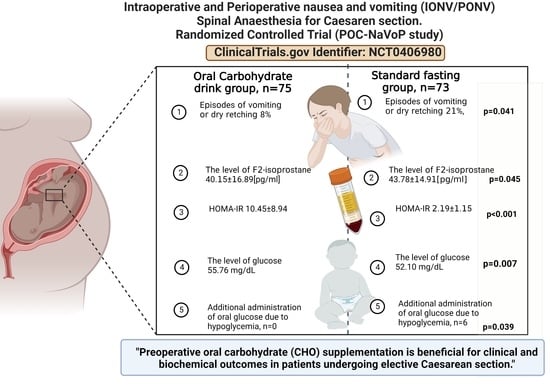
Preoperative Oral Carbohydrate (CHO) Supplementation Is Beneficial for Clinical and Biochemical Outcomes in Patients Undergoing Elective Cesarean Delivery under Spinal Anaesthesia—A Randomized Controlled Trial
Abstract
1. Introduction
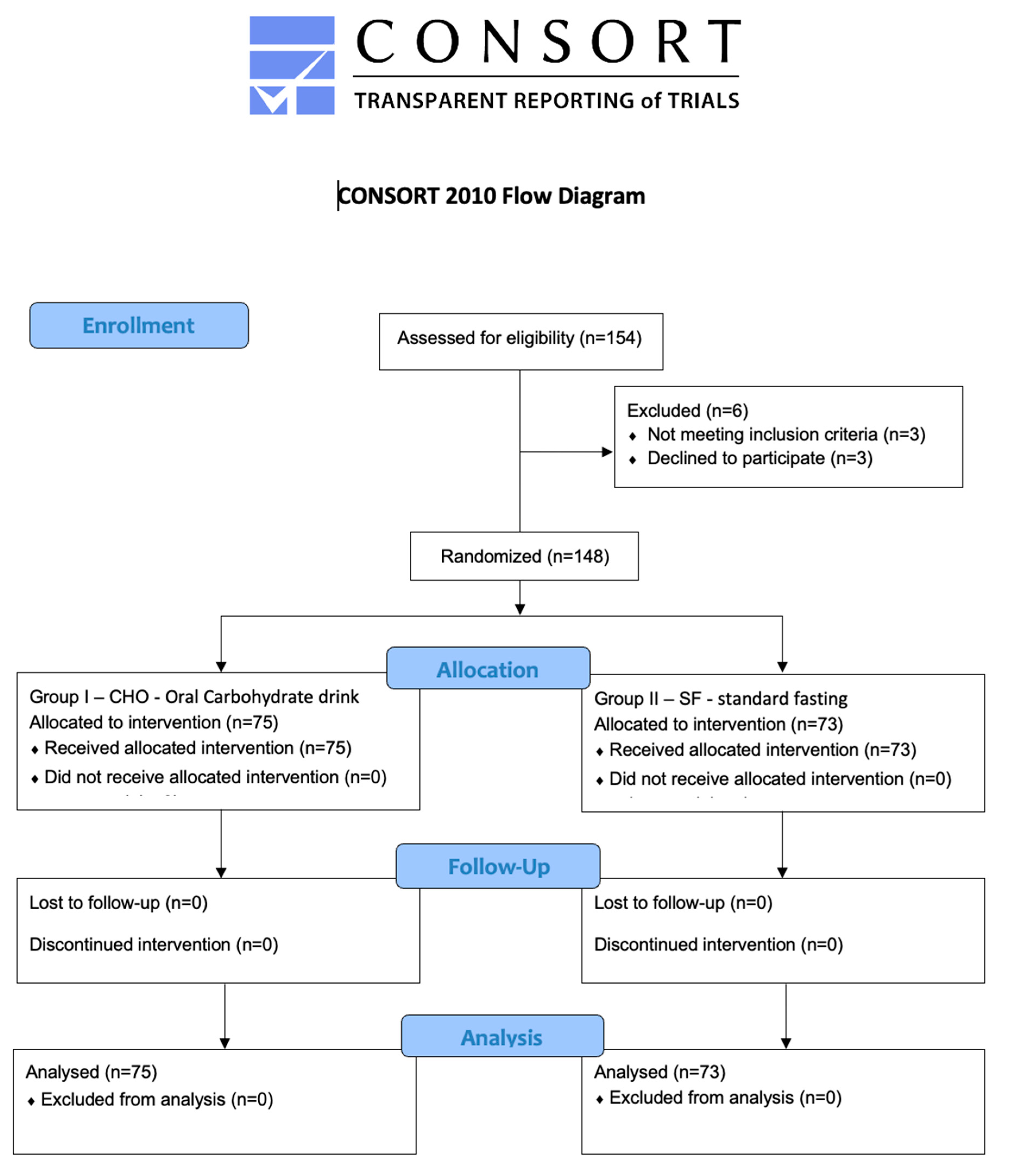
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Patients
2.4. Interventions
2.4.1. Randomization
2.4.2. Perioperative Care
2.5. Data Collection
2.5.1. Demographic and Perioperative Data
Maternal Data
Neonatal Data
2.6. Biochemical Analysis
2.7. Main Outcome Measures
Study Endpoints
2.8. Additional Assessments
2.9. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. Intraoperative Factors
3.3. Primary Outcome Measures
3.4. Secondary Outcome Measures
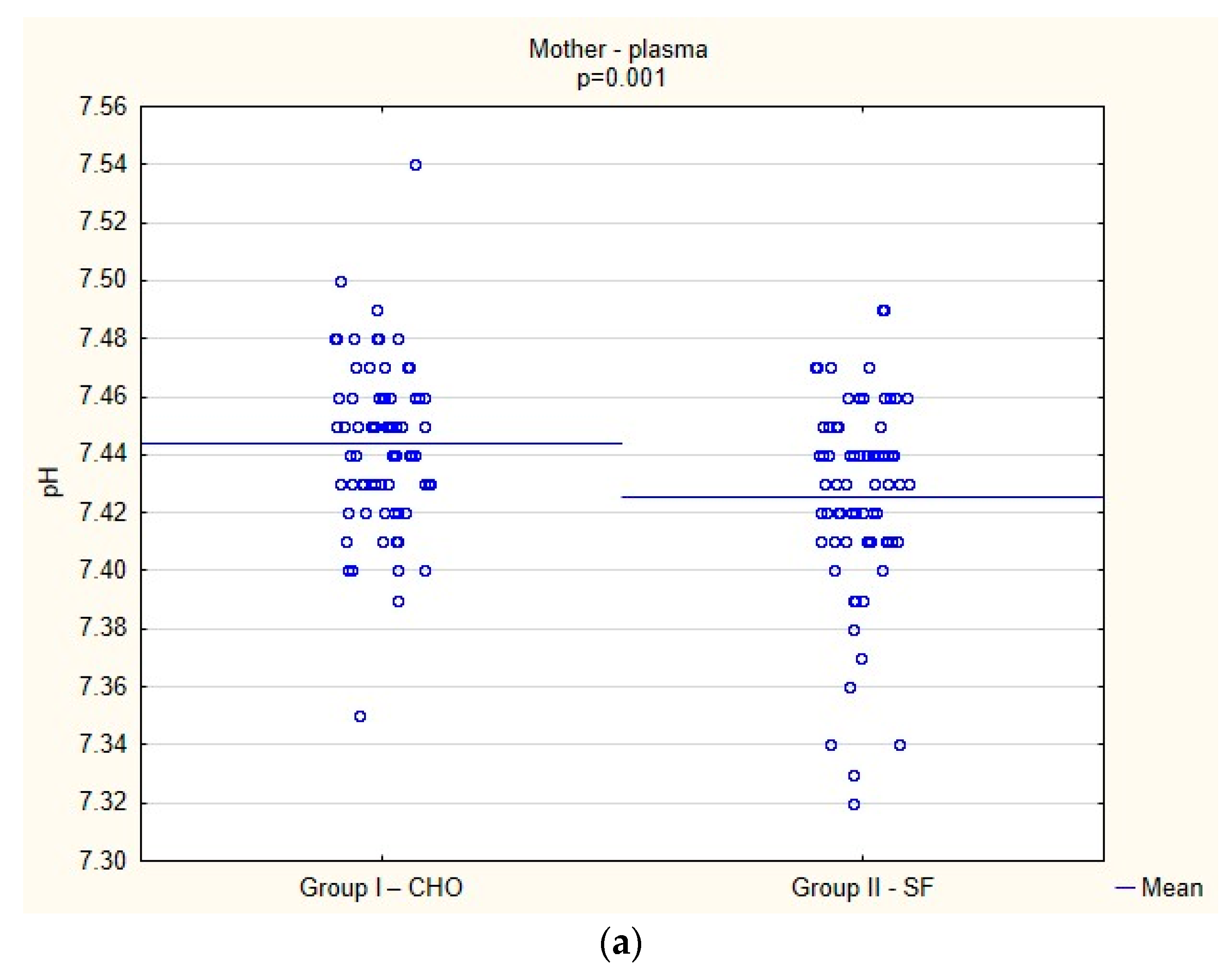
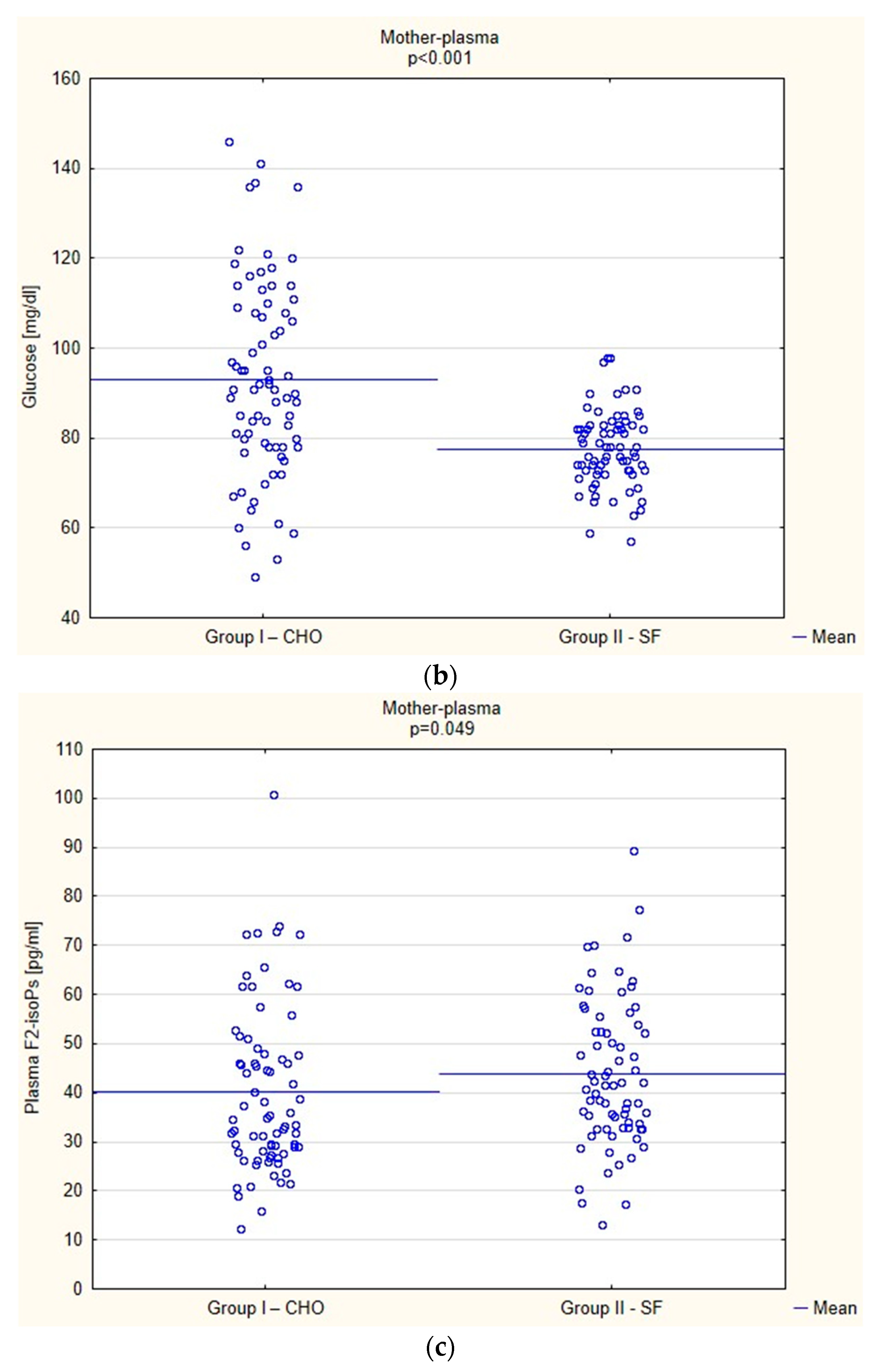
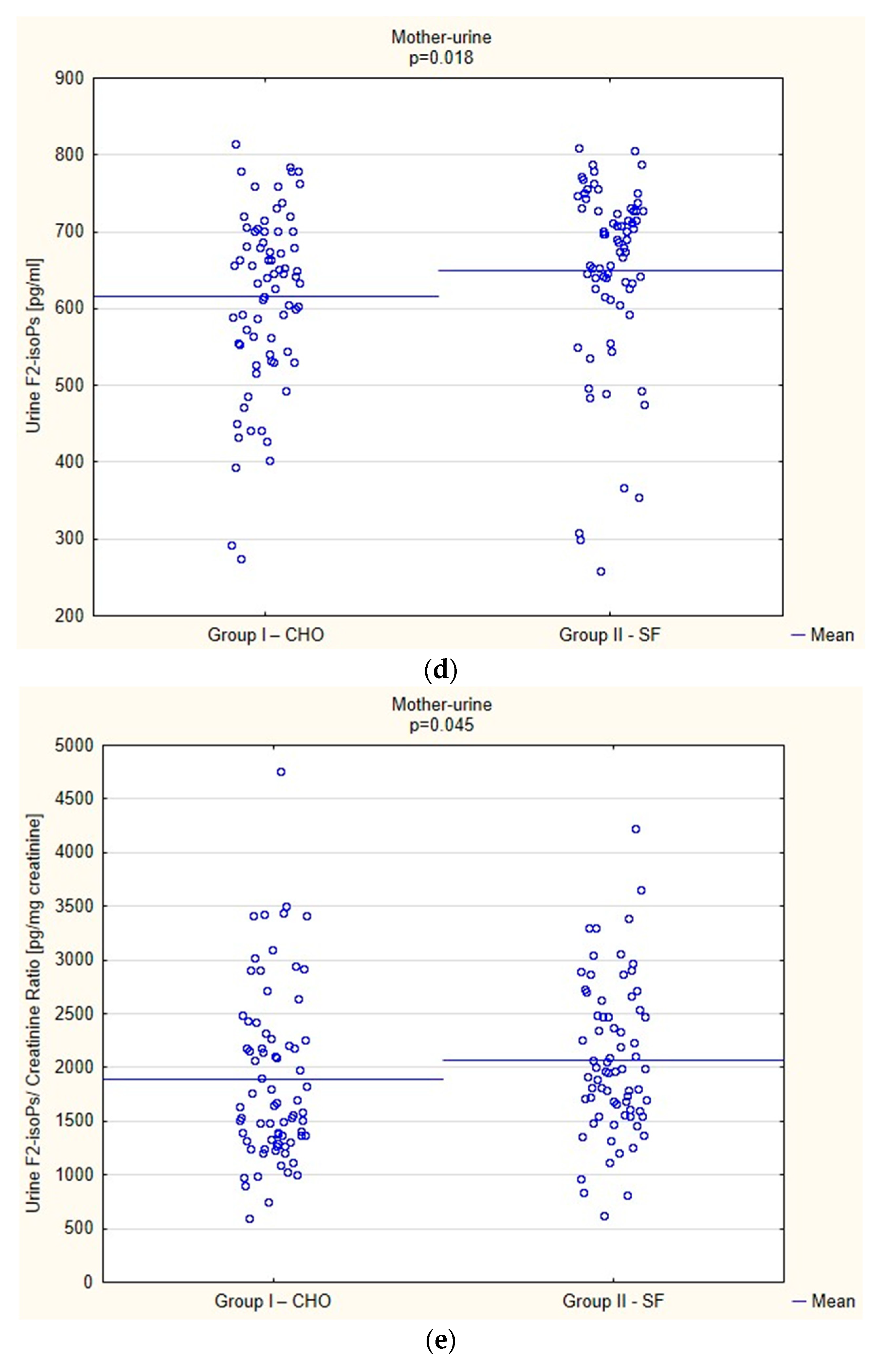
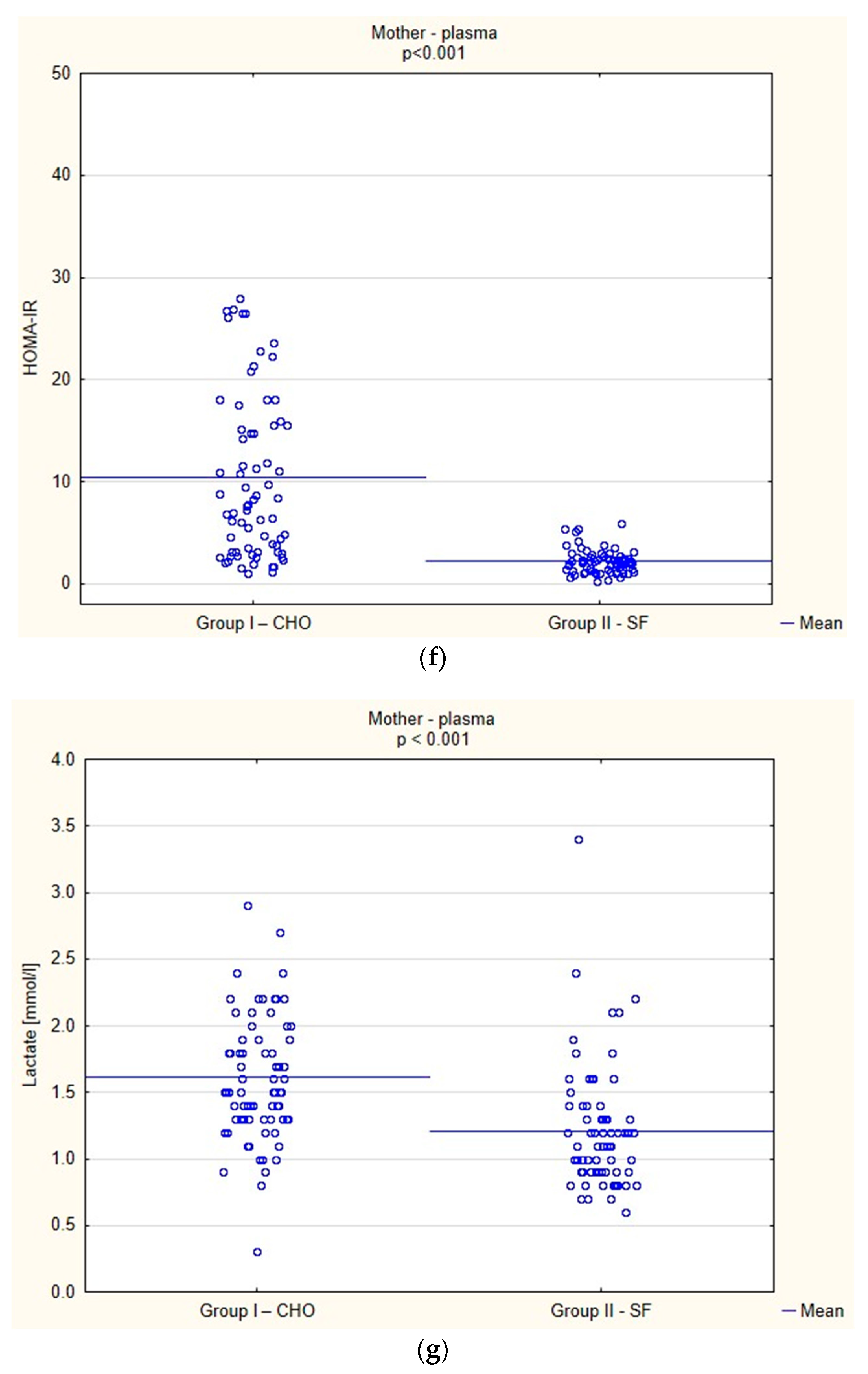
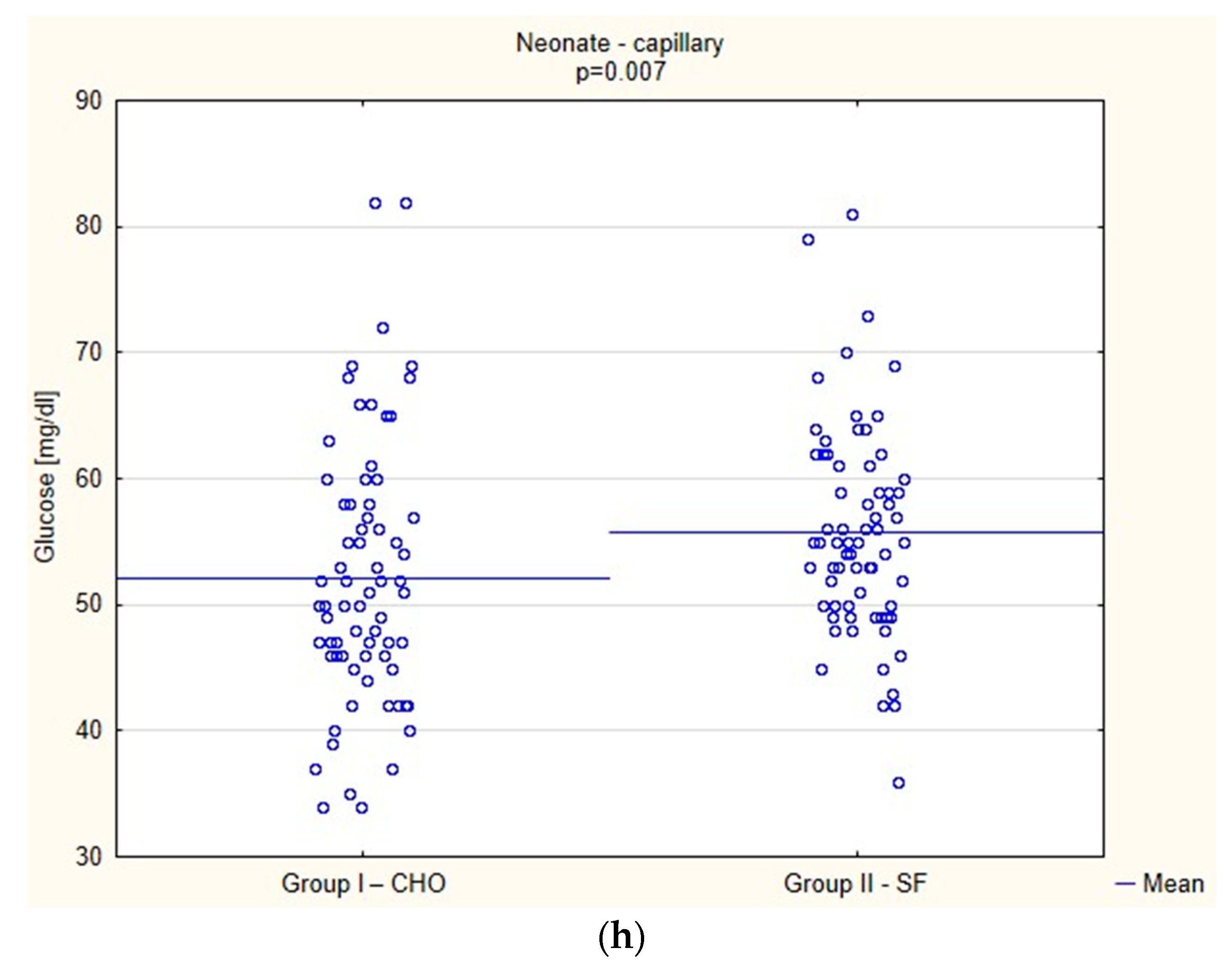
Biochemical Parameters
3.5. Clinical Parameters
3.6. Patient Satisfaction
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jelting, Y.; Klein, C.; Harlander, T.; Eberhart, L.; Roewer, N.; Kranke, P. Preventing nausea and vomiting in women undergoing regional anesthesia for cesarean section: Challenges and solutions. Local Reg. Anesth. 2017, 10, 83–90. [Google Scholar] [CrossRef]
- Balki, M.; Carvalho, J.C. Intraoperative nausea and vomiting during cesarean section under regional anesthesia. Int. J. Obstet. Anesth. 2005, 14, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Batova, R.; Georgiev, S. Impact of spinal needle design and approach to postdural puncture headache and spinal anesthesia failure in obstetrics. Anaesthesiol. Intensive Ther. 2019, 51, 81–86. [Google Scholar]
- Voigt, M.; Frolich, C.W.; Huttel, C.; Kranke, P.; Mennen, J.; Boessneck, O.; Lenz, C.; Erbes, T.; Ernst, J.; Kerger, H. Prophylaxis of intra and postoperative nausea and vomiting in patients during cesarean section in spinal anesthesia. Med. Sci. Monit. 2013, 19, 993–1000. [Google Scholar] [PubMed]
- Mendelson, C. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am. J. Obstet. Gynecol. 1946, 52, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Maltby, J.R. Fasting from midnight: The history behind the dogma. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 363–378. [Google Scholar] [CrossRef]
- Bouvet, L.; Cercueil, E.; Barnoud, S.; Lilot, M.; Desgranges, F.; Chassard, D. Relationship between the regurgitated and the aspirated volume of water. A manikin study. Anaesthesiol. Intensive Ther. 2019, 51, 125–129. [Google Scholar] [CrossRef]
- Horosz, B.; Nawrocka, K.; Malec-Milewska, M. Anaesthetic perioperative management according to the enhanced recovery after surgery protocol. Anaesthesiol. Intensive Ther. 2016, 48, 49–54. [Google Scholar] [CrossRef]
- Brady, M.C.; Kinn, S.; Stuart, P.; Ness, V. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst. Rev. 2003, 4, CD004423. [Google Scholar] [CrossRef]
- Wendling, A.L.; Byun, S.Y.; Koenig, M.; Vasipouloulos, T. Impact of oral carbohydrate consumption prior to cesarean delivery on preoperative well-being: A randomized interventional study. Arch. Gynecol. Obstet. 2020, 301, 179–187. [Google Scholar] [CrossRef]
- Nygren, J. The metabolic effects of fasting and surgery. Best. Pract. Res. Clin. Anaesthesiol. 2006, 20, 429–438. [Google Scholar] [CrossRef]
- Awad, S.; Constantin-Teodosiu, D.; Macdonald, I.A.; Lobo, D.N. Short-term starvation and mitochondrial dysfunction—a possible mechanism leading to postoperative insulin resistance. Clin. Nutr. 2009, 28, 497–499. [Google Scholar] [CrossRef]
- Toohill, J.; Soong, B.; Fleandy, V. Interventions for ketosis in labour. Cochrane Database Syst. Rev. 2008, 3, CD004230. [Google Scholar]
- Smith, I.; Kranke, P.; Murat, I.; Smith, A.; O’Sullivan, G.; Søreide, E.; Spies, C. European Society of Anaesthesiology. Perioperative fasting in adults and children: Guidelines from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. 2011, 28, 556–569. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: Application to healthy patients undergoing elective procedures: An updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology 2011, 114, 495–511. [Google Scholar]
- Lambert, E.; Carey, S. Practice guideline recommendations on perioperative fasting: A systematic review. JPEN J. Parenter. Enter. Nutr. 2016, 40, 1158–1165. [Google Scholar]
- Wilson, R.D.; Caughey, A.B.; Wood, S.L.; Macones, G.A.; Wrench, I.J.; Huang, J.; Norman, M.; Pettersson, K.; Fawcett, W.J.; Shalabi, M.M.; et al. Guidelines for Antenatal and Preoperative care in Cesarean Delivery: Enhanced Recovery After Surgery Society Recommendations (Part 1). Am. J. Obstet. Gynecol. 2018, 219, 523.e1–523.e15. [Google Scholar] [CrossRef]
- Smith, M.D.; McCall, J.; Plank, L.; Herbison, P.G.; Soop, M.; Nygren, J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst. Rev. 2014, 8, CD009161. [Google Scholar]
- Morrison, C.E.; Ritchie-McLean, S.; Jha, A.; Mythen, M. Two hours too long: Time to review fasting guidelines for clear fluids. Br. J. Anaesth. 2020, 17, 363–366. [Google Scholar] [CrossRef]
- Kotfis, K.; Jamioł-Milc, D.; Skonieczna-Żydecka, K.; Folwarski, M.; Stachowska, E. The Effect of Preoperative Carbohydrate Loading on Clinical and Biochemical Outcomes after Cardiac Surgery: A Systematic Review and Meta-Analysis of Randomized Trials. Nutrients 2020, 12, 3105. [Google Scholar] [CrossRef]
- O’Sullivan, G.; Liu, B.; Hart, D.; Seed, P.; Shennan, A. Effect of food intake during labour on obstetric outcome: Randomised controlled trial. BMJ 2009, 338, b784. [Google Scholar] [CrossRef]
- Rahmani, R.; Khakbazan, Z.; Yavari, P.; Granmayeh, M.; Yavari, L. Effect of oral carbohydrate intake on labor progress: Randomized controlled trial. Iran. J. Public Health 2012, 41, 59–66. [Google Scholar]
- Scheepers, H.C.; Thans, M.C.; de Jong, P.A.; Essed, G.G.; Kanhai, H.H. The effects of oral carbohydrate administration on fetal acid base balance. J. Perinat. Med. 2002, 30, 400–404. [Google Scholar] [CrossRef]
- Malin, G.L.; Bugg, G.J.; Thornton, J.; Taylor, M.A.; Grauwen, N.; Devlieger, R.; Kardel, K.R.; Kubli, M.; Tranmer, J.E.; Jones, N.W. Does oral carbohydrate supplementation improve labour outcome? A systematic review and individual patient data meta-analysis. BJOG 2016, 123, 510–517. [Google Scholar] [CrossRef]
- Clark, A.; Litchfield, K.; Hannah, S.; Love, C.; Slade, K.; Lake, K.; Agaram, R. Pre-operative carbohydrate loading prior to elective caesarean delivery: A randomised controlled trial. Int. J. Obstet. Anesth. 2021, 45, 21–27. [Google Scholar] [CrossRef]
- Bellwood, H.; Rozdarz, K.M.; Riordan, J. Incidence of urinary ketosis and the effect of carbohydrate drink supplementation during fasting for elective caesarean section: Audit. J. Perioper. Pract. 2022, 32, 280–285. [Google Scholar] [CrossRef]
- Awad, S.; Varadhan, K.K.; Ljungqvist, O.; Lobo, D.N. A meta-analysis of randomized controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin. Nutr. 2013, 32, 34–44. [Google Scholar] [CrossRef]
- Faris, M.A.; Hussein, R.N.; Al-Kurd, R.A.; Al-Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K. Impact of ramadan intermittent fasting on oxidative stress measured by urinary 15-f(2t)-isoprostane. J. Nutr. Metab. 2012, 2012, 802924. [Google Scholar] [CrossRef]
- Greco, A.; Minghetti, L.; Puopolo, M.; Pietrobon, B.; Franzoi, M.; Chiandetti, L.; Suppiej, A. Plasma levels of 15-F(2t)-isoprostane in newborn infants are affected by mode of delivery. Clin. Biochem. 2007, 40, 1420–1422. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, B.; Dong, Q.; Zhao, Z.; Yu, Y. Effect of Preoperative Oral Carbohydrate Administration on Patients Undergoing Cesarean Section with Epidural Anesthesia: A Pilot Study. J. Perianesth. Nurs. 2021, 36, 30–35. [Google Scholar] [CrossRef]
- He, Y.; Liu, C.; Han, Y.; Huang, Y.; Zhou, J.; Xie, Q. The impact of oral carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes—A randomized clinical trial. BMC Pregnancy Childbirth 2021, 21, 682. [Google Scholar] [CrossRef]
- Bilku, D.R.; Dennison, A.R.; Hall, T.C.; Metcalfe, M.S.; Garcea, G. Role of preoperative carbohydrate loading: A systematic review. Ann. R. Coll. Surg. Engl. 2014, 96, 15–22. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Monti, L.D.; Genzano, C.B.; Fontana, B.; Galluccio, E.; Spadoni, S.; Magistro, A.; Bosi, E.; Piatti, P. Association between new markers of cardiovascular risk and hepatic insulin resistance in those at high risk of developing type 2 diabetes. Endocrine 2022, 75, 409–417. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Gyllenhammer, L.E.; Entringer, S.; Wadhwa, P.D. Rate of Gestational Weight Gain and Glucose-Insulin Metabolism Among Hispanic Pregnant Women With Overweight and Obesity. J. Clin. Endocrinol. Metab. 2022, 107, e734–e744. [Google Scholar] [CrossRef]
- Tayanloo-Beik, A.; Roudsari, P.P.; Rezaei-Tavirani, M.; Biglar, M.; Tabatabaei-Malazy, O.; Arjmand, B.; Larijani, B. Diabetes and Heart Failure: Multi-Omics Approaches. Front. Physiol. 2021, 12, 705424. [Google Scholar] [CrossRef]
- McIntyre, C.; Johnston, A.; Foley, D.; Lawler, J.; Bucholc, M.; Flanagan, L.; Sugrue, M. Readmission to hospital following laparoscopic cholecystectomy: A meta-analysis. Anaesthesiol. Intensive Ther. 2020, 52, 47–55. [Google Scholar] [CrossRef]
- Fard, R.K.; Tabassi, Z.; Qorbani, M.; Hosseini, S. The Effect of Preoperative Oral Carbohydrate on Breastfeeding After Cesarean Section: A Double-Blind, Randomized Controlled Clinical Trial. J. Diet. Suppl. 2018, 15, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.N.; Cassady, B.A.; Hegazi, R.A.; Wischmeyer, P.E. Preoperative carbohydrate loading in surgical patients with type 2 diabetes: Are concerns supported by data? Clin. Nutr. ESPEN 2021, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lafflin, M.R.; Shuai, L.; Brisebois, R.; Senior, P.A.; Wang, H. The use of a preoperative carbohydrate drink in patients with diabetes mellitus: A prospective, non-inferiority, cohort study. World J. Surg. 2018, 42, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jin, Y.; Wang, X.; Xiang, Z.; Zhang, L.; Feng, S. Safety, and feasibility of oral carbohydrate consumption before cesarean delivery on patients with gestational diabetes mellitus: A parallel, randomized controlled trial. J. Obstet. Gynaecol. Res. 2021, 47, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
| Data | Group I—CHO (n = 75) | Group II—SF (n = 73) | ||
|---|---|---|---|---|
| Demographic data | ||||
| Age, Me, Q1–Q3 | 31.00 | 28.00–36.00 | 34.00 | 29.00–36.00 |
| Height, Me, Q1–Q3 | 167.0 | 161.00–170.00 | 165.00 | 160.00–170.00 |
| Weight, Me, Q1–Q3 | 77.0 | 69.50–84.0 | 78.00 | 72.00–85.00 |
| BMI, Me, Q1–Q3 | 28.04 | 25.51–30.12 | 28.65 | 26.47–31.22 |
| Parity, Me, Q1–Q3 | 2.0 | 1.00–2.00 | 2.00 | 2.00–3.00 |
| Birth, Me, Q1–Q3 | 2.0 | 1.00–2.00 | 2.00 | 1.00–2.00 |
| Pregnancy week, Me, Q1–Q3 | 39.0 | 39.00–40.00 | 39.00 | 39.00–39.00 |
| Pre-pregnancy medical history | ||||
| Hypertension, n (%) | 1 | (1.33%) | 1 | (1.37%) |
| Valvular disease, n (%) | 0 | (0.00%) | 1 | (1.37%) |
| Thrombosis, n (%) | 1 | (1.33%) | 1 | (1.37%) |
| Thrombophilia, n (%) | 2 | (2.67%) | 0 | (0.00%) |
| Varicose veins, n (%) | 1 | (1.33%) | 2 | (2.74%) |
| Hypothyroidism, n (%) | 11 | (14.67%) | 12 | (16.44%) |
| Hyperthyroidism, n (%) | 1 | (1.33%) | 0 | (0.00%) |
| Hashimoto disease, n (%) | 2 | (2.67%) | 2 | (2.74%) |
| Multiple sclerosis, n (%) | 1 | (1.33%) | 0 | (0.00%) |
| Epilepsy, n (%) | 0 | (0.00%) | 1 | (1.33%) |
| Asthma, n (%) | 2 | (2.67%) | 2 | (2.74%) |
| Medical problems in pregnancy | ||||
| None, n (%) | 54 | (72.00%) | 36 | (49.32%) |
| Hypertension | 2 | (2.67%) | 5 | (6.85%) |
| Hypothyroidism | 8 | (10.67%) | 11 | (15.07%) |
| Infection | 2 | (2.67%) | 6 | (8.22%) |
| Other | 3 | (4.00%) | 9 | (12.33%) |
| Obstetric | 6 | (8.00%) | 6 | (8.22%) |
| Medications | ||||
| Methyldopa, n (%) | 5 | (6.67%) | 5 | (6.85%) |
| Metoprolol, n (%) | 0 | (0.00%) | 1 | (1.37%) |
| Enoxaparin, n (%) | 3 | (4.00%) | 3 | (4.17%) |
| Levothyroxine, n (%) | 19 | (25.33%) | 23 | (31.51%) |
| Levetiracetam, n (%) | 1 | (1.33%) | 0 | (0.00%) |
| Salbutamol, n (%) | 2 | (2.67%) | 3 | (4.11%) |
| Intraoperative Factors | Group I—CHO (n = 75) | Group II—SF (n = 73) | p-Value | |||
|---|---|---|---|---|---|---|
| Cesarean delivery duration time (min), Me, Q1–Q3 | 28.00 | 24.00–33.00 | 26.00 | 21.00–33.00 | 0.132 | |
| Anesthesia care duration time (min), Me, Q1–Q3 | 45.00 | 400.0–56.0 | 43.00 | 38.00–52.00 | 0.283 | |
| Marcaine 0.5% Spinal Heavy (mL), Me, Q1–Q3 | 2.08 | 2.60–3.00 | 2.90 | 2.80–3.20 | 0.314 | |
| Bupivacaine 0.5% hydrochloride Spinal Heavy (mL), Me, Q1–Q3 | 2.80 | 2.60–30.0 | 2.90 | 2.80–3.00 | 0.132 | |
| Morphine sulfate Spinal 0.1% (mcg), Me, Q1–Q3 | 200.0 | 200.0 | 200.0 | 200.0 | 0.998 | |
| Spinal blockade level prior to cesarean delivery (Th), n (%) | 2 | 1 | (1.33%) | 0 | (0.00%) | 0.802 |
| 3 | 1 | (1.33%) | 0 | (0.00%) | ||
| 4 | 21 | (28.00%) | 21 | (28.77%) | ||
| 5 | 8 | (10.67%) | 8 | (10.96%) | ||
| 6 | 37 | (49.33%) | 37 | (50.68%) | ||
| 7 | 7 | (99.33%) | 6 | (8.22%) | ||
| 8 | 0 | (0.00%) | 1 | (1.37%) | ||
| Spinal blockade level prior after cesarean delivery (Th), n (%) | 2 | 15 | (20.00%) | 16 | (21.92%) | 0.563 |
| 3 | 27 | (36.00%) | 29 | (39.73%) | ||
| 4 | 26 | (34.67%) | 21 | (28.77%) | ||
| 5 | 1 | (1.33%) | 4 | (5.48%) | ||
| 6 | 5 | (6.67%) | 3 | (4.11%) | ||
| 8 | 1 | (1.33%) | 0 | (0.00%) | ||
| Crystalloids n (%) | Optilyte | 73 | (97.33%) | 73 | (100.00%) | 0.484 |
| Sterofundin | 2 | (2.67%) | 0 | (0.00%) | ||
| Volume of crystalloids (mL), Me, Q1–Q3 | 1000.0 | 1000.00–1000.0 | 1000.0 | 1000.0–1000.0 | 0.135 | |
| Colloids (4% Gelaspan), n (%) | No | 68 | (90.67%) | 68 | (93.15%) | 0.801 |
| Yes | 7 | (9.33%) | 5 | (6.85%) | ||
| Volume of colloids (mL), Me, Q1–Q3 | 500.0 | 500.00–1000.0 | 500.0 | 500.0–500.0 | 0.508 | |
| ION, n (%) | No | 47 | (62.67%) | 37 | (50.68%) | 0.192 |
| Yes | 28 | (37.33%) | 36 | (49.32%) | ||
| IOV, n (%) | No | 70 | (93.33%) | 70 | (95.89%) | 0.746 |
| Yes | 5 | (6.67%) | 3 | (4.11%) | ||
| Antiemetics n (%) | No | 67 | (89.33%) | 64 | (87.67%) | 0.936 |
| Yes | 8 | (10.67%) | 9 | (12.33%) | ||
| Ondansetron | 5 | (6.67%) | 6 | (8.22%) | ||
| Metoclopramide | 3 | (4.00%) | 3 | (4.11%) | ||
| Ephedrine dose (mg), Me, Q1–Q3 | 20.0 | 10.00–25.00 | 20.00 | 10.00–25.00 | 0.872 | |
| Wengritzky Scale | Group I—CHO (n = 75) | Group II—SF (n = 73) | p-Value | |
|---|---|---|---|---|
| Nausea 6 h after cesarean delivery | ||||
| Q1 (6), n (%) | 0 points | 47 (62.67%) | 53 (72.60%) | 0.197 |
| 2 points | 16 (21.33%) | 15 (20.55%) | ||
| 50 points | 12 (16.00%) | 5 (6.85%) | ||
| Q2 (6), n (%) | 0 points | 37 (49.33%) | 38 (52.05%) | 0.172 |
| 1 point | 26 (34.67%) | 31 (42.47%) | ||
| 2 points | 10 (13.33%) | 4 (5.48%) | ||
| 25 points | 2 (2.67%) | 0 (0.00%) | ||
| Q3 (6), n (%) | 1 point | 32 (84.21%) | 34 (97.14%) | 0.139 |
| 2 points | 6 (15.79%) | 1 (2.86%) | ||
| Q4 (6): (h) Me (Q1–Q3) | 0.10 (0.00–0.40) | 0.00 (0.00–0.10) | 0.339 | |
| Result A (6), Me, (Q1–Q3) | 0.10 (0.00–2.00) | 0.00 (0.00–0.40) | 0.252 | |
| Nausea 24 h after cesarean delivery | ||||
| Q1 (24), n (%) | 0 points | 52 (69.33%) | 49 (67.12%) | 0.041 |
| 2 points | 17 (22.67%) | 9 (12.33%) | ||
| 50 points | 6 (8.00%) | 15 (20.55%) | ||
| Q2 (24), n (%) | 0 points | 44 (59.46%) | 37 (50.68%) | 0.369 |
| 1 point | 23 (31.08%) | 25 (34.25%) | ||
| 2 points | 6 (8.11%) | 11 (15.07%) | ||
| 25 points | 1 (1.35%) | 0 (0.00%) | ||
| Q3 (24), n (%) | 1 point | 26 (86.67%) | 34 (94.44%) | 0.506 |
| 2 points | 4 (13.33%) | 2 (5.56%) | ||
| Q4 (24): (h), Me, (Q1–Q3) | 0.00 (0.00–0.15) | 0.00 (0.00–0.30) | 0.264 | |
| Result B (24), Me, (Q1–Q3) | 0.00 (0.00–0.20) | 0.00 (0.00–1.00) | 0.196 | |
| Combined Result A + B, Me, (Q1–Q3) | 0.20 (0.00–10.00) | 0.30 (0.00–9.00) | 0.574 | |
| Postoperative Data—Mother | Group I—CHO (n = 75) | Group II—SF (n = 73) | p-Value | |||
|---|---|---|---|---|---|---|
| Withdrawal from drinking after CD (h), Me, Q1–Q3 | 3.00 | 2.30–3.70 | 3.00 | 2.50–4.00 | 0.614 | |
| Withdrawal from eating after CD (h), Me, Q1–Q3 | 9.20 | 7.30–12.00 | 8.00 | 5.50–11.0 | 0.035 | |
| Total withdrawal from drinking (h), Me, Q1–Q3 | 6.10 | 5.50–7.30 | 14.80 | 11.50–17.10 | <0.001 | |
| Total withdrawal from eating (h), Me, Q1–Q3 | 23.20 | 21.00–26.60 | 23.20 | 19.70–25.70 | 0.568 | |
| Antiemetics after CD, n (%) | No | 50 | (66.67%) | 48 | (65.75%) | 0.358 |
| Yes | 25 | (33.34%) | 25 | (34.25%) | ||
| Ondansetron | 20 | (26.67%) | 23 | (31.51%) | ||
| Metoclopramide | 2 | (2.67%) | 2 | (2.74%) | ||
| Both drugs | 3 | (4.00%) | 0 | (0.00%) | ||
| Time to first peristalsis (h), Me, Q1–Q3 | 10.00 | 7.00–13.00 | 11.70 | 6.70–17.00 | 0.213 | |
| Time to first bowel movement (h), n (%) | <72 h | 4 | (5.33%) | 4 | (5.48%) | 0.746 |
| >72 h | 71 | (94.67%) | 69 | (94.52%) | ||
| Fluids iv, n (%) | No | 6 | (8.00%) | 14 | (19.18%) | 0.077 |
| Yes | 69 | (92.00%) | 59 | (80.82%) | ||
| Crystalloids | 69 | (92.00%) | 58 | (79.45%) | ||
| Crystalloids and Colloids | 0 | (0.00%) | 1 | (1.37%) | ||
| Volume of iv fluids (mL), Me, Q1–Q3 | 1000.0 | 500–1000.00 | 1000.0 | 500.0–1000.00 | 0.807 | |
| Postoperative data—neonate | Group I—CHO (n = 75) | Group II—SF (n = 73) | p | |||
| Time between spinal anesthesia and birth (min), Me, Q1–Q3 | 9.00 | 8.00–11.00 | 9.00 | 8.00–11.00 | 0.664 | |
| Gender, n (%) | female | 30 | (40.00%) | 29 | (39.73%) | 0.973 |
| male | 45 | (60.00%) | 44 | (60.27%) | ||
| Apgar I, Me, Q1–Q3 | 9.00 | 8.00–10.00 | 10.0 | 10.00–10.00 | 0.182 | |
| Apgar II, Me, Q1–Q3 | 10.00 | 10.00–10.00 | 10.0 | 10.00–10.00 | 0.264 | |
| Apgar III, Me, Q1–Q3 | 10.00 | 10.00–10.00 | 10.0 | 10.00–10.00 | 0.332 | |
| Birth weight (g), Me, Q1–Q3 | 3510.0 | 3200.0–3730.0 | 3350.0 | 3080.0–3640.0 | 0.033 | |
| Lowest weight after birth (g) Me, Q1–Q3 | 3240.0 | 3010.0–3480.0 | 3140.0 | 2795.0–3405.0 | 0.063 | |
| Decrease in body weight after birth (%), Me, Q1–Q3 | 7.63 | 6.37–8.90 | 7.26 | 6.13–8.26 | 0.163 | |
| Breastfeeding, n (%) | No | 7 | (9.33%) | 2 | (2.74%) | 0.182 |
| Yes | 68 | (90.67%) | 71 | (97.26%) | ||
| Additional feeding, Day I, n (%) | No | 59 | (78.67%) | 52 | (71.23%) | 0.039 |
| MM | 16 | (21.33%) | 15 | (20.55%) | ||
| G | 0 | (0.00%) | 6 | (8.22%) | ||
| Additional feeding, Day II, n (%) | No | 47 | (62.67%) | 44 | (60.27%) | 0.665 |
| MM | 25 | (33.33%) | 28 | (38.36%) | ||
| G | 2 | (2.67%) | 1 | (1.37%) | ||
| BMM | 1 | (1.33%) | 0 | (0.00%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotfis, K.; Wojciechowska, A.; Zimny, M.; Jamioł-Milc, D.; Szylińska, A.; Kwiatkowski, S.; Kaim, K.; Dołęgowska, B.; Stachowska, E.; Zukowski, M.; et al. Preoperative Oral Carbohydrate (CHO) Supplementation Is Beneficial for Clinical and Biochemical Outcomes in Patients Undergoing Elective Cesarean Delivery under Spinal Anaesthesia—A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 4978. https://doi.org/10.3390/jcm12154978
Kotfis K, Wojciechowska A, Zimny M, Jamioł-Milc D, Szylińska A, Kwiatkowski S, Kaim K, Dołęgowska B, Stachowska E, Zukowski M, et al. Preoperative Oral Carbohydrate (CHO) Supplementation Is Beneficial for Clinical and Biochemical Outcomes in Patients Undergoing Elective Cesarean Delivery under Spinal Anaesthesia—A Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(15):4978. https://doi.org/10.3390/jcm12154978
Chicago/Turabian StyleKotfis, Katarzyna, Arleta Wojciechowska, Małgorzata Zimny, Dominika Jamioł-Milc, Aleksandra Szylińska, Sebastian Kwiatkowski, Karolina Kaim, Barbara Dołęgowska, Ewa Stachowska, Maciej Zukowski, and et al. 2023. "Preoperative Oral Carbohydrate (CHO) Supplementation Is Beneficial for Clinical and Biochemical Outcomes in Patients Undergoing Elective Cesarean Delivery under Spinal Anaesthesia—A Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 15: 4978. https://doi.org/10.3390/jcm12154978
APA StyleKotfis, K., Wojciechowska, A., Zimny, M., Jamioł-Milc, D., Szylińska, A., Kwiatkowski, S., Kaim, K., Dołęgowska, B., Stachowska, E., Zukowski, M., Pankowiak, M., Torbé, A., & Wischmeyer, P. (2023). Preoperative Oral Carbohydrate (CHO) Supplementation Is Beneficial for Clinical and Biochemical Outcomes in Patients Undergoing Elective Cesarean Delivery under Spinal Anaesthesia—A Randomized Controlled Trial. Journal of Clinical Medicine, 12(15), 4978. https://doi.org/10.3390/jcm12154978