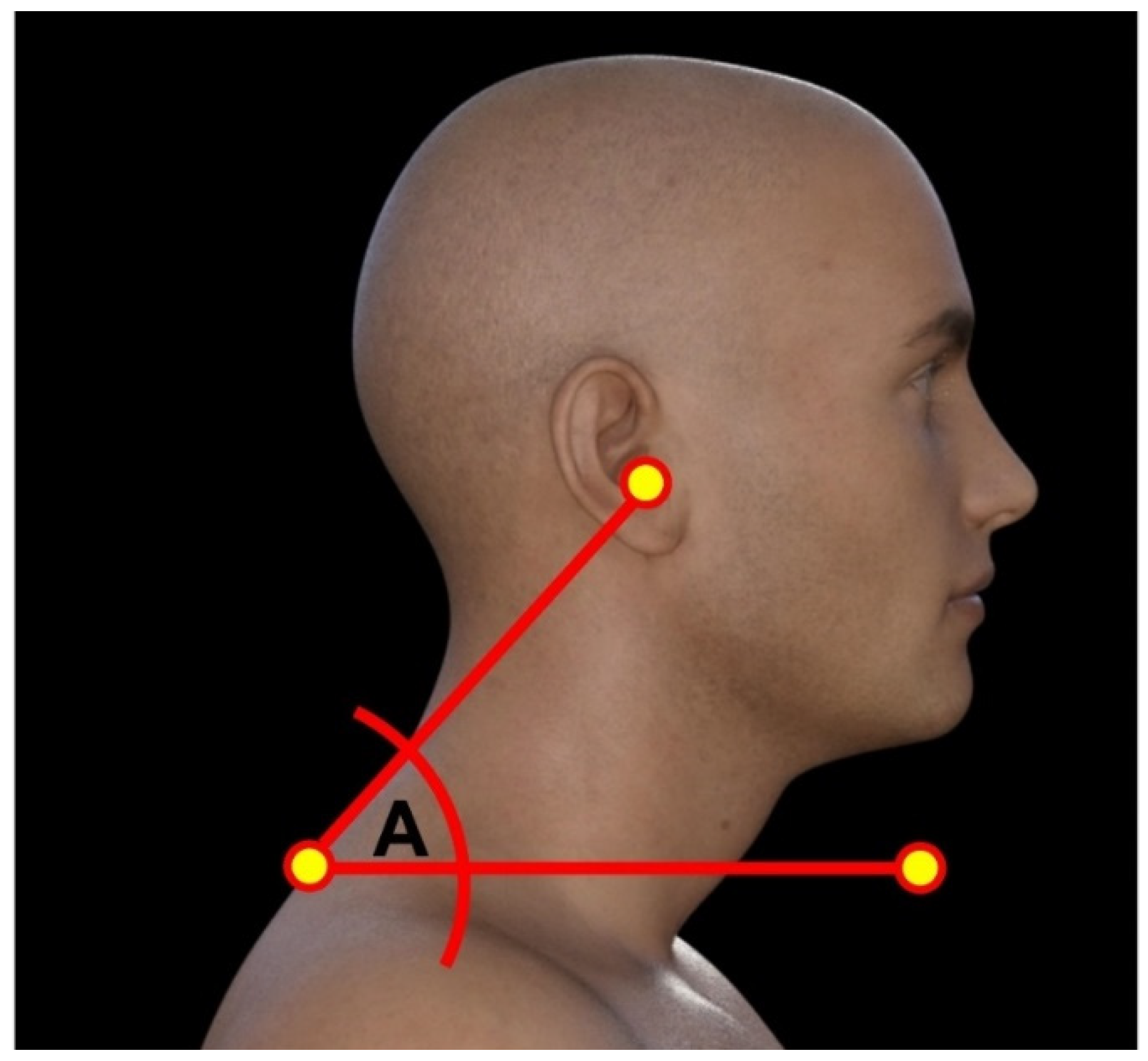
2.2.2. Craniovertebral Angle (CVA)
2.2.3. Numerical Rating Score (NRS)
2.2.4. Neck Disability Index
2.2.5. Sensorimotor Control Measures
- a.
- Cervical Joint Position Sense Testing
- b.
- Head and eye movement control: smooth pursuit neck torsion test (SPNT)
- c.
- Postural stability
2.2.6. Sympathetic Skin Response (SSR)
2.3. Sample Size Determination
2.4. Statistical Analysis
3. Results
3.1. Participant Demographics and Characteristics
3.2. Between Group Analysis
3.2.1. ICT-ITL (Max)
3.2.2. NRS and NDI
3.2.3. Sensorimotor Control Variables
3.2.4. SSR Latency and Amplitude
3.3. Correlations
Craniovertebral Angle (CVA)
4. Discussion
4.1. Thoracic Kyphosis
4.2. CVA, Pain, Disability, and Sensorimotor Control
4.3. SSR
4.4. Clinical Relevance
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, S.P. Epidemiology, diagnosis, and treatment of neck pain. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2015; pp. 284–299. [Google Scholar]
- Oxland, T.R. Fundamental biomechanics of the spine-What we have learned in the past 25 years and future directions. J. Biomech. 2016, 49, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Kaya, F.; Celenay, S. An investigation of sagittal thoracic spinal curvature and mobility in subjects with and without chronic neck pain: Cut-off points and pain relationship. Turk. J. Med. Sci. 2017, 47, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, T.F.; Peterson, D.H. Chiropractic Technique Principles and Procedures; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Norlander, S.; Gustavsson, B.; Lindell, J.; Nordgren, B. Reduced mobility in the cervico-thoracic motion segment: A risk factor for musculoskeletal neck-shoulder pain: A two-year prospective follow-up study. Scand. J. Rehabil. Med. 1997, 29, 167–174. [Google Scholar] [PubMed]
- Norlander, S.; Aste-Norlander, U.; Nordgren, B.; Sahlstedt, B. Mobility in the cervico-thoracic motion segment: An indicative factor of musculo-skeletal neckshoulder pain. Scand. J. Rehabil. Med. 1996, 28, 183–192. [Google Scholar]
- Fernández-de-las-Peñas, C.; Fernández-Carnero, J.; Fernández, A.P.; Lomas-Vega, R.; Miangolarra-Page, J.C. Dorsal manipulation in whiplash injury treatment. J. Whiplash Relat. Disord. 2004, 3, 55–72. [Google Scholar] [CrossRef]
- Tsang, S.M.H.; Szeto, G.P.Y.; Lee, R.Y.W. Normal kinematics of the neck: The interplay between the cervical and thoracic spines. Man. Ther. 2013, 18, 431–437. [Google Scholar] [CrossRef]
- Garni, A.D.; Al-Saran, Y.; Al-Moawi, A.; Bin Dous, A.; Al-Ahaideb, A.; Kachanathu, S.J. The prevalence of and factors associated with neck, shoulder, and low-back pains among medical students at university hospitals in central Saudi Arabia. Pain Res. Treat. 2017, 2017, 1235706. [Google Scholar]
- Alshagga, M.A.; Nimer, A.R.; Yan, L.P.; Ibrahim, I.A.; Al-Ghamdi, S.S.; Radman Al-Dubai, S.A. Prevalence and factors associated with neck, shoulder and low back pains among medical students in a Malaysian medical college. BMC Res. Notes 2013, 6, 244. [Google Scholar] [CrossRef]
- Almhdawi, K.A.; Mathiowetz, V.; Al-Hourani, Z.; Khader, Y.; Kanaan, S.F.; Alhasan, M. Musculoskeletal pain symptoms among allied health professions’ students: Prevalence rates and associated factors. J. Back Musculoskelet. Rehabil. 2017, 30, 1291–1301. [Google Scholar] [CrossRef]
- Cleland, J.; Selleck, B.; Stowell, T. Short-term effects of thoracic manipulation on lower trapezius muscle strength. J. Man. Manip. Ther. 2004, 12, 82–90. [Google Scholar] [CrossRef]
- Quek, J.; Pua, Y.H.; Clark, R.A.; Bryant, A.L. Effects of thoracic kyphosis and forward head posture on cervical range of motion in older adults. Man. Ther. 2013, 18, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.T.; Cheung, K.Y.; Chan kwok, B.; Chan, M.H.; Lo, K.Y.; Wing Chiu, T.T. Relationships between sagittal postures of thoracic and cervical spine, presence of neck pain, neck pain severity and disability. Man. Ther. 2010, 15, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Balthillaya, G.; Neelapala, Y.V.R. Thoracic posture and mobility in mechanical neck pain population: A review of the literature. Asian Spine J. 2019, 13, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Artz, N.J.; Adams, M.A.; Dolan, P. Sensorimotor function of the cervical spine in healthy volunteers. Clin. Biomech. 2015, 30, 260–268. [Google Scholar] [CrossRef]
- Treleaven, J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man. Ther. 2008, 13, 2–11. [Google Scholar] [CrossRef]
- Kristjansson, E.; Treleaven, J. Sensorimotor function and dizziness in neck pain: Implications for assessment and management. J. Orthop. Sport. Phys. Ther. 2009, 39, 364–377. [Google Scholar] [CrossRef]
- Röijezon, U.; Jull, G.; Blandford, C.; Daniels, A.; Michaelson, P.; Karvelis, P.; Treleaven, J. Proprioceptive disturbance in chronic neck pain: Discriminate validity and reliability of performance of the clinical cervical movement sense test. Front. Pain. Res. 2022, 3, 908414. [Google Scholar] [CrossRef]
- Sittikraipong, K.; Silsupadol, P.; Uthaikhup, S. Slower reaction and response times and impaired hand-eye coordination in individuals with neck pain. Musculoskelet. Sci. Pract. 2020, 50, 102273. [Google Scholar] [CrossRef]
- Asiri, F.; Reddy, R.S.; Tedla, J.S.; ALMohiza, M.A.; Alshahrani, M.S.; Govindappa, S.C.; Sangadala, D.R. Kinesiophobia and its correlations with pain, proprioception, and functional performance among individuals with chronic neck pain. PLoS ONE 2021, 16, e0254262. [Google Scholar] [CrossRef]
- Hellström, F.; Roatta, S.; Thunberg, J.; Passatore, M.; Djupsjöbacka, M. Responses of muscle spindles in feline dorsal neck muscles to electrical stimulation of the cervical sympathetic nerve. Exp. Brain Res. 2005, 165, 328–342. [Google Scholar] [CrossRef]
- Corneil, B.D.; Olivier, E.; Munoz, D.P. Neck muscle responses to stimulation of monkey superior colliculus. II. Gaze shift initiation and volitional head movements. J. Neurophysiol. 2002, 88, 2000–2018. [Google Scholar] [CrossRef] [PubMed]
- Bolton, P.S.; Kerman, I.A.; Woodring, S.F.; Yates, B.J. Influences of neck afferents on sympathetic and respiratory nerve activity. Brain Res. Bull. 1998, 47, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Budgell, B.S. Reflex effects of subluxation: The autonomic nervous system. J. Manip. Physiol. Ther. 2000, 23, 104–106. [Google Scholar] [CrossRef]
- Petcharaporn, M.; Pawelek, J.; Bastrom, T.; Lonner, B.; Newton, P.O. The relationship between thoracic hyperkyphosis and the scoliosis research society outcomes instrument. Spine 2007, 32, 2226–2231. [Google Scholar] [CrossRef]
- Nissinen, M.; Heliövaara, M.; Seitsamo, J.; Poussa, M. Left handedness and risk of thoracic hyperkyphosis in prepubertal schoolchildren. Int. J. Epidemiol. 1995, 24, 1178–1181. [Google Scholar] [CrossRef]
- Pellisé, F.; Vila-Casademunt, A.; Ferrer, M.; Domingo-Sàbat, M.; Bagó, J.; Pérez-Grueso, F.J.S.; Alanay, A.; Mannion, A.F.; Acaroglu, E.; European Spine Study Group; et al. Impact on health related quality of life of adult spinal deformity (ASD) compared with other chronic conditions. Eur. Spine J. 2015, 24, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bess, S.; Line, B.; Fu, K.M.; McCarthy, I.; Lafage, V.; Schwab, F.; Shaffrey, C.; Ames, C.; Akbarnia, B.; Jo, H.; et al. The health impact of symptomatic adult spinal deformity: Comparison of deformity types to United States population norms and chronic diseases. Spine 2016, 41, 224–233. [Google Scholar] [CrossRef]
- McDaniels-Davidson, C.; Davis, A.; Wing, D.; Macera, C.; Lindsay, S.P.; Schousboe, J.T.; Nichols, J.F.; Kado, D.M. Kyphosis and incident falls among community-dwelling older adults. Osteoporos Int. 2018, 29, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Van Der Jagt-Willems, H.C.; De Groot, M.H.; Van Campen, J.P.C.M.; Lamoth, C.J.C.; Lems, W.F. Associations between vertebral fractures, increased thoracic kyphosis, a flexed posture and falls in older adults: A prospective cohort study. BMC Geriatr. 2015, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Lason, G.; Peeters, L.; Vandenberghe, K.; Byttebier, G.; Comhaire, F. Reassessing the accuracy and reproducibility of Diers formetric measurements in healthy volunteers. Int. J. Osteopath. Med. 2015, 18, 247–254. [Google Scholar] [CrossRef]
- Knott, P.; Sturm, P.; Lonner, B.; Cahill, P.; Betsch, M.; McCarthy, R.; Kelly, M.; Lenke, L.; Betz, R. Multicenter comparison of 3D spinal measurements using surface topography with those from conventional radiography. Spine Deform. 2016, 4, 98–103. [Google Scholar] [CrossRef]
- Harrison, D.E.; Janik, T.J.; Harrison, D.D.; Cailliet, R.; Harmon, S.F. Can the thoracic kyphosis be modeled with a simple geometric shape. J. Spinal Disord. Technol. 2002, 15, 2130220. [Google Scholar] [CrossRef] [PubMed]
- Krott, N.L.; Wild, M.; Betsch, M. Meta-analysis of the validity and reliability of rasterstereographic measurements of spinal posture. Eur. Spine J. 2020, 29, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.H.T.; Chiu, T.T.W.; Poon, A.T.K. The relationship between head posture and severity and disability of patients with neck pain. Man Ther. 2008, 13, 148–154. [Google Scholar] [CrossRef]
- Van Niekerk, S.M.; Louw, Q.; Vaughan, C.; Grimmer-Somers, K.; Schreve, K. Photographic measurement of upper-body sitting posture of high school students: A reliability and validity study. BMC Musculoskelet Disord. 2008, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Lundeberg, T.; Lund, I.; Dahlin, L.; Borg, E.; Gustafsson, C.; Sandin, L.; Rosén, A.; Kowalski, J.; Eriksson, S.V. Reliability and responsiveness of three different pain assessments. J. Rehabil. Med. 2001, 33, 279–283. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Walton, D.M.; Avery, S.; Blanchard, A.; Etruw, E.; McAlpine, C.; Goldsmith, C.H. Measurement properties of the neck disability index: A systematic review. J. Orthop. Sports Phys. Ther. 2009, 39, 400–417. [Google Scholar] [CrossRef]
- Loudon, J.K.; Ruhl, M.; Field, E. Ability to reproduce head position after whiplash injury. Spine 1997, 22, 865–868. [Google Scholar] [CrossRef]
- Treleaven, J.; Jull, G.; Sterling, M. Dizziness and unsteadiness following whiplash injury: Characteristic features and relationship with cervical joint position error. J. Rehabil. Med. 2003, 35, 36–43. [Google Scholar] [CrossRef]
- Tjell, C.; Rosenhall, U. Smooth pursuit neck torsion test: A specific test for cervical dizziness. Am. J. Otol. 1998, 19, 76–81. [Google Scholar]
- Arnold, B.L.; Schmitz, R.J. Examination of balance measures produced by the biodex stability system. J. Athl. Train. 1998, 33, 323. [Google Scholar]
- Schmitz, R.; Arnold, B. Intertester and intratester reliability of a dynamic balance protocol using the biodex stability system. J. Sport Rehabil. 1998, 7, 95–101. [Google Scholar] [CrossRef]
- Elie, B.; Guiheneuc, P. Sympathetic skin response: Normal results in different experimental conditions. Electroencephalogr. Clin. Neurophysiol. 1990, 76, 258–267. [Google Scholar] [CrossRef] [PubMed]
- On, A.Y.; Colakoglu, Z.; Hepguler, S.; Aksit, R. Local heat effect on sympathetic skin responses after pain of electrical stimulus. Arch. Phys. Med. Rehabil. 1997, 78, 1196–1199. [Google Scholar] [PubMed]
- Kucera, P.; Goldenberg, Z.; Kurca, E. Sympathetic skin response: Review of the method and its clinical use. Bratisl. Lek Listy. 2004, 105, 108–116. [Google Scholar]
- Chroni, E.; Argyriou, A.A.; Polychronopoulos, P.; Sirrou, V. The effect of stimulation technique on sympathetic skin responses in healthy subjects. Clin. Auton. Res. 2006, 16, 396–400. [Google Scholar] [CrossRef]
- Wyrwich, K.W.; Tierney, W.M.; Wolinsky, F.D. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J. Clin. Epidemiol. 1999, 52, 861–873. [Google Scholar] [CrossRef]
- Wolinsky, F.D.; Wan, G.J.; Tierney, W.M. Changes in the SF-36 in 12 months in a clinical sample of disadvantaged older adults. Med. Care 1998, 36, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Tarlov, A.R. Individual-patient monitoring in clinical practice: Are available health status surveys adequate? Qual. Life Res. 1995, 4, 293–307. [Google Scholar] [CrossRef]
- González-Gálvez, N.; Gea-García, G.M.; Marcos-Pardo, P.J. Effects of exercise programs on kyphosis and lordosis angle: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0216180. [Google Scholar] [CrossRef]
- Bezalel, T.; Carmeli, E.; Levi, D.; Kalichman, L. The effect of Schroth therapy on thoracic kyphotic curve and quality of life in Scheuermann’s patients: A randomized controlled trial. Asian Spine J. 2019, 13, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Rivett, D.A.; McKiernan, S.; Weerasekara, I.; Snodgrass, S.J. Is the inclinometer a valid measure of thoracic kyphosis? A cross-sectional study. Braz. J. Phys. Ther. 2018, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, D.; Iizuka, Y.; Iizuka, H.; Nishinome, M.; Kobayashi, R.; Ara, T.; Yamamoto, A.; Takagishi, K. Associations between neck and shoulder pain (called katakori in Japanese) and sagittal spinal alignment parameters among the general population. J. Orthop. Sci. 2013, 18, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Szeto, G.P.Y.; Straker, L.M.; O’Sullivan, P.B. A comparison of symptomatic and asymptomatic office workers performing monotonous keyboard work - 2: Neck and shoulder kinematics. Man. Ther. 2005, 10, 281–291. [Google Scholar] [CrossRef]
- Cross, K.M.; Kuenze, C.; Grindstaff, T.; Hertel, J. Thoracic spine thrust manipulation improves pain, range of motion, and self-reported function in patients with mechanical neck pain: A systematic review. J. Orthop. Sports Phys. Ther. 2011, 41, 633–643. [Google Scholar] [CrossRef]
- Nejati, P.; Lotfian, S.; Moezy, A.; Nejati, M. The study of correlation between forward head posture and neck pain in Iranian office workers. Int. J. Occup. Med. Environ. Health 2015, 28, 295–303. [Google Scholar] [CrossRef]
- Smart, K.M.; Blake, C.; Staines, A.; Doody, C. Clinical indicators of “nociceptive”, “peripheral neuropathic” and “central” mechanisms of musculoskeletal pain. A delphi survey of expert clinicians. Man Ther. 2010, 15, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Jones, E.W.; Janik, T.J.; Harrison, D.D. Evaluation of axial and flexural stresses in the vertebral body cortex and trabecular bone in lordosis and two sagittal cervical translation configurations with an elliptical shell model. J. Manip. Physiol. Ther. 2002, 25, 391–401. [Google Scholar] [CrossRef]
- Patwardhan, A.G.; Khayatzadeh, S.; Havey, R.M.; Voronov, L.I.; Smith, Z.A.; Kalmanson, O.; Ghanayem, A.J.; Sears, W. Cervical sagittal balance: A biomechanical perspective can help clinical practice. Eur. Spine J. 2018, 27 (Suppl. S1), 25–38. [Google Scholar] [CrossRef]
- Smith, J.S.; Lafage, V.; Ryan, D.J.; Shaffrey, C.I.; Schwab, F.J.; Patel, A.A.; Brodke, D.S.; Arnold, P.M.; Riew, K.D.; Traynelis, V.C.; et al. Association of myelopathy scores with cervical sagittal balance and normalized spinal cord volume: Analysis of 56 preoperative cases from the AOSpine North America myelopathy study. Spine 2013, 38 (Suppl. S1), S161–S170. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Alonso-Blanco, C.; Cuadrado, M.; Pareja, J. Forward head posture and neck mobility in chronic tension-type headache. Cephalalgia 2006, 26, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Khayatzadeh, S.; Kalmanson, O.A.; Schuit, D.; Havey, R.M.; Voronov, L.I.; Ghanayem, A.J.; Patwardhan, A.G. Cervical spine muscle-tendon unit length differences between neutral and forward head postures: Biomechanical study using human cadaveric specimens. Phys. Ther. 2017, 97, 756–766. [Google Scholar] [CrossRef]
- Thigpen, C.A.; Padua, D.A.; Michener, L.A.; Guskiewicz, K.; Giuliani, C.; Keener, J.D.; Stergiou, N. Head and shoulder posture affect scapular mechanics and muscle activity in overhead tasks. J. Electromyogr. Kinesiol. 2010, 20, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.R.; Leake, H.B.; Chalmers, K.J.; Moseley, G.L. Evidence of impaired proprioception in chronic, idiopathic neck pain: Systematic review and meta-analysis. Phys. Ther. 2016, 96, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.F.; Hassan, K.A.; Abdelmajeed, S.F.; Moustafa, I.M.; Silva, A.G. The relationship between forward head posture and neck pain: A systematic review and meta-analysis. Curr. Rev. Musculoskelet. Med. 2019, 12, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Rani, B.; Paul, A.; Chauhan, A.; Pradhan, P.; Dhillon, M.S. Is neck pain related to sagittal head and neck posture? A systematic review and meta-analysis. Indian J. Orthop. 2023, 57, 371–403. [Google Scholar] [CrossRef]
- Pacheco, J.; Raimundo, J.; Santos, F.; Ferreira, M.; Lopes, T.; Ramos, L.; Silva, A.G. Forward head posture is associated with pressure pain threshold and neck pain duration in university students with subclinical neck pain. Somat. Mot. Res. 2018, 35, 103–108. [Google Scholar] [CrossRef]
- Moustafa, I.M.; Diab, A.A.; Hegazy, F.; Harrison, D.E. Demonstration of central conduction time and neuroplastic changes after cervical lordosis rehabilitation in asymptomatic subjects: A randomized, placebo-controlled trial. Sci. Rep. 2021, 11, 15379. [Google Scholar] [CrossRef]
- Hayano, J.; Yuda, E. Pitfalls of Assessment of Autonomic Function by Heart Rate Variability. J. Physiol. Anthropol. 2019, 38, 3. [Google Scholar] [CrossRef]
- Ke, J.Q.; Shao, S.M.; Zheng, Y.Y.; Fu, F.W.; Zheng, G.Q.; Liu, C.F. Sympathetic Skin Response and Heart Rate Variability in Predicting Autonomic Disorders in Patients with Parkinson Disease. Medicine 2017, 96, e6523. [Google Scholar] [CrossRef] [PubMed]
- Oakley, P.A.; Moustafa, I.M.; Harrison, D.E. The influence of sagittal plane spine alignment on neurophysiology and sensorimotor control measures: Optimization of function through structural correction. In Therapy Approaches in Neurological Disorders; Bernardo-Filho, M., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Moustafa, I.M.; Shousha, T.M.; Walton, L.M.; Raigangar, V.; Harrison, D.E. Reduction of thoracic hyper-kyphosis improves short and long term outcomes in patients with chronic nonspecific neck pain: A randomized controlled trial. J. Clin. Med. 2022, 11, 6028. [Google Scholar] [CrossRef] [PubMed]


| Variables | Entire Kyphotic (n = 80) | Normal (n = 80) | p Value |
|---|---|---|---|
| Age (years) | 25.1 ± 3 | 24 ± 4.6 | 0.07 |
| Weight (kg) | 66 ± 10 | 60 ± 9 | 0.9 |
| Sex | |||
| Male | 38 | 32 | 0.2 |
| Female | 42 | 48 | |
| Marital status | |||
| Single | 61 | 59 | 0.3 |
| Married | 19 | 21 | |
| Separated, divorced, or widowed | 0 | 0 | |
| Pain duration (months) | 18 ± 4 | 17 ± 5 | 0.16 |
| Smoking | |||
| Light smoker | 29 | 32 | 0.4 |
| Heavy smoker | 14 | 15 | |
| No Smoker | 37 | 33 | |
| Variables | Postural Kyphosis N = 35 | Scheuermann’s kyphosis N = 45 | Normal (n = 80) | p Value |
|---|---|---|---|---|
| Age (years) | 25 ± 3.2 | 25.3 ± 3 | 24 ± 4.6 | 0.16 |
| Weight (kg) | 65 ± 11 | 67 ± 9 | 60 ± 9 | 0.6 |
| Sex | ||||
| Male | 18 | 20 | 32 | 0.5 |
| Female | 17 | 25 | 48 | |
| Marital status | ||||
| Single | 27 | 33 | 59 | 0.6 |
| Married | 8 | 12 | 21 | |
| Separated, divorced, or widowed | 0 | 0 | 0 | |
| Pain duration (months) | 17 ± 3 | 18.7 ± 4.5 | 17 ± 5 | 0.1 |
| Smoking | ||||
| Light smoker | 15 | 14 | 32 | 0.15 |
| Heavy smoker | 8 | 6 | 15 | |
| No Smoker | 12 | 25 | 33 | |
| Kyphotic angle | 66.5 ± 3 | 67.5 ± 4.9 | 49 ± 3 | <0.001 * |
| Variables | Entire Kyphotic Group (n = 80) | Normal Group (n = 80) | Cohen’s d Effect Size | p Value (95% CI) |
|---|---|---|---|---|
| NDI | 37.3 ± 4.1 | 29.8 ± 2.4 | 2.2 | <0.001 * [−8.5, −6.45] |
| Pain intensity | 5.3 ± 2.0 | 4.9 ± 1.8 | 0.20 | 0.18 [−0.99, 0.19] |
| Postural Kyphosis N = 35 | Scheuermann’s Kyphosis N = 45 | Normal Group (n = 80) | F-Value/ p-Value | Post Hoc | |
|---|---|---|---|---|---|
| NDI | 35.2 ± 2.4 | 39.1 ± 4.5 | 29.8 ± 2.4 | 132.67/ <0.001 * | Group 1 vs. Group 2: Diff = 3.9, 95% CI = 2.22 to 5.57, p < 0.001 * Group 1 vs. Group 3: Diff = −5.4, 95% CI = −6.90 to −3.89, p < 0.001 * Group 2 vs. Group 3: Diff = −9.3, 95% CI = −10.68 to −7.91, p < 0.001 * |
| Pain intensity | 4.6 ± 1.4 | 5.9 ± 2.3 | 4.9 ± 1.8 | 2.68/0.07 |
| Variables | Kyphotic Group | Normal Group | Cohen’s d Effect Size | p Value [95% CI] |
|---|---|---|---|---|
| CVA (°) | 41 ± 5 | 53 ± 4 | 2.65 | <0.001 * [10.6, 13.4] |
| Smooth pursuit neck torsion test (% error) | 0.41 ± 0.17 | 0.31 ± 0.14 | 0.6 | <0.001 * [−0.15, −0.05] |
| ** Overall stability index (refer to methods) | 0.62 ± 0.2 | 0.42 ± 0.1 | 1.26 | <0.001 * [−0.05, −0.14] |
| Head repositioning accuracy (°) Right | 4.0 ± 1.5 | 3.0 ± 1.2 | 0.74 | <0.001 * [−0.57, −1.42] |
| Head repositioning accuracy (°) Left | 4.3 ± 1.8 | 3.3 ± 1.5 | 0.6 | <0.001 * [−0.45, −1.58] |
| Sympathetic skin resistance Amplitude | 2.9 ± 0.9 | 2.1 ± 0.7 | 0.87 | <0.001 * [−0.54, −1.05] |
| Sympathetic skin resistance Latency | 1.2 ± 0.4 | 1.3 ± 0.3 | 0.2 | 0.07 [−0.01, 0.21] |
| Variables | Postural Kyphosis N = 35 | Scheuermann’s Kyphosis N = 45 | Normal Group N = 80 | F-Value/ p-Value | Post Hoc |
|---|---|---|---|---|---|
| CVA (°) | 44 ± 4 | 38.5 ± 4.5 | 53 ± 4 | 187.4/ <0.001 * | Group 1 vs. Group 2: Diff = −5.5, 95% CI = −8.58 to −2.4, p = 0.0002 * Group 1 vs. Group 3: Diff = 9, 95% CI = 5.7 to 12.27, p < 0.001 * Group 2 vs. Group 3: Diff = 14.5, 95% CI = 11.3 to 17.6, p < 0.001 * |
| Smooth pursuit neck torsion test (% error) | 0.34 ± 0.13 | 0.48 ± 0.18 | 0.31 ± 0.14 | 19.1/<0.001 * | Group 1 vs. Group 2: Diff = 0.14, 95% CI = 0.059 to 0.22, p = 0.0002 * Group 1 vs. Group 3: Diff = −0.03, 95% CI = −0.10 to 0.04, p = 0.5 Group 2 vs. Group 3: Diff = −0.17, 95% CI = −0.24 to −0.10, p < 0.001 * |
| ** Overall stability index (refer to methods) | 0.56 ± 0.2 | 0.68 ± 0.3 | 0.42 ± 0.1 | 25.7/<0.001 * | Group 1 vs. Group 2: Diff = 0.12, 95% CI = 0.015 to 0.23, p = 0.02 * Group 1 vs. Group 3: Diff = −0.14, 95% CI = −0.23 to −0.045, p = 0.0017 * Group 2 vs. Group 3: Diff = −0.26, 95% CI = −0.35 to −0.17, p < 0.001 * |
| Head repositioning accuracy (°) Right | 3 ± 0.7 | 4.8 ± 1.6 | 3.0 ± 1.2 | 33.84/ <0.001 * | Group 1 vs. Group 2: Diff = 1.8, 95% CI = 1.14 to 2.5, p < 0.001 * Group 1 vs. Group 3: Diff = 0.0, 95% CI = −0.59 to 0.59, p = 0.99 Group 2 vs. Group 3: Diff = −1.8, 95% CI = −2.34 to −1.25, p < 0.001 * |
| Head repositioning accuracy (°) Left | 3.8 ± 2 | 4.7 ± 1.6 | 3.3 ± 1.5 | 10.39/0.04 * | Group 1 vs. Group 2: Diff = 0.9, 95% CI = 0.02 to 1.77, p = 0.04 * Group 1 vs. Group 3: Diff = −0.5, 95% CI = −1.29 to 0.29, p = 0.29 Group 2 vs. Group 3: Diff = −1.4, 95% CI = −2.12 to −0.67, p < 0.001 * |
| Sympathetic skin resistance Amplitude | 2.4 ± 0.6 | 3.3 ± 1 | 2.1 ± 0.7 | 34.68/<0.001 * | Group 1 vs. Group 2: Diff = 0.9, 95% CI = 0.48 to 1.31, p < 0.001 * Group 1 vs. Group 3: Diff = −0.3, 95% CI = −0.67 to 0.07, p = 0.14 Group 2 vs. Group 3: Diff = −1.2, 95% CI = −1.54 to −0.85, p < 0.001 * |
| Sympathetic skin resistance Latency | 1.3 ± 0.3 | 1.2 ± 0.5 | 1.3 ± 0.3 | 1.19/0.3 | NA |
| Correlation between Variables | Postural Kyphosis r (p Value) N = 35 | Scheuermann’s Kyphosis r (p Value) N = 45 | Normal Group r (p Value) N = 80 | Entire Sample r (p Value) N = 160 |
|---|---|---|---|---|
| CVA | −0.7 (<0.001) | −0.6 (<0.001) | −0.51 (<0.001) | −0.61 (<0.001) |
| NDI | 0.58 (<0.001) | 0.50 (<0.001) | 0.51 (<0.001) | 0.67 (<0.001) |
| Pain intensity (NRS) | 0.5 (<0.001) | 0.35 (0.03) | 0.34 (0.043) | 0.53 (<0.001) |
| Smooth pursuit neck torsion test | 0.54 (<0.001) | 0.50 (<0.001) | 0.50 (<0.001) | 0.58 (<0.001) |
| Overall stability index | 0.61 (<0.001) | 0.49 (<0.001) | 0.52 (<0.001) | 0.59 (<0.001) |
| Head repositioning accuracy (Right) | 0.7 (<0.001) | 0.54 (<0.001) | 0.61 (<0.001) | 0.74 (<0.001) |
| Head repositioning accuracy (Left) | 0.67 (<0.001) | 0.52 (<0.001) | 0.61 (<0.001) | 0.71 (<0.001) |
| Sympathetic skin resistance amplitude | 0.7 (<0.001) | 0.56 (<0.001) | 0.61 (<0.001) | 0.69 (<0.001) |
| Sympathetic skin resistance latency | −0.2 (0.05) | −0.5 (<0.001) | −0.36 (<0.001) | −0.49 (<0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustafa, I.M.; Shousha, T.; Arumugam, A.; Harrison, D.E. Is Thoracic Kyphosis Relevant to Pain, Autonomic Nervous System Function, Disability, and Cervical Sensorimotor Control in Patients with Chronic Nonspecific Neck Pain? J. Clin. Med. 2023, 12, 3707. https://doi.org/10.3390/jcm12113707
Moustafa IM, Shousha T, Arumugam A, Harrison DE. Is Thoracic Kyphosis Relevant to Pain, Autonomic Nervous System Function, Disability, and Cervical Sensorimotor Control in Patients with Chronic Nonspecific Neck Pain? Journal of Clinical Medicine. 2023; 12(11):3707. https://doi.org/10.3390/jcm12113707
Chicago/Turabian StyleMoustafa, Ibrahim M., Tamer Shousha, Ashokan Arumugam, and Deed E. Harrison. 2023. "Is Thoracic Kyphosis Relevant to Pain, Autonomic Nervous System Function, Disability, and Cervical Sensorimotor Control in Patients with Chronic Nonspecific Neck Pain?" Journal of Clinical Medicine 12, no. 11: 3707. https://doi.org/10.3390/jcm12113707
APA StyleMoustafa, I. M., Shousha, T., Arumugam, A., & Harrison, D. E. (2023). Is Thoracic Kyphosis Relevant to Pain, Autonomic Nervous System Function, Disability, and Cervical Sensorimotor Control in Patients with Chronic Nonspecific Neck Pain? Journal of Clinical Medicine, 12(11), 3707. https://doi.org/10.3390/jcm12113707







