Saline versus Plasma Solution-A in Initial Resuscitation of Patients with Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Randomization
2.2. Trial Design and Intervention
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Fluid Administration and In-Hospital Treatments after Randomization
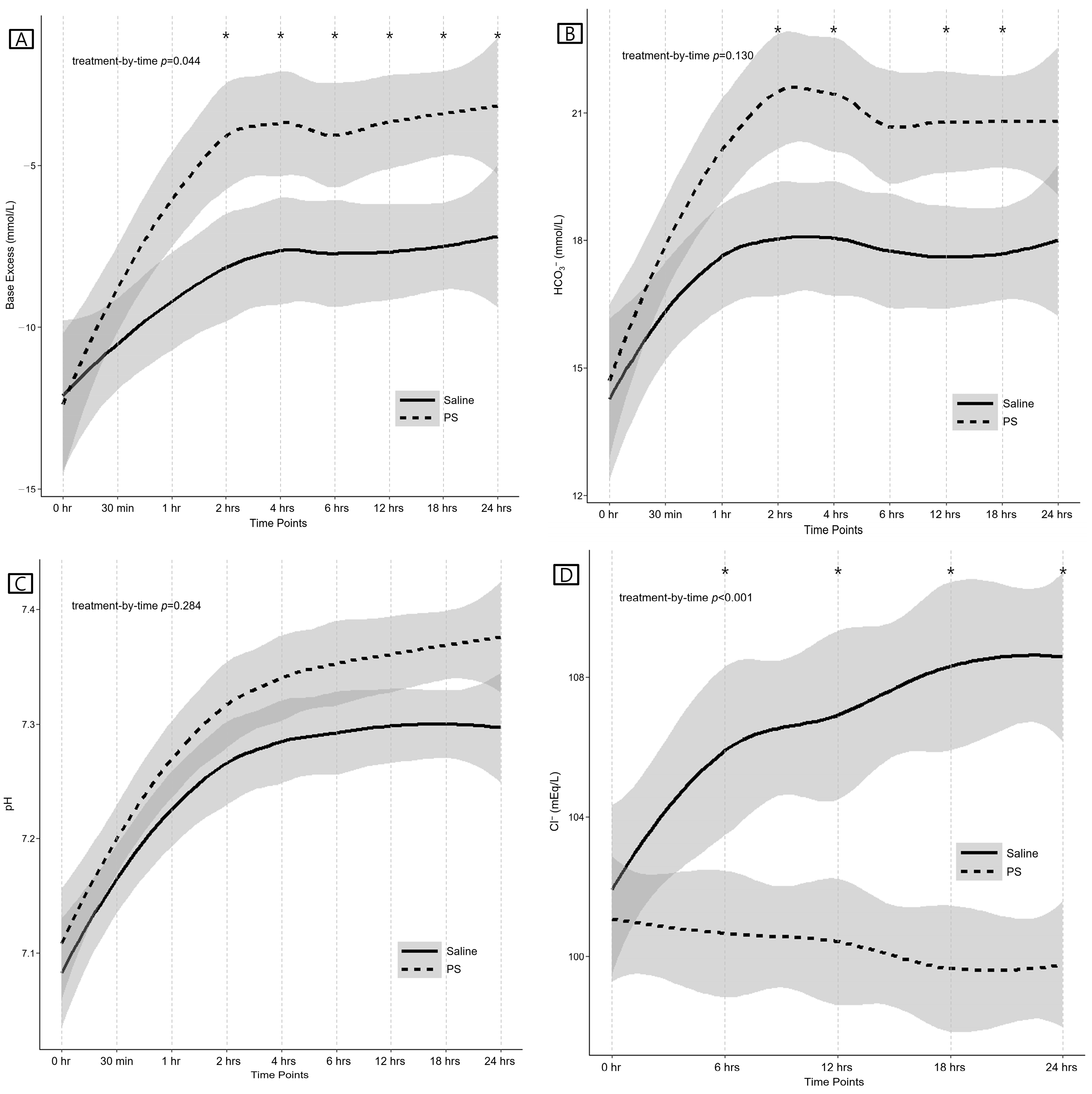
3.3. Primary Outcomes (Laboratory Results)
3.4. Secondary Outcomes (Clinical Outcomes)
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myburgh, J.A.; Mythen, M.G. Resuscitation fluids. N. Engl. J. Med. 2013, 369, 1243–1251. [Google Scholar] [CrossRef]
- Scheingraber, S.; Rehm, M.; Sehmisch, C.; Finsterer, U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 1999, 90, 1265–1270. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Li, J. Science review: Extracellular acidosis and the immune response: Clinical and physiologic implications. Crit. Care 2004, 8, 331. [Google Scholar] [CrossRef][Green Version]
- Chowdhury, A.H.; Cox, E.F.; Francis, S.T.; Lobo, D.N. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and Plasma-Lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann. Surg. 2012, 256, 18–24. [Google Scholar] [CrossRef]
- Chua, H.-R.; Venkatesh, B.; Stachowski, E.; Schneider, A.G.; Perkins, K.; Ladanyi, S.; Kruger, P.; Bellomo, R. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J. Crit. Care 2012, 27, 138–145. [Google Scholar] [CrossRef]
- Young, J.B.; Utter, G.H.; Schermer, C.R.; Galante, J.M.; Phan, H.H.; Yang, Y.; Anderson, B.A.; Scherer, L.A. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: A randomized trial. Ann. Surg. 2014, 259, 255–262. [Google Scholar] [CrossRef]
- Hammond, N.E.; Taylor, C.; Finfer, S.; Machado, F.R.; An, Y.; Billot, L.; Bloos, F.; Bozza, F.; Cavalcanti, A.B.; Correa, M.; et al. Patterns of intravenous fluid resuscitation use in adult intensive care patients between 2007 and 2014: An international cross-sectional study. PLoS ONE 2017, 12, e0176292. [Google Scholar] [CrossRef]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced crystalloids versus saline in critically ill adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef]
- Finfer, S.; Micallef, S.; Hammond, N.; Navarra, L.; Bellomo, R.; Billot, L.; Delaney, A.; Gallagher, M.; Gattas, D.; Li, Q.; et al. Balanced multielectrolyte solution versus saline in critically ill adults. N. Engl. J. Med. 2022, 386, 815–826. [Google Scholar] [CrossRef]
- Self, W.H.; Semler, M.W.; Wanderer, J.P.; Ehrenfeld, J.M.; Byrne, D.W.; Wang, L.; Atchison, L.; Felbinger, M.; Jones, I.D.; Russ, S.; et al. Saline versus balanced crystalloids for intravenous fluid therapy in the emergency department: Study protocol for a cluster-randomized, multiple-crossover trial. Trials 2017, 18, 178. [Google Scholar] [CrossRef]
- Orchard, C.H.; Cingolani, H.E. Acidosis and arrhythmias in cardiac muscle. Cardiovasc. Res. 1994, 28, 1312–1319. [Google Scholar] [CrossRef]
- Oh, J.; Cha, K.-C.; Lee, J.-H.; Park, S.; Kim, D.-H.; Lee, B.K.; Park, J.S.; Jung, W.J.; Lee, D.K.; Roh, Y.I.; et al. 2020 Korean Guidelines for Cardiopulmonary Resuscitation. Part 4. Adult advanced life support. Clin. Exp. Emerg. Med. 2021, 8, S26–S40. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. Section 2: AKI definition. Kidney Int. Suppl. 2012, 2, 19–36. [Google Scholar] [CrossRef]
- Song, G.; You, Y.; Jeong, W.; Lee, J.; Cho, Y.; Lee, S.; Ryu, S.; Lee, J.; Kim, S.; Yoo, I. Vasopressor requirement during targeted temperature management for out-of-hospital cardiac arrest caused by acute myocardial infarction without cardiogenic shock. Clin. Exp. Emerg. Med. 2016, 3, 20–26. [Google Scholar] [CrossRef]
- Trzeciak, S.; McCoy, J.V.; Dellinger, R.P.; Arnold, R.C.; Rizzuto, M.; Abate, N.L.; Shapiro, N.I.; Parrillo, J.E.; Hollenberg, S.M. Microcirculatory Alterations In on behalf of the Microcirculatory Alterations in Resuscitation and Shock (MARS) investigators Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensiv. Care Med. 2008, 34, 2210–2217. [Google Scholar] [CrossRef]
- Woo, J.-H.; Lim, Y.S.; Yang, H.J.; Hyun, S.Y.; Cho, J.S.; Kim, J.J.; Lee, G. The Relationship Between the Decreased Rate of Initial Blood Glucose and Neurologic Outcomes in Survivors of Out-of-Hospital Cardiac Arrest Receiving Therapeutic Hypothermia. Neurocritical Care 2016, 26, 402–410. [Google Scholar] [CrossRef]
- Reddy, S.; Weinberg, L.; Young, P. Crystalloid fluid therapy. Crit. Care 2016, 20, 59. [Google Scholar] [CrossRef]
- Kirkendol, R.L.; Pearson, J.E.; Bower, J.D.; Holbert, R.D. Myocardial depressant effects of sodium acetate. Cardiovasc. Res. 1978, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.D.; Bagshaw, S.M.; Goldstein, S.L.; Scherer, L.A.; Duan, M.; Schermer, C.R.; Kellum, J.A. Major Complications, Mortality, and Resource Utilization After Open Abdominal Surgery. Ann. Surg. 2012, 255, 821–829. [Google Scholar] [CrossRef]
- Handy, J.M.; Soni, N. Physiological effects of hyperchloraemia and acidosis. Br. J. Anaesth. 2008, 101, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; La Via, L.; Lanzafame, B.; Dezio, V.; Busalacchi, D.; Messina, A.; Ristagno, G.; Pelosi, P.; Astuto, M. Targeted Temperature Management after Cardiac Arrest: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. J. Clin. Med. 2021, 10, 3943. [Google Scholar] [CrossRef]
- Buijs, E.A.; Verboom, E.M.; Top, A.P.; Andrinopoulou, E.-R.; Buysse, C.M.; Ince, C.; Tibboel, D. Early microcirculatory impairment during therapeutic hypothermia is associated with poor outcome in post-cardiac arrest children: A prospective observational cohort study. Resuscitation 2013, 85, 397–404. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, B.K.; Lee, N.H.; Jung, Y.H.; Cho, Y.S.; Lee, S.M.; Lee, S.J.; Park, C.H.; Jeung, K.W. Association between lactate clearance during post-resuscitation care and neurologic outcome in cardiac arrest survivors treated with targeted temperature management. Clin. Exp. Emerg. Med. 2017, 4, 10–18. [Google Scholar] [CrossRef]
- Zhou, F.; Peng, Z.Y.; Bishop, J.V.; Cove, M.E.; Singbartl, K.; Kellum, J.A. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis*. Crit. Care Med. 2014, 42, e270–e278. [Google Scholar] [CrossRef]
- Mahler, S.A.; Conrad, S.A.; Wang, H.; Arnold, T.C. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am. J. Emerg. Med. 2011, 29, 670–674. [Google Scholar] [CrossRef]
- Kellum, J.A.; Song, M.; Venkataraman, R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest 2004, 125, 243–248. [Google Scholar] [CrossRef]
- Brown, R.M.; Semler, M.W. Fluid management in sepsis. J. Intensive Care Med. 2019, 34, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Chung, Y.E.; Lee, H.S.; You, J.S.; Chung, H.S.; Park, I.; Chung, S.P. Usefulness of chloride levels for fluid resuscitation in patients undergoing targeted temperature management after out-of-hospital cardiac arrest. Am. J. Emerg. Med. 2021, 43, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Jeung, K.W.; Kim, W.Y.; Park, Y.S.; Oh, J.S.; You, Y.H.; Lee, D.H.; Chae, M.K.; Jeong, Y.J.; Kim, M.C.; et al. 2020 Korean Guidelines for Cardiopulmonary Resuscitation. Part 5. Post-cardiac arrest care. Clin. Exp. Emerg. Med. 2021, 8, S41–S64. [Google Scholar] [CrossRef]
- Rabinstein, A.A. Treatment of cerebral edema. Neurologist 2006, 12, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Park, K.N.; Choi, S.P.; Lee, B.K.; Park, K.; Kim, J.; Kim, J.H.; Chung, S.P.; Hwang, S.O. Part 4. Post-cardiac arrest care: 2015 Korean Guidelines for Cardiopulmonary Resuscitation. Clin. Exp. Emerg. Med. 2016, 3, S27–S38. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Badenes, R.; Battaglini, D.; Ball, L.; Sanfilippo, F.; Brunetti, I.; Jakobsen, J.C.; Lilja, G.; Friberg, H.; Wendel-Garcia, P.D.; et al. Oxygen targets and 6-month outcome after out of hospital cardiac arrest: A pre-planned sub-analysis of the targeted hypothermia versus targeted normothermia after Out-of-Hospital Cardiac Arrest (TTM2) trial. Crit. Care 2022, 26, 323. [Google Scholar] [CrossRef]
- LA Via, L.; Astuto, M.; Bignami, E.G.; Busalacchi, D.; Dezio, V.; Girardis, M.; Lanzafame, B.; Ristagno, G.; Pelosi, P.; Sanfilippo, F. The effects of exposure to severe hyperoxemia on neurological outcome and mortality after cardiac arrest. Minerva Anestesiol. 2022, 88, s0375–s9393. [Google Scholar]
- Monnet, X.; Marik, P.; Teboul, J.L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- La Via, L.; Astuto, M.; Dezio, V.; Muscarà, L.; Palella, S.; Zawadka, M.; Vignon, P.; Sanfilippo, F. Agreement between subcostal and transhepatic longitudinal imaging of the inferior vena cava for the evaluation of fluid responsiveness: A systematic review. J. Crit. Care 2022, 71, 154108. [Google Scholar] [CrossRef]
- Alvarado Sanchez, J.I.; Caicedo Ruiz, J.D.; Diaztagle Fernandez, J.J.; Amaya Zuniga, W.F.; Ospina-Tascon, G.A.; Cruz Martinez, L.E. Predictors of fluid responsiveness in critically ill patients mechanically ventilated at low tidal volumes: Systematic review and meta-analysis. Ann. Intensive Care 2021, 11, 28. [Google Scholar] [CrossRef]
- Woo, J.H.; Cho, J.S.; Lee, C.A.; Kim, G.W.; Kim, Y.J.; Moon, H.J.; Park, Y.J.; Lee, K.M.; Jeong, W.J.; Choi, I.K.; et al. Survival and rearrest in out-of-hospital cardiac arrest patients with prehospital return of spontaneous circulation: A prospective multi-regional observational study. Prehospital Emerg. Care 2021, 25, 59–66. [Google Scholar]
- Kim, G.W.; Moon, H.J.; Lim, H.; Kim, Y.J.; Lee, C.A.; Park, Y.J.; Lee, K.M.; Woo, J.H.; Cho, J.S.; Jeong, W.J. Effects of Smart Advanced Life Support protocol implementation including CPR coaching during out-of-hospital cardiac arrest. Am. J. Emerg. Med. 2022, 56, 211–217. [Google Scholar]
- Tintinalli, J.E.; Ma, O.J.; Yealy, D.M.; Meckler, G.D.; Stapczynski, J.S.; Cline, D.M.; Thomas, S.H. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 8th ed.; McGraw Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. Section 5: Dialysis interventions for treatment of AKI. Kidney Int. Suppl. 2012, 2, 89–115. [Google Scholar]

| Variables | Saline Group (n = 27) | PS Group (n = 26) | p-Value |
|---|---|---|---|
| Age (years) | 59.0 ± 15.3 | 61.7 ± 14.9 | 0.526 |
| Sex (male), n (%) | 16 (59.3) | 18 (69.2) | 0.449 |
| Location of arrest (residence), n (%) | 13 (48.1) | 15 (57.7) | 0.487 |
| Bystander CPR, n (%) | 13 (48.1) | 15 (57.7) | 0.593 |
| Cardiac etiology, n (%) | 18 (66.7) | 19 (73.1) | 0.899 |
| Initial rhythm (shockable), n (%) | 13 (48.1) | 17 (65.4) | 0.206 |
| Interval from collapse to BLS (min) | 5.1 ± 4.5 | 4.9 ± 4.8 | 0.862 |
| Interval from BLS to ROSC (min) | 14.0 (9.0, 21.5) | 14.0 (9.0, 21.0) | 0.845 |
| Interval from collapse to ED arrival (min) | 26.9 ± 12.6 | 30.3 ± 11.7 | 0.317 |
| ROSC before ED arrival (yes), n (%) | 17 (63.0) | 21 (80.8) | 0.150 |
| Pre-existing illness, n (%) | |||
| Diabetes mellitus | 9 (33.3) | 6 (23.1) | 0.407 |
| Hypertension | 17 (63.0) | 17 (65.4) | 0.854 |
| CVA | 4 (14.8) | 1 (3.8) | 0.351 |
| Cardiac disease | 10 (37.0) | 7 (26.9) | 0.430 |
| Cancer | 1 (3.7) | 1 (3.8) | 1.000 |
| ACLS in prehospital settings (yes), n (%) | 13 (48.1) | 15 (57.7) | 0.487 |
| Fluid administration in prehospital settings (yes), n (%) | 12 (44.4) | 12 (46.2) | 0.901 |
| Fluids administered in prehospital settings (saline), n (%) | 12 (44.4) | 12 (46.2) | 0.901 |
| Volume of fluid administered in prehospital settings (mL) | 0.0 (0.0, 400.0) | 0.0 (0.0, 300.0) | 0.961 |
| In-hospital treatments within 24 h | |||
| Cumulative volume of the allocated crystalloid administered (mL) | 4029.0 (3562.0, 5650.0) | 3917.5 (3640.0, 5420.0) | 0.742 |
| Administration of diuretics (yes), n (%) | 5 (18.5) | 3 (11.5) | 0.704 |
| Administration of insulin (yes), n (%) | 11 (40.7) | 9 (34.6) | 0.646 |
| Administration of sodium bicarbonate (yes), n (%) | 0 (0.0) | 0 (0.0) | N/C |
| Intravenous potassium replacement (yes), n (%) | 3 (11.1) | 1 (3.8) | 0.610 |
| Intravenous calcium replacement (yes), n (%) | 3 (11.1) | 2 (7.7) | 1.000 |
| Intravenous magnesium replacement (yes), n (%) | 3 (11.1) | 1 (3.8) | 0.610 |
| Intravenous phosphate replacement (yes), n (%) | 1 (3.7) | 0 (0.0) | 1.000 |
| Transfusion (yes), n (%) | 2 (7.4) | 1 (3.8) | 1.000 |
| Targeted temperature management, n (%) | 24 (88.9) | 24 (92.3) | 1.000 |
| Variables | Saline Group (n = 27) | PS Group (n = 26) | Absolute Difference (95% CI) † | p-Value |
|---|---|---|---|---|
| Base excess | ||||
| at baseline (mmol/L) | −14.9 (−16.3, −9.3) | −12.8 (−15.4, −8.8) | −0.1 (−4.5 to 4.3) | 0.595 * |
| at 6 h (mmol/L) | −7.9 ± 5.5 | −4.1 ± 4.9 | 3.7 (0.8 to 6.6) | 0.032 * |
| at 24 h (mmol/L) | −7.2 ± 6.1 | −3.4 ± 5.5 | 3.9 (0.6 to 7.1) | 0.036 * |
| Increase in base excess (0–2 h, mmol/L) | 3.9 ± 7.3 | 8.4 ± 4.6 | 4.5 (0.7 to 8.3) | 0.021 |
| Increase in base excess (0–24 h, mmol/L) | 5.5 (1.4, 8.7) | 9.4 (5.9, 12.2) | 5.2 (1.2 to 9.2) | 0.025 |
| Arterial HCO3− | ||||
| at baseline (mmol/L) | 14.1 ± 4.5 | 14.8 ± 5.4 | 0.7 (−2.2 to 3.7) | 0.625 * |
| at 6 h (mmol/L) | 17.8 ± 5.1 | 20.5 ± 4.1 | 2.7 (0.1 to 5.3) | 0.062 * |
| at 24 h (mmol/L) | 18.8 (15.0, 20.1) | 20.2 (17.7, 22.8) | 2.5 (−0.2 to 5.3) | 0.062 * |
| Increase in arterial HCO3− (0–2 h, mmol/L) | 4.0 ± 3.8 | 6.8 ± 3.5 | 2.8 (0.6 to 5.0) | 0.015 |
| Increase in arterial HCO3− (0–24 h, mmol/L) | 2.8 ± 5.1 | 5.9 ± 4.3 | 3.0 (0.2 to 5.8) | 0.036 |
| Arterial pH | ||||
| at baseline | 7.08 ± 0.19 | 7.11 ± 0.18 | 0.03 (−0.08 to 0.13) | 0.620 * |
| at 6 h | 7.29 ± 0.11 | 7.35 ± 0.12 | 0.07 (0.00 to 0.13) | 0.108 * |
| at 24 h | 7.30 ± 0.11 | 7.38 ± 0.11 | 0.08 (0.02 to 0.14) | 0.099 * |
| Increase in arterial pH (0–2 h) | 0.18 ± 0.15 | 0.20 ± 0.18 | 0.02 (−0.08 to 0.11) | 0.718 |
| Increase in arterial pH (0–24 h) | 0.20 ± 0.17 | 0.27 ± 0.15 | 0.06 (−0.03 to 0.16) | 0.169 |
| Serum chloride | ||||
| at baseline (mEq/L) | 101.9 ± 6.3 | 101.1 ± 5.1 | −0.8 (−4.0 to 2.3) | 0.593 * |
| at 6 h (mEq/L) | 105.9 ± 6.3 | 100.7 ± 5.0 | −5.2 (−8.4 to −2.1) | 0.003 * |
| at 24 h (mEq/L) | 108.6 ± 5.7 | 99.8 ± 4.8 | −8.8 (−11.7 to −5.9) | <0.001 * |
| Change in serum chloride (0–24 h, mEq/L) | 7.0 (2.0, 9.5) | −1.0 (−3.0, 0.0) | −8.0 (−10.3 to −5.6) | <0.001 |
| Increase in serum chloride (0–24 h, yes), n (%) | 25 (92.6) | 5 (19.2) | −73.4 (−91.5 to −55.3) | <0.001 |
| Development of hyperchloremia within 24 h (yes), n (%) | 13 (48.1) | 0 (0.0) | N/C | <0.001 |
| Development of hypernatremia within 24 h (yes), n (%) | 1 (3.7) | 0 (0.0) | N/C | 1.000 |
| Development of hyperosmolar state within 24 h (yes), n (%) | 4 (14.8) | 4 (15.4) | 0.6 (−18.7 to 19.9) | 1.000 |
| Development of hypoosmolar state within 24 h (yes), n (%) | 16 (59.3) | 19 (73.1) | 13.8 (−11.4 to 39.0) | 0.288 |
| Serum creatinine | ||||
| at baseline (mg/dL) | 1.2 (0.9, 1.3) | 1.1 (0.9, 1.3) | −0.2 (−0.4 to 0.0) | 0.434 |
| at 24 h (mg/dL) | 0.7 (0.6, 1.0) | 0.7 (0.5, 0.9) | −0.2 (−0.6 to 0.1) | 0.413 |
| Change in serum creatinine (0–24 h, mg/dL) | −0.4 (−0.5, −0.2) | −0.4 (−0.5, −0.2) | −0.1 (−0.3 to 0.2) | 0.859 |
| Variables | Saline Group (n = 27) | PS Group (n = 26) | Absolute Difference (95% CI) † | p-Value |
|---|---|---|---|---|
| SBP at ICU admission (mmHg) | 125.9 ± 40.9 | 126.9 ± 46.8 | 1.0 (−23.2 to 25.2) | 0.934 |
| at 6 h after ED visit (mmHg) | 110.1 ± 28.0 | 123.9 ± 34.4 | 13.8 (−3.5 to 31.0) | 0.115 |
| at 12 h after ED visit (mmHg) | 121.3 ± 29.1 | 118.4 ± 29.4 | −2.9 (−19.0 to 13.2) | 0.722 |
| at 24 h after ED visit (mmHg) | 119.6 ± 21.7 | 115.2 ± 27.0 | −4.4 (−17.9 to 9.1) | 0.512 |
| Highest SBP within 24 h (mmHg) | 171.0 (157.5, 203.5) | 173.5 (151.0, 196.0) | −6.4 (−25.5 to 12.6) | 0.702 |
| Lowest SBP within 24 h (mmHg) | 71.4 ± 15.4 | 75.3 ± 15.0 | 3.9 (−4.5 to 12.3) | 0.355 |
| HR at ICU admission (per min) | 91.1 ± 25.8 | 89.5 ± 23.6 | −1.6 (−15.3 to 12.1) | 0.814 |
| at 6 h after ED visit (per min) | 79.7 ± 29.7 | 83.5 ± 24.5 | 3.8 (−11.3 to 18.8) | 0.615 |
| at 12 h after ED visit (per min) | 74.0 ± 24.9 | 77.2 ± 22.2 | 3.2 (−9.8 to 16.2) | 0.625 |
| at 24 h after ED visit (per min) | 70.0 (58.0, 85.0) | 70.0 (62.0, 80.0) | −4.1 (−16.2 to 7.9) | 0.624 |
| Highest HR within 24 h (per min) | 115.3 ± 24.1 | 125.8 ± 27.1 | 10.5 (−3.6 to 24.6) | 0.142 |
| Lowest HR within 24 h (per min) | 60.0 (45.5, 80.0) | 53.0 (46.0, 66.0) | −3.3 (−16.1 to 9.5) | 0.682 |
| CVI at ICU admission | 0.0 (0.0, 3.0) | 0.0 (0.0, 4.0) | 1.0 (−0.4 to 2.5) | 0.274 |
| at 6 h after ED visit | 0.0 (0.0, 4.0) | 2.0 (0.0, 4.0) | 0.3 (−1.0 to 1.6) | 0.644 |
| at 12 h after ED visit | 2.0 (0.0, 4.0) | 2.0 (0.0, 4.0) | 0.4 (−1.4 to 2.2) | 0.904 |
| at 24 h after ED visit | 2.0 (0.0, 3.5) | 1.5 (0.0, 4.0) | 0.0 (−1.9 to 1.8) | 0.661 |
| Total urine output within 24 h (mL) | 3220.5 ± 1919.0 | 3686.8 ± 2235.2 | 466.3 (−681.1 to 1613.7) | 0.418 |
| Development of AKI within 72 h, n (%) | 11 (40.7) | 7 (26.9) | −13.8 (−39.0 to 11.4) | 0.288 |
| Development of MAKE30, n (%) | 14 (51.9) | 7 (26.9) | −24.9 (−50.3 to 0.5) | 0.064 |
| Survival to hospital discharge, n (%) | 14 (51.9) | 19 (73.1) | 21.2 (−4.2 to 46.6) | 0.111 |
| Survival at 6 months, n (%) | 14 (51.9) | 17 (65.4) | 13.5 (−12.7 to 39.8) | 0.318 |
| CPC 1, 2 at discharge, n (%) | 13 (48.1) | 17 (65.4) | 17.2 (−9.0 to 43.5) | 0.206 |
| CPC 1, 2 at 6 months, n (%) | 13 (48.1) | 16 (61.5) | 13.4 (−13.2 to 39.9) | 0.328 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, J.-H.; Lim, Y.S.; Cho, J.S.; Yang, H.J.; Jang, J.H.; Choi, J.Y.; Choi, W.S. Saline versus Plasma Solution-A in Initial Resuscitation of Patients with Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. J. Clin. Med. 2023, 12, 5040. https://doi.org/10.3390/jcm12155040
Woo J-H, Lim YS, Cho JS, Yang HJ, Jang JH, Choi JY, Choi WS. Saline versus Plasma Solution-A in Initial Resuscitation of Patients with Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. Journal of Clinical Medicine. 2023; 12(15):5040. https://doi.org/10.3390/jcm12155040
Chicago/Turabian StyleWoo, Jae-Hyug, Yong Su Lim, Jin Seong Cho, Hyuk Jun Yang, Jae Ho Jang, Jea Yeon Choi, and Woo Sung Choi. 2023. "Saline versus Plasma Solution-A in Initial Resuscitation of Patients with Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial" Journal of Clinical Medicine 12, no. 15: 5040. https://doi.org/10.3390/jcm12155040
APA StyleWoo, J.-H., Lim, Y. S., Cho, J. S., Yang, H. J., Jang, J. H., Choi, J. Y., & Choi, W. S. (2023). Saline versus Plasma Solution-A in Initial Resuscitation of Patients with Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. Journal of Clinical Medicine, 12(15), 5040. https://doi.org/10.3390/jcm12155040







