Comparable and Complimentary Modalities for Treatment of Small-Sized HCC: Surgical Resection, Radiofrequency Ablation, and Microwave Ablation
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Zhao, Y.; Li, B.; Xu, D.; Yin, Z.; Xie, F.; Yang, J. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular Carcinoma Incidence, Mortality, and Survival Trends in the United States From 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, N.N.; Park, J.O.; Farjah, F.; Yeung, R.S.W.; Symons, R.G.; Vaughan, T.L.; Baldwin, L.M.; Flum, D.R. Trends in the Utilization and Impact of Radiofrequency Ablation for Hepatocellular Carcinoma. J. Am. Coll. Surg. 2010, 210, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Dong, B.; Yu, X.; Yu, D.; Wang, Y.; Feng, L.; Xiao, Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005, 235, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Yu, J.; Yu, X.L.; Wang, X.H.; Wei, Q.; Yu, S.Y.; Li, H.X.; Sun, H.T.; Zhang, Z.X.; Liu, H.C.; et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: A multicentre analysis of 1363 treatment-naïve lesions in 1007 patients in China. Gut 2012, 61, 1100–1101. [Google Scholar] [CrossRef]
- Dou, J.P.; Yu, J.; Yang, X.H.; Cheng, Z.G.; Han, Z.Y.; Liu, F.Y.; Yu, X.L.; Liang, P. Outcomes of microwave ablation for hepatocellular carcinoma adjacent to large vessels: A propensity score analysis. Oncotarget 2017, 8, 28758–28768. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, Q.; Wang, N.; Wu, P.P.; Huang, B.; Kuang, M.; Qian, G.J. Microwave ablation is as effective as radiofrequency ablation for very-early-stage hepatocellular carcinoma. Chin. J. Cancer 2017, 36, 14. [Google Scholar] [CrossRef]
- Thamtorawat, S.; Hicks, R.M.; Yu, J.; Siripongsakun, S.; Lin, W.C.; Raman, S.S.; McWilliams, J.P.; Douek, M.; Bahrami, S.; Lu, D.S. Preliminary Outcome of Microwave Ablation of Hepatocellular Carcinoma: Breaking the 3-cm Barrier? J. Vasc. Interv. Radiol. 2016, 27, 623–630. [Google Scholar] [CrossRef]
- Sun, A.X.; Cheng, Z.L.; Wu, P.P.; Sheng, Y.H.; Qu, X.J.; Lu, W.; Zhao, C.G.; Qian, G.J. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J. Gastroenterol. 2015, 21, 2997–3004. [Google Scholar] [CrossRef]
- Ziemlewicz, T.J.; Hinshaw, J.L.; Lubner, M.G.; Brace, C.L.; Alexander, M.L.; Agarwal, P.; Lee, F.T., Jr. Percutaneous microwave ablation of hepatocellular carcinoma with a gas-cooled system: Initial clinical results with 107 tumors. J. Vasc. Interv. Radiol. 2015, 26, 62–68. [Google Scholar] [CrossRef][Green Version]
- Baker, E.H.; Thompson, K.; McKillop, I.H.; Cochran, A.; Kirks, R.; Vrochides, D.; Martinie, J.B.; Swan, R.Z.; Iannitti, D.A. Operative microwave ablation for hepatocellular carcinoma: A single center retrospective review of 219 patients. J. Gastrointest. Oncol. 2017, 8, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.; Elbaz, T.; Shousha, H.I.; Mahmoud, S.; Ibrahim, M.; Abdelmaksoud, A.; Nabeel, M. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: An Egyptian multidisciplinary clinic experience. Surg. Endosc. 2014, 28, 3429–3434. [Google Scholar] [CrossRef]
- Wang, T.; Lu, X.J.; Chi, J.C.; Ding, M.; Zhang, Y.; Tang, X.Y.; Li, P.; Zhang, L.; Zhang, X.Y.; Zhai, B. Microwave ablation of hepatocellular carcinoma as first-line treatment: Long term outcomes and prognostic factors in 221 patients. Sci. Rep. 2016, 6, 32728. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, Q.; Wang, N.; Liu, P.; Wu, P.; Peng, Z.; Qian, G. Percutaneous microwave ablation of 5-6 cm unresectable hepatocellular carcinoma: Local efficacy and long-term outcomes. Int. J. Hyperth. 2017, 33, 247–254. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, N.; Shen, Q.; Cheng, W.; Qian, G.J. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS ONE 2013, 8, e76119. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Li, W.; Zhao, P.; Wang, Y.; Zheng, J. Treatment efficacy of CT-guided percutaneous microwave ablation for primary hepatocellular carcinoma. Clin. Radiol. 2017, 72, 136–140. [Google Scholar] [CrossRef]
- Yang, W.; Yan, K.; Wu, G.X.; Wu, W.; Fu, Y.; Lee, J.C.; Zhang, Z.Y.; Wang, S.; Chen, M.H. Radiofrequency ablation of hepatocellular carcinoma in difficult locations: Strategies and long-term outcomes. World J. Gastroenterol. 2015, 21, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.M.; Lee, J.Y.; Kim, S.H.; Yoon, J.H.; Kim, Y.J.; Han, J.K.; Choi, B.I. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: Long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014, 270, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Osaki, Y.; Nishikawa, H. Treatment for hepatocellular carcinoma in Japan over the last three decades: Our experience and published work review. Hepatol. Res. 2015, 45, 59–74. [Google Scholar] [CrossRef]
- Lencioni, R.; Cioni, D.; Crocetti, L.; Franchini, C.; Pina, C.D.; Lera, J.; Bartolozzi, C. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005, 234, 961–967. [Google Scholar] [CrossRef]
- Tateishi, R.; Shiina, S.; Teratani, T.; Obi, S.; Sato, S.; Koike, Y.; Fujishima, T.; Yoshida, H.; Kawabe, T.; Omata, M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005, 103, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Yan, K.; Yang, W.; Gao, W.; Dai, Y.; Huo, L.; Zhang, H.; Huang, X.F. Long term (5 years) outcome of radiofrequency ablation for hepatocellular carcinoma in 256 cases. Beijing Da Xue Xue Bao Yi Xue Ban 2005, 37, 671–672. (In Chinese) [Google Scholar] [PubMed]
- Yoon, S.K.; Chun, H.G. Status of hepatocellular carcinoma in South Korea. Chin. Clin. Oncol. 2013, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef]
- N’Kontchou, G.; Mahamoudi, A.; Aout, M.; Ganne-Carrié, N.; Grando, V.; Coderc, E.; Vicaut, E.; Trinchet, J.C.; Sellier, N.; Beaugrand, M.; et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009, 50, 1475–1483. [Google Scholar] [CrossRef]
- Shiina, S.; Teratani, T.; Obi, S.; Sato, S.; Tateishi, R.; Fujishima, T.; Ishikawa, T.; Koike, Y.; Yoshida, H.; Kawabe, T.; et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005, 129, 122–130. [Google Scholar] [CrossRef]
- Brunello, F.; Veltri, A.; Carucci, P.; Pagano, E.; Ciccone, G.; Moretto, P.; Sacchetto, P.; Gandini, G.; Rizzetto, M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand. J. Gastroenterol. 2008, 43, 727–735. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Cheng, Z.; Wu, H.; Du, L.; Wang, J.; Xu, Y.; Zeng, Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 2010, 252, 903–912. [Google Scholar] [CrossRef]
- Feng, K.; Yan, J.; Li, X.; Xia, F.; Ma, K.; Wang, S.; Bie, P.; Dong, J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 2012, 57, 794–802. [Google Scholar] [CrossRef]
- Vivarelli, M.; Guglielmi, A.; Ruzzenente, A.; Cucchetti, A.; Bellusci, R.; Cordiano, C.; Cavallari, A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann. Surg. 2004, 240, 102–107. [Google Scholar] [CrossRef]
- Choi, D.; Lim, H.K.; Rhim, H.; Kim, Y.S.; Lee, W.J.; Paik, S.W.; Koh, K.C.; Lee, J.H.; Choi, M.S.; Yoo, B.C. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: Long-term results and prognostic factors in a large single-institution series. Eur. Radiol. 2007, 17, 684–692. [Google Scholar] [CrossRef]
- Hiraoka, A.; Horiike, N.; Yamashita, Y.; Koizumi, Y.; Doi, K.; Yamamoto, Y.; Hasebe, A.; Ichikawa, S.; Yano, M.; Miyamoto, Y.; et al. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology 2008, 55, 2171–2174. [Google Scholar]
- Yun, W.K.; Choi, M.S.; Choi, D.; Rhim, H.C.; Joh, J.W.; Kim, K.H.; Jang, T.H.; Lee, J.H.; Koh, K.C.; Paik, S.W.; et al. Superior long-term outcomes after surgery in child-pugh class a patients with single small hepatocellular carcinoma compared to radiofrequency ablation. Hepatol. Int. 2011, 5, 722–729. [Google Scholar] [CrossRef]
- Hung, H.H.; Chiou, Y.Y.; Hsia, C.Y.; Su, C.W.; Chou, Y.H.; Chiang, J.H.; Kao, W.Y.; Huo, T.I.; Huang, Y.H.; Su, Y.H.; et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin. Gastroenterol. Hepatol. 2011, 9, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Inuzuka, T.; Takeda, H.; Nakajima, J.; Matsuda, F.; Sakamoto, A.; Henmi, S.; Hatamaru, K.; Ishikawa, T.; Saito, S.; et al. Comparison of percutaneous radiofrequency thermal ablation and surgical resection for small hepatocellular carcinoma. BMC Gastroenterol. 2011, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Wang, C.C.; Hung, C.H.; Chen, C.L.; Lu, S.N. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J. Hepatol. 2012, 56, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.W.; Lin, X.J.; Zhang, Y.J.; Liang, H.H.; Guo, R.P.; Shi, M.; Chen, M.S. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: A retrospective comparative study. Radiology 2012, 262, 1022–1033. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kokudo, N.; Makuuchi, M.; Izumi, N.; Ichida, T.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; Matsui, O.; et al. Comparison of resection and ablation for hepatocellular carcinoma: A cohort study based on a Japanese nationwide survey. J. Hepatol. 2013, 58, 724–729, Erratum in: J. Hepatol. 2013, 59, 641. [Google Scholar] [CrossRef]
- Nagasue, N.; Kohno, H.; Chang, Y.C.; Taniura, H.; Yamanoi, A.; Uchida, M.; Kimoto, T.; Takemoto, Y.; Nakamura, T.; Yukaya, H. Liver resection for hepatocellular carcinoma. Results of 229 consecutive patients during 11 years. Ann. Surg. 1993, 217, 375–384. [Google Scholar] [CrossRef]
- Takenaka, K.; Kawahara, N.; Yamamoto, K.; Kajiyama, K.; Maeda, T.; Itasaka, H.; Shirabe, K.; Nishizaki, T.; Yanaga, K.; Sugimachi, K. Results of 280 liver resections for hepatocellular carcinoma. Arch. Surg. 1996, 131, 71–76. [Google Scholar] [CrossRef]
- Makuuchi, M.; Takayama, T.; Kubota, K.; Kimura, W.; Midorikawa, Y.; Miyagawa, S.; Kawasaki, S. Hepatic resection for hepatocellular carcinoma—Japanese experience. Hepatogastroenterology 1998, 45 (Suppl. S3), 1267–1274. [Google Scholar] [PubMed]
- Shimozawa, N.; Hanazaki, K. Longterm prognosis after hepatic resection for small hepatocellular carcinoma. J. Am. Coll. Surg. 2004, 198, 356–365. [Google Scholar] [CrossRef]
- Franco, D.; Capussotti, L.; Smadja, C.; Bouzari, H.; Meakins, J.; Kemeny, F.; Grange, D.; Dellepiane, M. Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology 1990, 98, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, J.; Regimbeau, J.M.; Durand, F.; Kianmanesh, A.R.; Dondero, F.; Terris, B.; Sauvanet, A.; Farges, O.; Degos, F. Resection of hepatocellular carcinoma: A European experience on 328 cases. Hepatogastroenterology 2002, 49, 41–46. [Google Scholar] [PubMed]
- Ercolani, G.; Grazi, G.L.; Ravaioli, M.; Del Gaudio, M.; Gardini, A.; Cescon, M.; Varotti, G.; Cetta, F.; Cavallari, A. Liver resection for hepatocellular carcinoma on cirrhosis: Univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann. Surg. 2003, 237, 536–543. [Google Scholar] [CrossRef]
- Cha, C.; Fong, Y.; Jarnagin, W.R.; Blumgart, L.H.; DeMatteo, R.P. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J. Am. Coll. Surg. 2003, 197, 753–758. [Google Scholar] [CrossRef]
- Ringe, B.; Pichlmayr, R.; Wittekind, C.; Tusch, G. Surgical treatment of hepatocellular carcinoma: Experience with liver resection and transplantation in 198 patients. World J. Surg. 1991, 15, 270–285. [Google Scholar] [CrossRef]
- Pitre, J.; Houssin, D.; Kracht, M. Resection of hepatocellular carcinomas. Analysis of prognostic factors of a multicenter series of 153 patients. Gastroenterol. Clin. Biol. 1993, 17, 200–206. [Google Scholar]
- Di Carlo, V.; Ferrari, G.; Castoldi, R.; Nadalin, S.; Marenghi, C.; Molteni, B.; Taccagni, G.; Castrucci, M. Surgical treatment and prognostic variables of hepatocellular carcinoma in 122 cirrhotics. Hepatogastroenterology 1995, 42, 222–229. [Google Scholar]
- Lise, M.; Bacchetti, S.; Da Pian, P.; Nitti, D.; Pilati, P.L.; Pigato, P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: Results in a series of 100 Italian patients. Cancer 1998, 82, 1028–1036. [Google Scholar] [CrossRef]
- Grazi, G.L.; Ercolani, G.; Pierangeli, F.; Del Gaudio, M.; Cescon, M.; Cavallari, A.; Mazziotti, A. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann. Surg. 2001, 234, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.; Fan, S.T.; Lo, C.M.; Ng, I.O.; Liu, C.L.; Lam, C.M.; Wong, J. Improving survival results after resection of hepatocellular carcinoma: A prospective study of 377 patients over 10 years. Ann. Surg. 2001, 234, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kanematsu, T.; Furui, J.; Yanaga, K.; Okudaira, S.; Shimada, M.; Shirabe, K. A 16-year experience in performing hepatic resection in 303 patients with hepatocellular carcinoma: 1985–2000. Surgery 2002, 131 (Suppl. S1), S153–S158. [Google Scholar] [CrossRef]
- Wayne, J.D.; Lauwers, G.Y.; Ikai, I.; Doherty, D.A.; Belghiti, J.; Yamaoka, Y.; Regimbeau, J.M.; Nagorney, D.M.; Do, K.A.; Ellis, L.M.; et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann. Surg. 2002, 235, 722–730; discussion 730–731. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.N.; Chen, M.F.; Lee, W.C.; Jeng, L.B. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: Univariate and multivariate analysis. J. Surg. Oncol. 2002, 81, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Capussotti, L.; Muratore, A.; Amisano, M.; Polastri, R.; Bouzari, H.; Massucco, P. Liver resection for hepatocellular carcinoma on cirrhosis: Analysis of mortality, morbidity and survival--a European single center experience. Eur. J. Surg. Oncol. 2005, 31, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.; Schulick, R.D.; Choti, M.A.; Pawlik, T.M. Predictors of survival after resection of early hepatocellular carcinoma. Ann. Surg. 2009, 249, 799–805. [Google Scholar] [CrossRef]
- Arii, S.; Yamaoka, Y.; Futagawa, S.; Inoue, K.; Kobayashi, K.; Kojiro, M.; Makuuchi, M.; Nakamura, Y.; Okita, K.; Yamada, R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: A retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 2000, 32, 1224–1229. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, H.; Zhang, J.; He, L.; Ye, X.; Li, X. Comparison of microwave ablation and hepatic resection for hepatocellular carcinoma: A meta-analysis. OncoTargets Ther. 2017, 10, 4829–4839. [Google Scholar] [CrossRef]
- Cucchetti, A.; Piscaglia, F.; Cescon, M.; Ercolani, G.; Pinna, A.D. Systematic review of surgical resection vs radiofrequency ablation for hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 4106–4118. [Google Scholar] [CrossRef]
- Malagó, M.; Sotiropoulos, G.C.; Nadalin, S.; Valentin-Gamazo, C.; Paul, A.; Lang, H.; Radtke, A.; Saner, F.; Molmenti, E.; Beckebaum, S.; et al. Living donor liver transplantation for hepatocellular carcinoma: A single-center preliminary report. Liver Transplant. 2006, 12, 934–940. [Google Scholar] [CrossRef]
- Zamora-Valdes, D.; Taner, T.; Nagorney, D.M. Surgical Treatment of Hepatocellular Carcinoma. Cancer Control 2017, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shamim, F.; Asghar, A.; Tauheed, S.; Yahya, M. Radiofrequency ablation of hepatocellular carcinomas: A new spectrum of anesthetic experience at a tertiary care hospital in Pakistan. Saudi J. Anaesth. 2017, 11, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-x.; Huang, J.-w.; Liao, M.-h.; Zeng, Y. Treatment strategy for hepatocellular carcinoma in China: Radiofrequency ablation versus liver resection. Jpn. J. Clin. Oncol. 2016, 46, 1075–1080. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, E.C.H.; Tang, C.N. Radiofrequency ablation versus hepatic resection for hepatocellular carcinoma within the Milan criteria—A comparative study. Int. J. Surg. 2013, 11, 77–80. [Google Scholar] [CrossRef]
- Poggi, G.; Tosoratti, N.; Montagna, B.; Picchi, C. Microwave ablation of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 2578–2589. [Google Scholar] [CrossRef]
- Shin, S.W.; Ahn, K.S.; Kim, S.W.; Kim, T.S.; Kim, Y.H.; Kang, K.J. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma within the Milan Criteria: A Systematic Review and Meta-Analysis. Ann. Surg. 2021, 273, 656–666. [Google Scholar] [CrossRef]
- Takayama, T.; Hasegawa, K.; Izumi, N.; Kudo, M.; Shimada, M.; Yamanaka, N.; Inomata, M.; Kaneko, S.; Nakayama, H.; Kawaguchi, Y.; et al. Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial). Liver Cancer 2022, 11, 209–218. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Zhang, D.Z.; Wu, S.S.; Hong, Z.X.; He, G.B.; Yang, H.; Xiang, B.D.; Li, X.; Jiang, T.A.; et al. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3–5-cm HCC. Hepatology 2022, 76, 66–77. [Google Scholar] [CrossRef] [PubMed]





| Region | Number of Patients | Median Follow-Up Period (mo.) | Mean Tumor Size (cm) | Overall Survival Rates | Major Complication Rate (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1-Year (%) | 2-Year (%) | 3-Year (%) | 4-Year (%) | 5-Year (%) | |||||
| Liang [4] | 288 | 31.41 | 93 | 82 | 72 | 63 | 51 | ||
| Liang [5] | 1007 | 38.0 | 91.2 | 72.5 | 59.8 | ||||
| Jian-Ping [6] | 293 113 | 29.3 | 2.6 | 98.1 98.0 | 73.7 82.0 | 48.2 46.9 | 3.2 | ||
| Xu [7] | 301 | 53.0 | 1.7 | 99.3 | 90.4 | 78.3 | 0.7 | ||
| Thamtorawat [8] | 129 | 11.8 | 91.3 | 81.7 | 2.2 | ||||
| Sun [9] | 182 | 3.7 | 89 | 74 | 60 | 2.7 | |||
| Ziemlewicz [10] | 75 | 14.0 12.0 | 2.1 3.7 | 76 | 0 | ||||
| Baker [11] | 219 | 10.9 | 3.2 | 80 | 61.5 | 3.2 | |||
| Abdelaziz [12] | 66 | <5 | 91.6 | 86.1 | 3.2 | ||||
| Wang [13] | 221 | 41.0 | <5 | 87.1 | 63.2 | 3.8 | |||
| Xu [14] | 82 | <5 | 92.7 | 63.4 | 41.1 | 3.7 | |||
| Zhang [15] | 77 | <5 | 92.2 | 51.7 | 38.5 | 2.6 | |||
| Yin [16] | 220 | 95.5 | 89.1 | ||||||
| Total/Mean | 3273 | 27.9 | 91.1 | 76.3 | 69.1 | 63.0 | 50.7 | 2.5 | |
| Region | Number of Patients | Median Follow-Up Period (mo.) | Mean Tumor Size (cm) | Overall Survival Rates | Major Complication Rate (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1-Year (%) | 2-Year (%) | 3-Year (%) | 4-Year (%) | 5-Year (%) | |||||
| Yang [17] | 382 88 | 28.0 | 3.4 | 84.3 92.5 | 54.4 60.3 | 41.2 43.2 | 4.9 0.8 | ||
| Lee [18] | 162 | 49.0 | 2.59 | 94.4 | 84.1 | 67.9 | 3.1 | ||
| Osaki [19] | 1161 | <4 | 96.0 | 76.2 | 56.0 | ||||
| Lencioni [20] | 206 | 24.0 | <5 | 97.0 | 71.0 | 48.0 | |||
| Tateishi [21] | 319 | <5 | 94.7 | 86.1 | 77.7 | 67.4 | 54.3 | 4.0 | |
| Chen [22] | 256 | 53.0 | 1.7 | 83.3 | 66.9 | 41.2 | 2.4 | ||
| Yoon [23] | 570 | <5 | 95.2 | 82.9 | 69.5 | 60.8 | 58.0 | 1.9 | |
| Livraghi [24] | 218 | 31.0 | <4 | 68.5 | 1.8 | ||||
| N’Kontc [25] | 235 | 27.0 | 3.0 | 60.0 | 40.0 | 0.9 | |||
| Shiina [26] | 118 | 37.0 | <4 | 81.0 | 74.0 | 3.2 | |||
| Brunell [27] | 70 | <3 | 63.0 | 14 | |||||
| Huang [28] | 115 | <5 | 87.0 | 76.5 | 69.6 | 66.1 | 54.8 | ||
| Feng [29] | 84 | <4 | 93.1 | 83.1 | 67.2 | ||||
| Viva [30] | 79 | 15.6 | <5 | 78 | 33 | ||||
| Cho [31] | 99 | 3.1 | 95.8 | 86.8 | 80 | ||||
| Hiraok [32] | 105 | 2.0 | 87.8 | 59.3 | 0 | ||||
| Yun [33] | 255 | 42 | 2.1 | 92 | 87 | ||||
| Hung [34] | 190 | 14.5 | 2.4 | 67.4 | |||||
| Nishi [35] | 162 | 37 | 2.0 | 95.4 | 79.6 | 63.1 | |||
| Wang [36] | 345 | <3 | 80.3 | 72 | |||||
| Peng [37] | 71 | 59 | 1.2 | 90.5 | 70.9 | 62.1 | 19 | ||
| Hasegawa [38] | 5548 | 26 | <3 | 81.0 | 61.1 | ||||
| Total/Mean | 10,838 | 32.3 | 91.2 | 81.8 | 72.8 | 67.1 | 59.6 | 3.4 | |
| Region | Number of Patients | Mean Tumor Size (cm) | Overall Survival Rates | Major Complication Rate (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 1-Year (%) | 2-Year (%) | 3-Year (%) | 4-Year (%) | 5-Year (%) | ||||
| Nagasue [39] | 229 | <4 | 80 | 51 | 26 | 24 | ||
| Takenaka [40] | 280 | <5 | 88 | 70 | 50 | 50 | ||
| Makuuchi [41] | 352 | <4 | 92 | 73 | 47 | |||
| Shimozawa [42] | 135 | <3 | 95 | 73 | 55 | 25 | ||
| Franco [43] | 72 | <4 | 68 | 51 | 48 | |||
| Belghiti [44] | 300 | <5 | 81 | 57 | 37 | |||
| Ercolani [45] | 224 | 4.0 | 83 | 63 | 42 | 36.2 | ||
| Cha [46] | 164 | <4 | 79 | 51 | 40 | |||
| Ringe [47] | 131 | <5 | 42.3 | 35.8 | ||||
| Pitre [48] | 153 | <5 | 30.1 | 17.9 | 38.5 | |||
| DiCarl [49] | 122 | <4 | 42.6 | 23.3 | 30 | |||
| Lise [50] | 100 | <5 | 38 | 16 | ||||
| Grazi [51] | 264 | 4.4 | 57 | 37 | 47 | |||
| Zhou [1] | 1000 | <5 | 62.7 | |||||
| Poon [52] | 241 | <5 | 82 | 62 | 49 | |||
| Kanem [53] | 303 | 84 | 67 | 51 | ||||
| Chen [22] | 252 | 80 | 54.3 | 34.2 | ||||
| Wayne [54] | 249 | <5 | 83 | 41.1 | ||||
| Yeh [55] | 218 | <5 | 63 | 42 | 32 | 15.6 | ||
| Capuss [56] | 216 | 51 | 34 | 38.4 | ||||
| Natha [57] | 788 | 3.2 | 39 | |||||
| Yang [17] | 260 | 87 | 56 | 38 | ||||
| Yun [33] | 215 | 2.1 | 98 | 94 | ||||
| Hung [34] | 229 | 2.9 | 79.3 | |||||
| Wang [36] | 260 | <3 | 98 | 91.5 | ||||
| Hasegawa [38] | 5361 | <3 | 85.3 | 71.1 | ||||
| Peng [37] | 74 | 1.1 | 98.5 | 87.7 | 71.9 | 51.4 | ||
| Arii [58] | 1318 502 2722 1548 | <2 <2 2–5 2–5 | 96 92 95 95 | 72 56 58 45 | ||||
| Total/Mean | 18,282 | 85.3 | 61.9 | 49.0 | 35.0 | |||
| Modality | Number of Patients | Overall Survival Rates | Major Complication Rate (%) | ||||
|---|---|---|---|---|---|---|---|
| 1-Year | 2-Year | 3-Year | 4-Year | 5-Year | |||
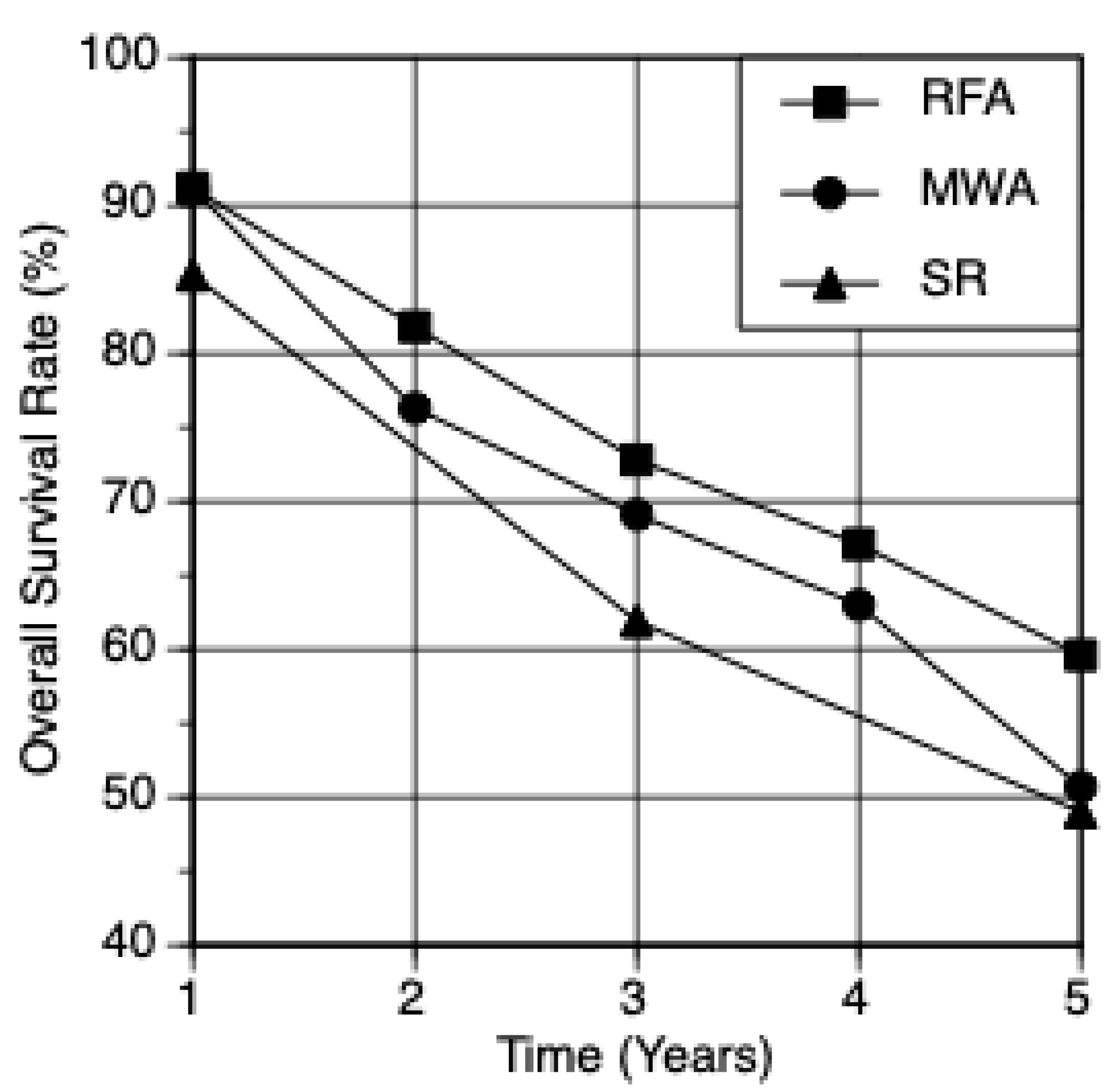
| MWA | 3725 | 91.1 | 76.3 | 69.1 | 63.0 | 50.7 | 3.2 |
| RFA | 10,838 | 91.2 | 81.8 | 72.8 | 67.1 | 59.6 | 2.6 |
| SR | 18,282 | 85.3 | 61.9 | 49.0 | 32.9 | ||
| MWA | 1 Year Survival Rate (%) | 3 Year Survival Rate (%) | 5 Year Survival Rate (%) | Complication Rate (%) |
|---|---|---|---|---|
| <3 cm | 92.9 | 82 | 57.8 | 2 |
| <5 cm | 91.1 | 69.1 | 50.7 | 2.5 |
| 3–5 cm | 90.4 | 61.4 | 43.5 | 3.1 |
| RFA | 1 year survival rate (%) | 3 year survival rate (%) | 5 year survival rate (%) | Complication rate (%) |
| <3 cm | 90.9 | 76.6 | 62.2 | 6.6 |
| <5 cm | 91.2 | 72.8 | 59.6 | 4.7 |
| 3–5 cm | 91.4 | 68.6 | 55.6 | 2.5 |
| SR | 1 year survival rate (%) | 3 year survival rate (%) | 5 year survival rate (%) | Complication rate (%) |
| <3 cm | 95.3 | 88.4 | 69.4 | 38.2 |
| <5 cm | 85.6 | 62.6 | 49.9 | 35 |
| 3–5 cm | 80.3 | 54.0 | 39 | 34.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wicks, J.S.; Dale, B.S.; Ruffolo, L.; Pack, L.J.; Dunne, R.; Laryea, M.A.; Hernandez-Alejandro, R.; Sharma, A.K. Comparable and Complimentary Modalities for Treatment of Small-Sized HCC: Surgical Resection, Radiofrequency Ablation, and Microwave Ablation. J. Clin. Med. 2023, 12, 5006. https://doi.org/10.3390/jcm12155006
Wicks JS, Dale BS, Ruffolo L, Pack LJ, Dunne R, Laryea MA, Hernandez-Alejandro R, Sharma AK. Comparable and Complimentary Modalities for Treatment of Small-Sized HCC: Surgical Resection, Radiofrequency Ablation, and Microwave Ablation. Journal of Clinical Medicine. 2023; 12(15):5006. https://doi.org/10.3390/jcm12155006
Chicago/Turabian StyleWicks, Jeffrey S., Benjamin S. Dale, Luis Ruffolo, Ludia J. Pack, Richard Dunne, Marie A. Laryea, Roberto Hernandez-Alejandro, and Ashwani Kumar Sharma. 2023. "Comparable and Complimentary Modalities for Treatment of Small-Sized HCC: Surgical Resection, Radiofrequency Ablation, and Microwave Ablation" Journal of Clinical Medicine 12, no. 15: 5006. https://doi.org/10.3390/jcm12155006
APA StyleWicks, J. S., Dale, B. S., Ruffolo, L., Pack, L. J., Dunne, R., Laryea, M. A., Hernandez-Alejandro, R., & Sharma, A. K. (2023). Comparable and Complimentary Modalities for Treatment of Small-Sized HCC: Surgical Resection, Radiofrequency Ablation, and Microwave Ablation. Journal of Clinical Medicine, 12(15), 5006. https://doi.org/10.3390/jcm12155006





